Abstract
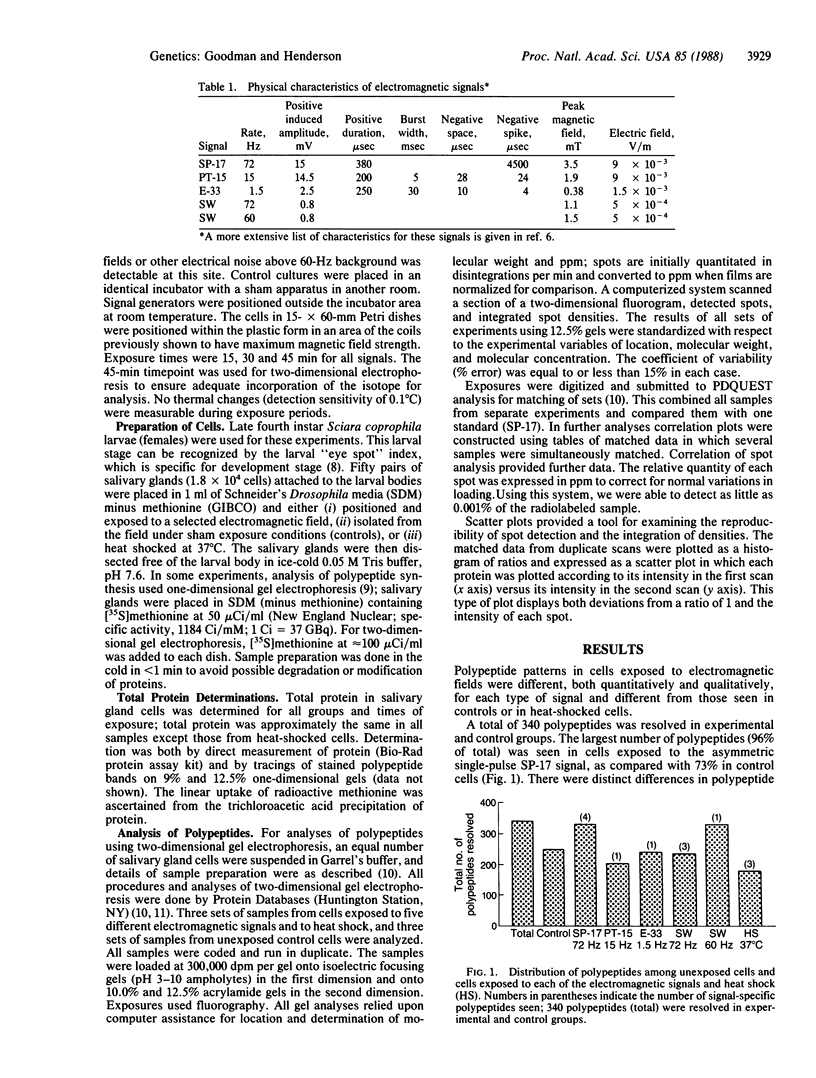
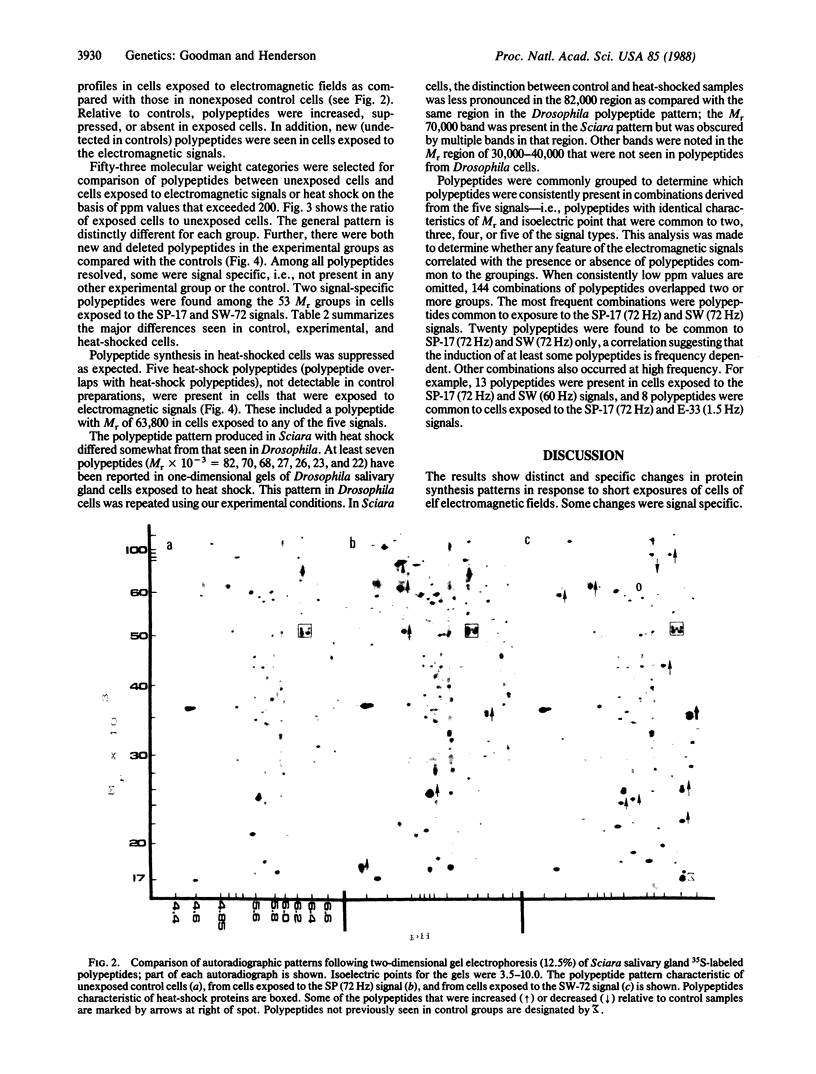
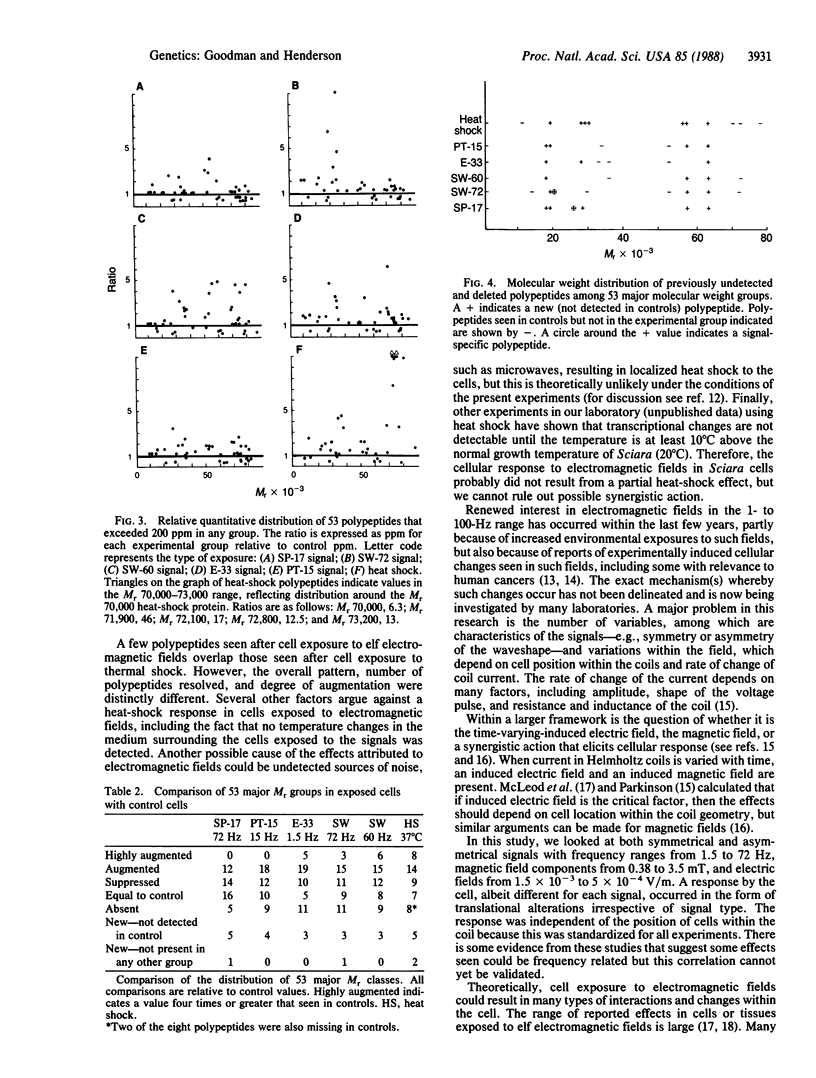
This study demonstrates that exposure of cells to extremely low-frequency electromagnetic fields can cause measurable changes in protein synthesis. Sciara coprophila salivary gland cells were exposed to five low-frequency (1.5-72 Hz) electromagnetic signals: three signals (1.5, 15, and 72 Hz) produced pulsed asymmetric electromagnetic fields and two signals (60 and 72 Hz) were sinusoidal. Subsequent analyses of two-dimensional gels showed that cell exposure to either type of low-frequency electromagnetic field resulted in both qualitative and quantitative changes in patterns of protein synthesis. Thus, signals producing diverse waveform characteristics induced previously undetectable polypeptides, some of which were signal specific and augmented or suppressed other polypeptides as compared with nonexposed cells. The pattern of polypeptide synthesis differed from that seen with heat shock: only five polypeptides in cells exposed to electromagnetic signals overlap those polypeptides exposed to heat shock, and the suppression of protein synthesis characteristic of heat shock does not occur.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adey W. R. Tissue interactions with nonionizing electromagnetic fields. Physiol Rev. 1981 Apr;61(2):435–514. doi: 10.1152/physrev.1981.61.2.435. [DOI] [PubMed] [Google Scholar]
