Abstract
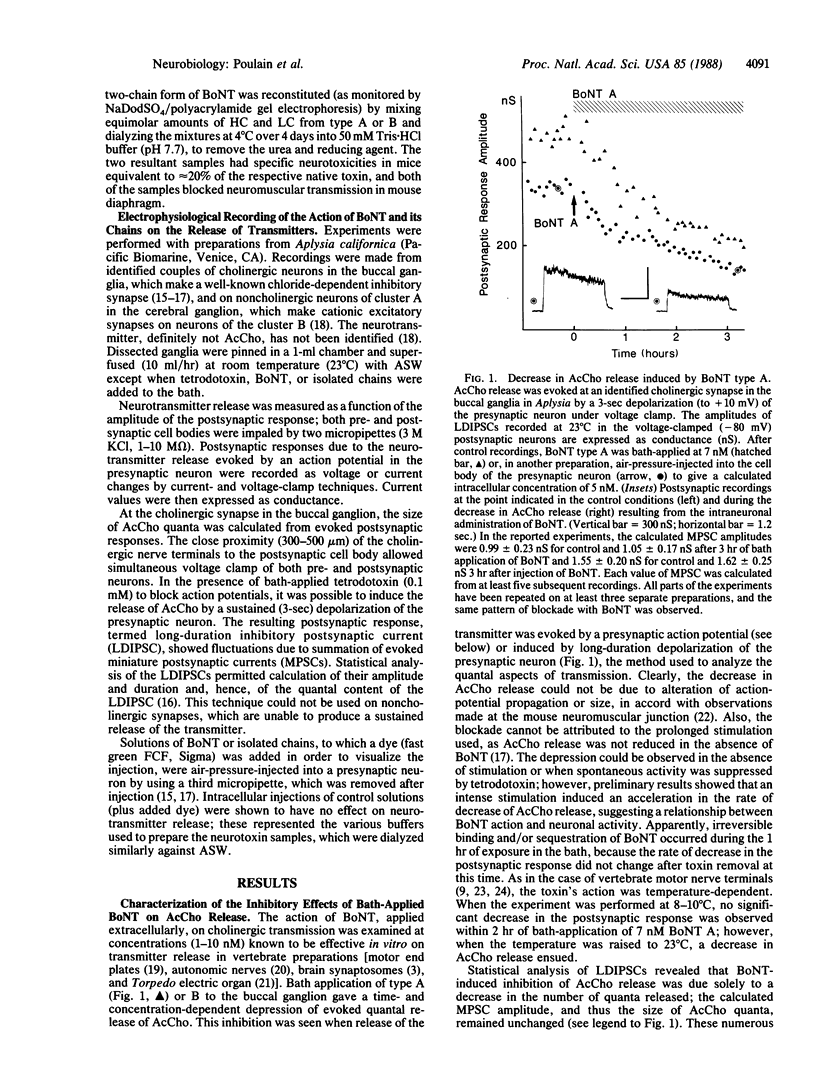
Botulinum neurotoxins (types A and B), which are microbial proteins consisting of two disulfide-linked chains, inhibit specifically and with high potency the release of acetylcholine from peripheral nerve terminals. As a prerequisite for a long-term development of effective treatments for botulism, the internalization and inhibitory action of the toxin and its constituent chains were examined by electrophysiological methods at identified synapses in Aplysia preparations that allow both intracellular and bath application of the neurotoxins. Intracellular recordings from cholinergic cells of the buccal ganglion demonstrated that extra- or intracellular application of low doses of botulinum neurotoxin results in a specific blockade of evoked transmitter release, without changing the quantal size; an intraneuronal site of action has thus been established. In contrast, release from noncholinergic neurons of cerebral ganglion was prevented by the neurotoxin only after injection into the cell. Purified preparations of the individual renatured chains, shown to be nontoxic in a mouse bioassay, failed to affect acetylcholine release when applied extra- or intracellularly. However, inhibition of release was observed after intracellular administration of both chains or when the light chain was injected and the heavy chain was bath-applied. These findings show that both chains are required on the cytosolic side of the neuronal plasma membrane for expression of toxicity and that the cholinergic specificity of the neurotoxin is attributable to its heavy chain, which mediates targeting and subsequent neuronal uptake.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black J. D., Dolly J. O. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol. 1986 Aug;103(2):521–534. doi: 10.1083/jcb.103.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. D., Dolly J. O. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. II. Autoradiographic evidence for its uptake into motor nerves by acceptor-mediated endocytosis. J Cell Biol. 1986 Aug;103(2):535–544. doi: 10.1083/jcb.103.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. D., Dolly J. O. Selective location of acceptors for botulinum neurotoxin A in the central and peripheral nervous systems. Neuroscience. 1987 Nov;23(2):767–779. doi: 10.1016/0306-4522(87)90094-7. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Cole H. A. Diphtheria toxin subunit active in vitro. Science. 1969 Jun 6;164(3884):1179–1181. doi: 10.1126/science.164.3884.1179. