Abstract
In bacteria, archaea, and the eukaryote nucleus, the endonuclease ribonuclease P (RNase P) is composed of a catalytic RNA that is assisted by protein subunits. Holzmann et al. (2008) now provide evidence that the human mitochondrial RNase P is an entirely protein-based enzyme.
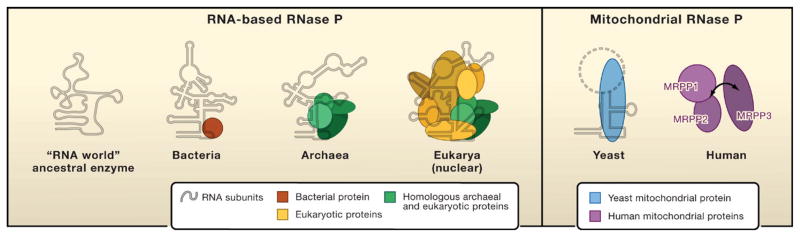
Ribonuclease P (RNase P) is responsible for the 5′ maturation of precursor transfer RNAs (pre-tRNAs). The catalytic RNA subunit of bacterial RNase P was one of the first examples of RNA-based catalysis. All forms of RNase P characterized to date have a fundamentally similar RNA subunit that retains catalytic activity. This catalytic RNA is widely presumed to be a remnant of the hypothesized “RNA world” in which RNA is thought to have been the original functional macromolecule preceding the evolution of protein. Although the nature of the RNase P catalytic RNA seems evolutionarily conserved, the number of proteins associated with the RNA actually increases with the complexity of the organism—ranging from one in bacteria to at least four in archaea and to at least nine in eukaryotes (Figure 1) (reviewed in Walker and Engelke, 2006). In contrast, the composition of RNase P in endosymbiont organelles (mitochondria and chloroplasts) has been less clear. Yeast mitochondrial RNase P (mtRNase P) is a ribonucleoprotein composed of an RNA encoded by the mitochondrial genome and a single protein encoded by the nuclear genome. However, biochemical studies of RNase P activity from plant chloroplasts and human mitochondria have suggested protein-only enzymatic activity (Rossmanith and Karwan, 1998; Thomas et al., 1995). In particular, there has been considerable debate over whether the metazoan mtRNase P also contains RNA (Rossmanith and Potuschak, 2001). One study reported a probable protein-only activity for the human mtRNase P (Rossmanith and Karwan, 1998), whereas another study suggested that the human mtRNase P contained RNA identical to that found in human nuclear RNase P (Puranam and Attardi, 2001). Given our knowledge about the highly conserved RNA-based enzymes, the notion of a protein-only RNase P was not widely accepted. Now, in this issue, Holzmann et al. (2008) identify components of human mitochondrial RNase P and demonstrate that the catalytic activity of this enzyme derives entirely from proteins.
Figure 1. The Evolution of RNase P.
(Left) The compositions of characterized RNA-based RNase P enzymes from bacteria, archaea, and eukarya show an increase in protein content with increased complexity of the organism. The sites of interaction between RNase P subunits are not known in most cases and are represented schematically. The structure of the proposed ancestral RNA-only RNase P is not known and is assumed to have the critical structural elements conserved in all forms of RNase P RNA. (Right) The composition of the fully characterized mitochondrial RNase P is shown for yeast (S. cerevisiae) and human (H. sapiens). Human mtRNase P is composed only of proteins (mitochondrial RNase P proteins 1, 2, 3) (Holzmann et al., 2008). The third subunit of the human mtRNase P (MRPP3) binds to the two-protein subcomplex weakly and may associate dynamically (arrow). Although key structural elements of the RNA subunit are preserved in various yeast mtRNase P enzymes (solid line), the entire RNA structure is not well defined (dashed line).
Using a combination of partial purification and proteomic techniques, Holzmann et al. were able to identify multiple human mtRNase P candidate proteins associated with mtRNase P activity. Ultimately, affinity tagging of selected candidate proteins and purification under mild conditions led to the identification of a rather unstable complex that conferred mtRNase P activity. Surprisingly, this mitochondrial enzyme contains no RNA and consists only of three protein subunits that the authors term mitochondrial RNase P proteins 1, 2, and 3 (MRPP1–3). Holzmann and colleagues were successful in reconstituting mtRNase P activity in vitro using only recombinant MRPP1, 2, and 3 proteins expressed and purified from bacteria. The reconstituted MRPP protein complex produced authentic chemical cleavage products consistent with mtRNase P activity. Essential controls ruled out the possibility of contamination of the recombinant protein preparations with bacterial RNase P. The authors also used small-interfering RNAs (siRNAs) to deplete each MRPP protein individually in cultured human cells. Loss of any of the three proteins resulted in a defect in mitochondrial tRNA processing. With these combined data, the existence of protein-only RNase P activity in the human mitochondria now seems incontrovertible.
