Abstract
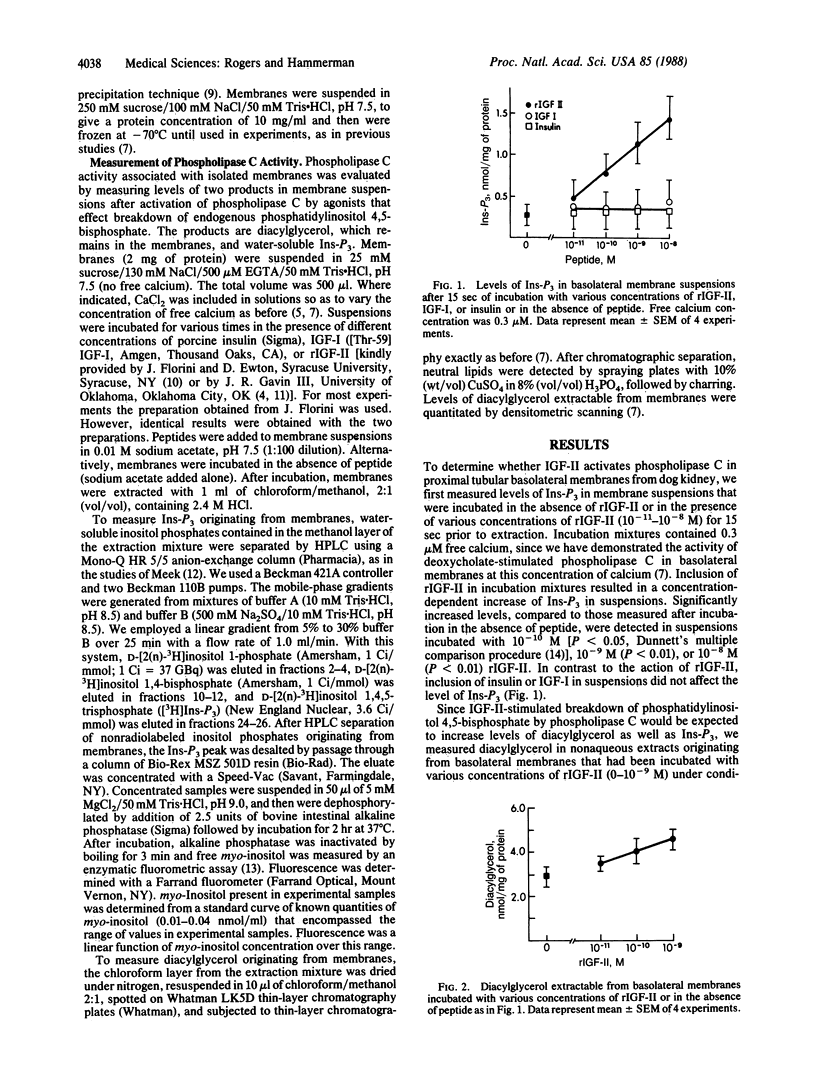
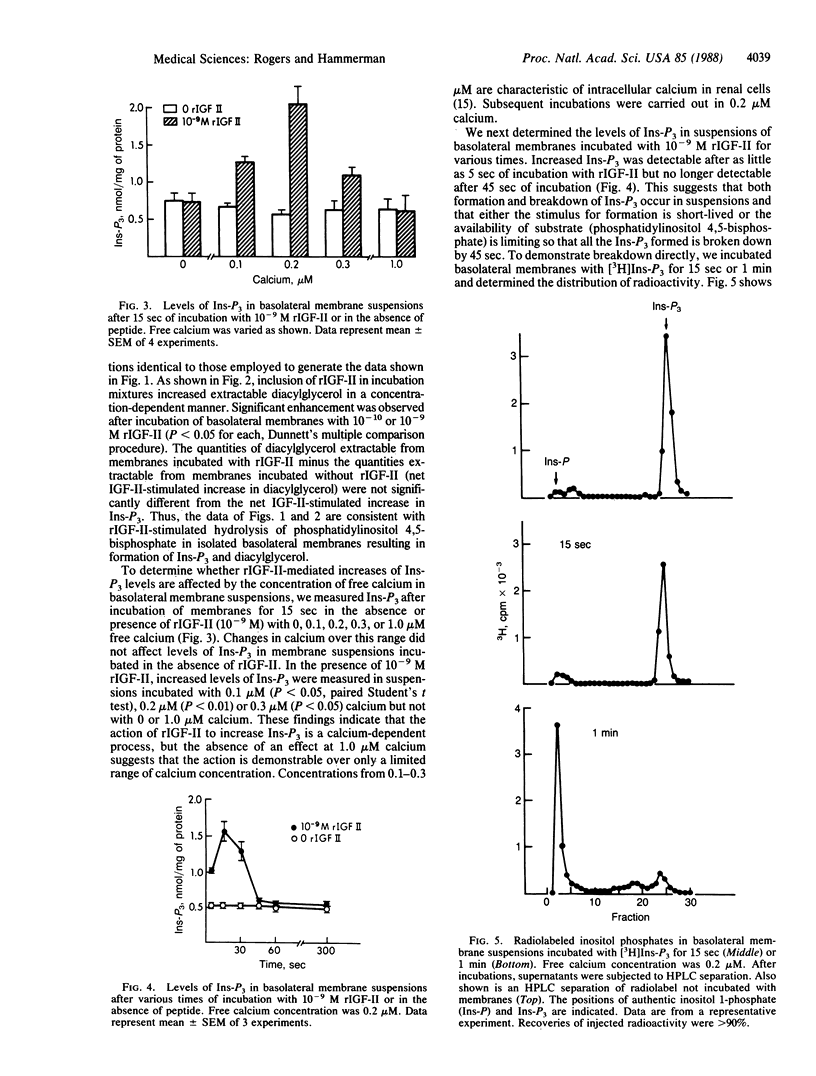
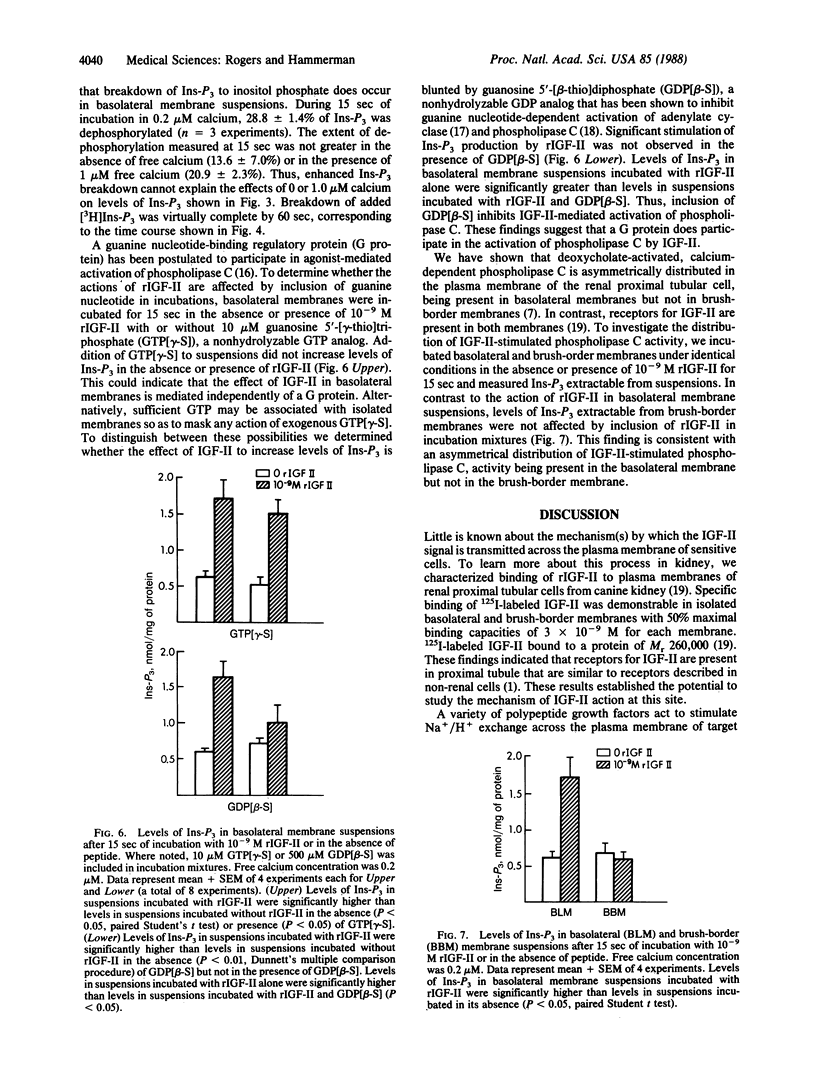
To determine whether insulin-like growth factor II (IGF-II) activates phospholipase C in the basolateral membrane of the renal proximal tubular cell, we incubated basolateral membranes isolated from canine kidney with rat IGF-II (rIGF-II) and measured levels of inositol trisphosphate (Ins-P3) in suspensions and of diacylglycerol extractable from the membranes. Incubation with rIGF-II increased levels of Ins-P3 and diacylglycerol in a concentration-dependent manner. Significant enhancement of Ins-P3 levels and extractable diacylglycerol occurred in suspensions incubated with as little as 10(-10) M rIGF-II. Elevated levels of Ins-P3 were measured after as little as 5 sec of incubation. Increases were no longer detectable after 45 sec of incubation, due to dephosphorylation of Ins-P3 in membrane suspensions. Incubation with either insulin or insulin-like growth factor I did not affect the level of Ins-P3. IGF-II-stimulated increases in Ins-P3 did not occur when basolateral membranes were suspended in the absence of free calcium. Increases were demonstrable in basolateral membrane suspensions in 0.1, 0.2, or 0.3 microM calcium, but not in 1.0 microM calcium. Inclusion of guanosine 5'-[gamma-thio]triphosphate in incubation mixtures did not increase levels of Ins-P3, nor did it enhance the action of rIGF-II in this regard. However, inclusion of guanosine 5'-[beta-thio]diphosphate inhibited rIGF-II stimulation of Ins-P3 production. In contrast to findings with basolateral membrane suspensions, incubation with rIGF-II did not