Abstract
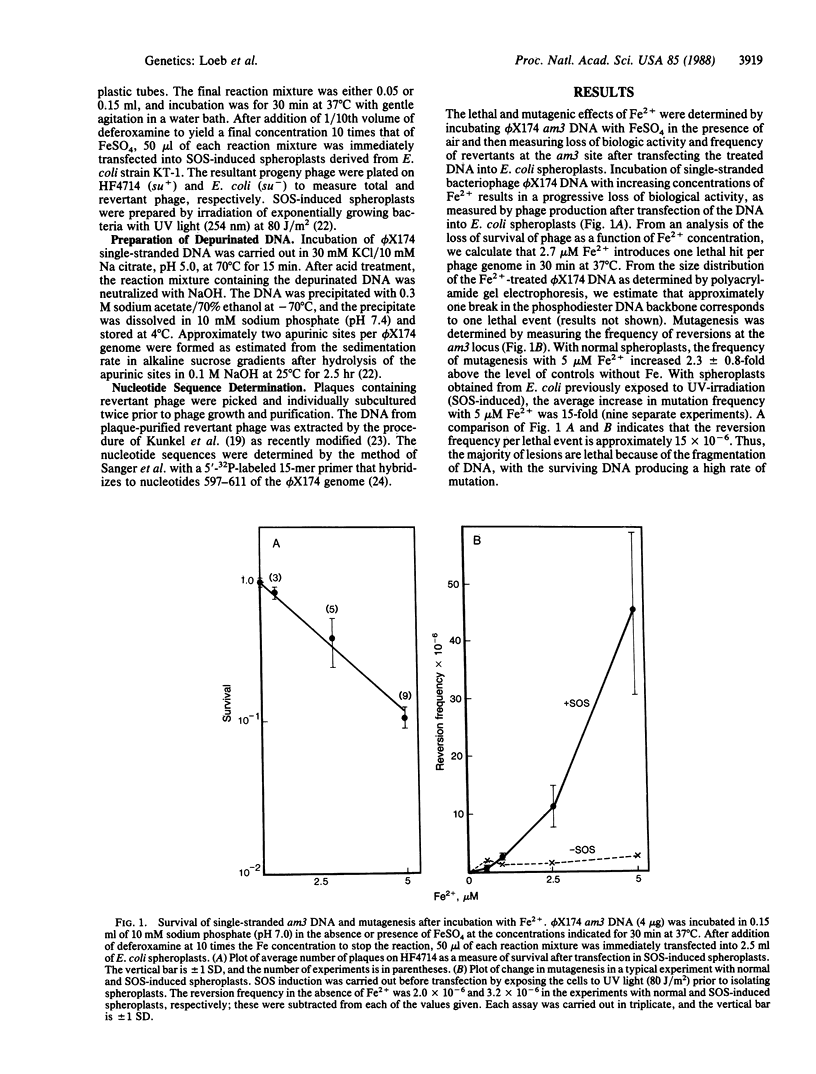
Oxygen free radicals are highly reactive species generated by many cellular oxidation-reduction processes. These radicals damage cellular constituents and have been causally implicated in the pathogenesis of many human diseases. We report here that oxygen free radicals generated by Fe2+ in aqueous solution are mutagenic. Aerobic incubation of luminal diameter X174 am3 (amber 3 mutation) DNA with Fe2+ results in decreased phage survival when the treated DNA is transfected into Escherichia coli spheroplasts. Transfection of the treated DNA into SOS-induced spheroplasts results in an increase in mutagenesis as great as 50-fold. Both killing and mutagenesis can be prevented by binding of Fe2+ with deferoxamine or by the addition of catalase or mannitol. These results suggest that DNA damage and mutagenesis brought about by Fe2+ are likely to occur by a Fenton-type mechanism that involves the generation of (i) hydrogen peroxide by the autoxidation of iron and (ii) hydroxyl radicals by the interaction of the hydrogen peroxide with Fe2+. DNA sequence analysis of the Fe2+-induced mutants indicates that reversion of the phage phenotype to wild type occurs largely by a transversion type of mutation involving substitution of deoxyadenosine for thymidine opposite a template deoxyadenosine. Mutagenesis is not abolished by incubation of Fe2+-treated luminal diameter X174 am3 DNA with an apurinic endonuclease and only partially abolished by incubation with alkali, suggesting that a large fraction of the mutagenesis by oxygen free radicals is not caused by formation of apurinic sites but instead involves an as-yet-to-be-defined alteration in deoxyadenosine. These findings raise the possibility that free iron localized in cellular DNA may cause mutations by the generation of oxygen free radicals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aft R. L., Mueller G. C. Hemin-mediated DNA strand scission. J Biol Chem. 1983 Oct 10;258(19):12069–12072. [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Andronikashvili E. L., Mosulishvili L. M., Belokobilski A. I., Kharabadze N. E., Tevzieva T. K., Efremova E. Y. Content of some trace elements in sarcoma M-1 DNA in dynamics of malignant growth. Cancer Res. 1974 Feb;34(2):271–274. [PubMed] [Google Scholar]
- Birnboim H. C. Factors which affect DNA strand breakage in human leukocytes exposed to a tumor promoter, phorbol myristate acetate. Can J Physiol Pharmacol. 1982 Nov;60(11):1359–1366. doi: 10.1139/y82-203. [DOI] [PubMed] [Google Scholar]
- Blumberg B. S., Lustbader E. D., Whitford P. L. Changes in serum iron levels due to infection with hepatitis B virus. Proc Natl Acad Sci U S A. 1981 May;78(5):3222–3224. doi: 10.1073/pnas.78.5.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn K., Fridovich I. DNA strand scission by enzymically generated oxygen radicals. Arch Biochem Biophys. 1981 Feb;206(2):414–419. doi: 10.1016/0003-9861(81)90108-9. [DOI] [PubMed] [Google Scholar]
- Cadet J., Berger M. Radiation-induced decomposition of the purine bases within DNA and related model compounds. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Feb;47(2):127–143. doi: 10.1080/09553008514550201. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. A. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1209–1215. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Lewis C. A. Hydroxyl free radical formation from hydrogen peroxide by ferrous iron-nucleotide complexes. Biochemistry. 1983 May 24;22(11):2645–2649. doi: 10.1021/bi00280a008. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Harris J., West M., Wong P. K. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986 Jun 13;137(2):841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K., Troll W., Teebor G. W., Steinberg J. J. Radiation-like modification of bases in DNA exposed to tumor promoter-activated polymorphonuclear leukocytes. Cancer Res. 1986 Nov;46(11):5533–5540. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Inoue S., Kawanishi S. Hydroxyl radical production and human DNA damage induced by ferric nitrilotriacetate and hydrogen peroxide. Cancer Res. 1987 Dec 15;47(24 Pt 1):6522–6527. [PubMed] [Google Scholar]