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Bassett C. A., Mitchell S. N., Gaston S. R. Pulsing electromagnetic field treatment in ununited fractures and failed arthrodeses. JAMA. 1982 Feb 5;247(5):623–628. [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., Rabinowitz J. R., House D. E., Joines W. T. A role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics. 1985;6(4):327–337. doi: 10.1002/bem.2250060402. [DOI] [PubMed] [Google Scholar]
- Byus C. V., Pieper S. E., Adey W. R. The effects of low-energy 60-Hz environmental electromagnetic fields upon the growth-related enzyme ornithine decarboxylase. Carcinogenesis. 1987 Oct;8(10):1385–1389. doi: 10.1093/carcin/8.10.1385. [DOI] [PubMed] [Google Scholar]
- GABRUSEWYCZ-GARCIA N. CYTOLOGICAL AND AUTORADIOGRAPHIC STUDIES IN SCIARA COPROPHILA SALIVARY GLAND CHROMOSOMES. Chromosoma. 1964 Aug 14;15:312–344. doi: 10.1007/BF00321517. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Goodman R., Abbott J., Henderson A. S. Transcriptional patterns in the X chromosome of Sciara coprophila following exposure to magnetic fields. Bioelectromagnetics. 1987;8(1):1–7. doi: 10.1002/bem.2250080102. [DOI] [PubMed] [Google Scholar]