increase levels of Ins-P3 in suspensions of isolated brush-border membranes. Our data are consistent with IGF-II-mediated activation of phospholipase C in isolated proximal tubular basolateral membranes. Such an action could reflect the mechanism by which the IGF-II "signal" is transmitted across the basolateral membrane of the renal proximal tubular cell and by which the actions of this peptide are mediated in renal and non-renal cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dragsten P. R., Handler J. S., Blumenthal R. Fluorescent membrane probes and the mechanism of maintenance of cellular asymmetry in epithelia. Fed Proc. 1982 Jan;41(1):48–53. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Ewton D. Z., Falen S. L., Florini J. R. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987 Jan;120(1):115–123. doi: 10.1210/endo-120-1-115. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Calcium signalling in cells--molecular mechanisms. Kidney Int Suppl. 1987 Dec;23:S68–S81. [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Gavin J. R., 3rd Binding of IGF I and IGF I-stimulated phosphorylation in canine renal basolateral membranes. Am J Physiol. 1986 Jul;251(1 Pt 1):E32–E41. doi: 10.1152/ajpendo.1986.251.1.E32. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Gavin J. R., 3rd Binding of insulin-like growth Factor ii and multiplication-stimulating activity-stimulated phosphorylation in basolateral membranes from dog kidney. J Biol Chem. 1984 Nov 10;259(21):13511–13517. [PubMed] [Google Scholar]