The identity of the mitochondrial RNase P proteins uncovered by Holzmann and colleagues is particularly surprising. The MRPP proteins are unrelated to proteins in any of the known bacterial, archaeal, or eukaryotic nuclear RNase P enzymes (the “RNA-based” enzymes). It appears that human mtRNase P is a composite of pre-existing protein enzymes. This “patchwork” enzyme most likely arose through a process of convergent evolution toward a viable protein-based pre-tRNA cleavage activity that could replace the function of the ancestral RNA-based RNase P. In the mtRNase P complex, MRPP1 is a probable tRNA methylase. The authors propose that MRPP1 provides tRNA-binding specificity to the RNase P enzyme. The function of MRPP2, which binds tightly to MRPP1 and is a member of the short chain dehydrogenase/reductase (SDR) protein family, is not yet clear. MRPP3 does not stably associate with the MRPP1/MRPP2 complex and is a previously uncharacterized protein (KIAA0391) with putative RNA-binding and metallonuclease domains (Figure 1). Holzmann and colleagues propose that MRPP3 may provide the enzymatic cleavage activity for the patchwork enzyme. This speculation on the functions of each subunit offers an appropriate starting point for biochemical characterization of the reconstituted mtRNase P enzyme and its individual constituents.
Why did only some mitochondria (and possibly chloroplasts) evolve a protein-based RNase P? Both mitochondria and chloroplasts are thought to be endosymbionts of early bacteria. As such, these organelles have undergone a reduction in overall complexity (including their genomes) as they became more specialized subcellular entities. The answer might be found in the degree to which the RNA-based enzymes have evolved increased protein complexity to fulfill additional functions in the cell. If the mtRNase P retained only the pre-tRNA cleavage function, it may have been simpler to replace than an enzyme with multiple functions. Even the bacterial RNase P holoenzyme, which contains only a large RNA and a single small protein, recognizes and cleaves a variety of non-tRNA substrates thought to structurally resemble pre-tRNA. There is also evidence that the eukaryotic nuclear RNase P, which contains RNA and nine proteins, participates in the processing of non-tRNA substrates (Coughlin et al., 2008). A related enzyme in eukaryotes, RNase MRP (mitochondrial RNA processing), also cleaves a variety of substrates (Gill et al., 2004). Expanding the functions in the RNA-based RNase P enzymes by adding “accessory proteins” might be comparable to the expansion of the eukaryotic transcription machinery, which retains the well-conserved catalytic subunits of bacterial RNA polymerases. Interestingly, the notion that the mitochondrial environment may confer a different selective pressure on RNase P function could also explain the rapid divergence of the RNA structure in yeast mitochondrial RNase P (Figure 1) (Seif et al., 2003).
The evolutionary departure of this ancient tRNA processing machinery in metazoan mitochondria provides unique insights into molecular evolution in the RNA and protein worlds. The findings of Holzmann et al. may also have implications in the study of disease. Mutations within mitochondrial tRNAs (mt-tRNAs) have been linked to a number of human diseases such as neuromuscular and neurodegenerative disorders (reviewed in Florentz et al., 2003). A limited analysis using partially purified human mtRNase P has shown that certain disease-linked mt-tRNA mutations significantly impair pre-tRNA cleavage in vitro. A functional reconstitution system for the human mtRNase P will allow a range of known pathological mt-tRNA mutations to be studied in detail and could be useful in guiding studies in mammalian model systems.
References
- Coughlin DJ, Pleiss JA, Walker SC, Whitworth GB, Engelke DR. Proc Natl Acad Sci USA. 2008;105:12218–12223. doi: 10.1073/pnas.0801906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentz C, Sohm B, Tryoen-Toth P, Putz J, Sissler M. Cell Mol Life Sci. 2003;60:1356–1375. doi: 10.1007/s00018-003-2343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T, Cai T, Aulds J, Wierzbicki S, Schmitt ME. Mol Cell Biol. 2004;24:945–953. doi: 10.1128/MCB.24.3.945-953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann J, Frank P, Löffler E, Bennett KL, Gerner C, Rosmanith W. Cell. 2008 doi: 10.1016/j.cell.2008.09.013. this issue. [DOI] [PubMed] [Google Scholar]
- Puranam RS, Attardi G. Mol Cell Biol. 2001;21:548–561. doi: 10.1128/MCB.21.2.548-561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmanith W, Karwan RM. Biochem Biophys Res Commun. 1998;247:234–241. doi: 10.1006/bbrc.1998.8766. [DOI] [PubMed] [Google Scholar]
- Rossmanith W, Potuschak T. Mol Cell Biol. 2001;21:8236–8237. doi: 10.1128/MCB.21.23.8236-8237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif ER, Forget L, Martin NC, Lang BF. RNA. 2003;9:1073–1083. doi: 10.1261/rna.5880403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BC, Gao L, Stomp D, Li X, Gegenheimer PA. Nucleic Acids Symp Ser. 1995;33:95–98. [PubMed] [Google Scholar]
- Walker SC, Engelke DR. Crit Rev Biochem Mol Biol. 2006;41:77–102. doi: 10.1080/10409230600602634. [DOI] [PMC free article] [PubMed] [Google Scholar]