- Goodman R., Bassett C. A., Henderson A. S. Pulsing electromagnetic fields induce cellular transcription. Science. 1983 Jun 17;220(4603):1283–1285. doi: 10.1126/science.6857248. [DOI] [PubMed] [Google Scholar]
- Goodman R., Henderson A. S. Sine waves enhance cellular transcription. Bioelectromagnetics. 1986;7(1):23–29. doi: 10.1002/bem.2250070104. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McLeod B. R., Pilla A. A., Sampsel M. W. Electromagnetic fields induced by Helmholtz aiding coils inside saline-filled boundaries. Bioelectromagnetics. 1983;4(4):357–370. doi: 10.1002/bem.2250040407. [DOI] [PubMed] [Google Scholar]
- Murray J. C., Farndale R. W. Modulation of collagen production in cultured fibroblasts by a low-frequency, pulsed magnetic field. Biochim Biophys Acta. 1985 Jan 28;838(1):98–105. doi: 10.1016/0304-4165(85)90255-7. [DOI] [PubMed] [Google Scholar]
- Parkinson W. C. Comments on the use of electromagnetic fields in biological studies. Calcif Tissue Int. 1985 Mar;37(2):198–207. doi: 10.1007/BF02554842. [DOI] [PubMed] [Google Scholar]
- Tenforde T. S. Thermoregulation in rodents exposed to high-intensity stationary magnetic fields. Bioelectromagnetics. 1986;7(3):341–346. doi: 10.1002/bem.2250070310. [DOI] [PubMed] [Google Scholar]
- Wertheimer N., Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979 Mar;109(3):273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]