- Hammerman M. R., Gavin J. R., 3rd Insulin-stimulated phosphorylation and insulin binding in canine renal basolateral membranes. Am J Physiol. 1984 Sep;247(3 Pt 2):F408–F417. doi: 10.1152/ajprenal.1984.247.3.F408. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Hruska K. A. Cyclic AMP-dependent protein phosphorylation in canine renal brush-border membrane vesicles is associated with decreased phosphate transport. J Biol Chem. 1982 Jan 25;257(2):992–999. [PubMed] [Google Scholar]
- Hammerman M. R., Rogers S. Distribution of IGF receptors in the plasma membrane of proximal tubular cells. Am J Physiol. 1987 Nov;253(5 Pt 2):F841–F847. doi: 10.1152/ajprenal.1987.253.5.F841. [DOI] [PubMed] [Google Scholar]
- Hammerman M. R., Rogers S., Morrissey J. J., Gavin J. R., 3rd Phorbol ester-stimulated phosphorylation of basolateral membranes from canine kidney. Am J Physiol. 1986 Jun;250(6 Pt 2):F1073–F1081. doi: 10.1152/ajprenal.1986.250.6.F1073. [DOI] [PubMed] [Google Scholar]
- Macara I. G. Oncogenes, ions, and phospholipids. Am J Physiol. 1985 Jan;248(1 Pt 1):C3–11. doi: 10.1152/ajpcell.1985.248.1.C3. [DOI] [PubMed] [Google Scholar]
- Meek J. L. Inositol bis-, tris-, and tetrakis(phosphate)s: analysis in tissues by HPLC. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4162–4166. doi: 10.1073/pnas.83.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellas J., Gavin J. R., 3rd, Hammerman M. R. Multiplication-stimulating activity-induced alkalinization of canine renal proximal tubular cells. J Biol Chem. 1986 Nov 5;261(31):14437–14442. [PubMed] [Google Scholar]
- Mellas J., Hammerman M. R. Phorbol ester-induced alkalinization of canine renal proximal tubular cells. Am J Physiol. 1986 Mar;250(3 Pt 2):F451–F459. doi: 10.1152/ajprenal.1986.250.3.F451. [DOI] [PubMed] [Google Scholar]