- Isildar M., Schuchmann M. N., Schulte-Frohlinde D., von Sonntag C. gamma-Radiolysis of DNA in oxygenated aqueous solutions: alterations at the sugar moiety. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Oct;40(4):347–354. doi: 10.1080/09553008114551301. [DOI] [PubMed] [Google Scholar]
- Kane C. M., Linn S. Purification and characterization of an apurinic/apyrimidinic endonuclease from HeLa cells. J Biol Chem. 1981 Apr 10;256(7):3405–3414. [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Wallace S. S. Exonuclease III recognizes urea residues in oxidized DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8354–8358. doi: 10.1073/pnas.82.24.8354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Mori F., Kasai H., Inoue H., Iwai S., Miura K., Ohtsuka E., Nishimura S. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987 May 7;327(6117):77–79. doi: 10.1038/327077a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. The accuracy of Escherichia coli DNA polymerase I in copying natural DNA in vitro. J Biol Chem. 1980 Oct 25;255(20):9961–9966. [PubMed] [Google Scholar]
- Lafleur M. V., Woldhuis J., Loman H. Alkali-labile sites in biologically active DNA: comparison of radiation induced potential breaks and apurinic sites. Int J Radiat Biol Relat Stud Phys Chem Med. 1981 Feb;39(2):113–118. doi: 10.1080/09553008114550131. [DOI] [PubMed] [Google Scholar]
- Larramendy M., Mello-Filho A. C., Martins E. A., Meneghini R. Iron-mediated induction of sister-chromatid exchanges by hydrogen peroxide and superoxide anion. Mutat Res. 1987 May;178(1):57–63. doi: 10.1016/0027-5107(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Lesko S. A., Lorentzen R. J., Ts'o P. O. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980 Jun 24;19(13):3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Michelson A. M. Studies in bioluminescence. 10. Chemical models of enzymic oxidations. Biochimie. 1973;55(4):465–479. doi: 10.1016/s0300-9084(73)80213-5. [DOI] [PubMed] [Google Scholar]
- Munkres K. D. Ageing of Neurospora crassa. VIII. Lethality and mutagenicity of ferrous ions, ascorbic acid, and malondialdehyde. Mech Ageing Dev. 1979 May;10(3-4):249–260. doi: 10.1016/0047-6374(79)90039-3. [DOI] [PubMed] [Google Scholar]
- Naqui A., Chance B., Cadenas E. Reactive oxygen intermediates in biochemistry. Annu Rev Biochem. 1986;55:137–166. doi: 10.1146/annurev.bi.55.070186.001033. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrzadeh S. M., Graf E., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984 Dec 10;259(23):14354–14356. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Dunn R. L. Spectra of spontaneous mutations in Escherichia coli strains defective in mismatch correction: the nature of in vivo DNA replication errors. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6220–6224. doi: 10.1073/pnas.84.17.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Koplitz R. M., Tkeshelashvili L. K., Loeb L. A. Metal-induced lethality and mutagenesis: possible role of apurinic intermediates. Mutat Res. 1987 Apr;177(2):179–188. doi: 10.1016/0027-5107(87)90001-7. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. G., Beasley R. P., Blumberg B. S. Iron-binding proteins and risk of cancer in Taiwan. J Natl Cancer Inst. 1986 Apr;76(4):605–610. doi: 10.1093/jnci/76.4.605. [DOI] [PubMed] [Google Scholar]
- Stoewe R., Prütz W. A. Copper-catalyzed DNA damage by ascorbate and hydrogen peroxide: kinetics and yield. Free Radic Biol Med. 1987;3(2):97–105. doi: 10.1016/s0891-5849(87)80003-5. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Teebor G. W., Frenkel K., Goldstein M. S. Ionizing radiation and tritium transmutation both cause formation of 5-hydroxymethyl-2'-deoxyuridine in cellular DNA. Proc Natl Acad Sci U S A. 1984 Jan;81(2):318–321. doi: 10.1073/pnas.81.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Iron(II) EDTA used to measure the helical twist along any DNA molecule. Science. 1985 Nov 8;230(4726):679–681. doi: 10.1126/science.2996145. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Weitberg A. B., Clark E. P., Stossel T. P. Phagocytes as carcinogens: malignant transformation produced by human neutrophils. Science. 1985 Mar 8;227(4691):1231–1233. doi: 10.1126/science.3975611. [DOI] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting R. F., Wei L., Stich H. F. Chromosome-damaging activity of ferritin and its relation to chelation and reduction of iron. Cancer Res. 1981 May;41(5):1628–1636. [PubMed] [Google Scholar]