- Nishimoto I., Hata Y., Ogata E., Kojima I. Insulin-like growth factor II stimulates calcium influx in competent BALB/c 3T3 cells primed with epidermal growth factor. Characteristics of calcium influx and involvement of GTP-binding protein. J Biol Chem. 1987 Sep 5;262(25):12120–12126. [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Apfeldorf W., Barrett P. Cellular mechanism of hormone action in the kidney: messenger function of calcium and cyclic AMP. Kidney Int. 1986 Jan;29(1):90–97. doi: 10.1038/ki.1986.11. [DOI] [PubMed] [Google Scholar]
- Rebecchi M. J., Rosen O. M. Stimulation of polyphosphoinositide hydrolysis by thrombin in membranes from human fibroblasts. Biochem J. 1987 Jul 1;245(1):49–57. doi: 10.1042/bj2450049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rogers S. A., Hammerman M. R. Calcium-activated phospholipase C associated with canine renal basolateral membranes. Am J Physiol. 1987 Jan;252(1 Pt 2):F74–F82. doi: 10.1152/ajprenal.1987.252.1.F74. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Morrison A. R., Lowry O. H. Enzymatic fluorometric assay for myo-inositol trisphosphate. Anal Biochem. 1987 May 1;162(2):562–568. doi: 10.1016/0003-2697(87)90434-9. [DOI] [PubMed] [Google Scholar]
- Weinman E. J., Shenolikar S. Protein kinase C activates the renal apical membrane Na+/H+ exchanger. J Membr Biol. 1986;93(2):133–139. doi: 10.1007/BF01870805. [DOI] [PubMed] [Google Scholar]
- Windus D. W., Cohn D. E., Klahr S., Hammerman M. R. Glutamine transport in renal basolateral vesicles from dogs with metabolic acidosis. Am J Physiol. 1984 Jan;246(1 Pt 2):F78–F86. doi: 10.1152/ajprenal.1984.246.1.F78. [DOI] [PubMed] [Google Scholar]