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Black J., Williams R. S., Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984 Feb 2;307(5950):457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- Dolly J. O., Lande S., Wray D. W. The effects of in vitro application of purified botulinum neurotoxin at mouse motor nerve terminals. J Physiol. 1987 May;386:475–484. doi: 10.1113/jphysiol.1987.sp016546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Mallart A., Brigant J. L. Botulinum A toxin and tetanus toxin do not affect presynaptic membrane currents in mammalian motor nerve endings. Brain Res. 1983 Jul 4;270(2):373–375. doi: 10.1016/0006-8993(83)90617-0. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Schmitt A. Transmitter release in tetanus and botulinum A toxin-poisoned mammalian motor endplates and its dependence on nerve stimulation and temperature. Pflugers Arch. 1983 Nov;399(3):228–234. doi: 10.1007/BF00656720. [DOI] [PubMed] [Google Scholar]
- Elston J. S., Lee J. P., Powell C. M., Hogg C., Clark P. Treatment of strabismus in adults with botulinum toxin A. Br J Ophthalmol. 1985 Oct;69(10):718–724. doi: 10.1136/bjo.69.10.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. M., Williams R. S., Shone C. C., Hambleton P., Melling J., Dolly J. O. Botulinum neurotoxin type B. Its purification, radioiodination and interaction with rat-brain synaptosomal membranes. Eur J Biochem. 1986 Jan 15;154(2):409–416. doi: 10.1111/j.1432-1033.1986.tb09413.x. [DOI] [PubMed] [Google Scholar]
- Gaillard W. D., Carpenter D. O. On the transmitter at the A-to-B cell in Aplysia californica. Brain Res. 1986 May 14;373(1-2):311–315. doi: 10.1016/0006-8993(86)90345-8. [DOI] [PubMed] [Google Scholar]
- Habermann E., Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Janicki P. K., Habermann E. Tetanus and botulinum toxins inhibit, and black widow spider venom stimulates the release of methionine-enkephalin-like material in vitro. J Neurochem. 1983 Aug;41(2):395–402. doi: 10.1111/j.1471-4159.1983.tb04755.x. [DOI] [PubMed] [Google Scholar]
- Knight D. E. Botulinum toxin types A, B and D inhibit catecholamine secretion from bovine adrenal medullary cells. FEBS Lett. 1986 Oct 27;207(2):222–226. doi: 10.1016/0014-5793(86)81492-2. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Togashi S., Sakaguchi G. Separation of Clostridium botulinum type A derivative toxin into two fragments. Jpn J Med Sci Biol. 1981 Apr;34(2):61–68. doi: 10.7883/yoken1952.34.61. [DOI] [PubMed] [Google Scholar]
- MacKenzie I., Burnstock G., Dolly J. O. The effects of purified botulinum neurotoxin type A on cholinergic, adrenergic and non-adrenergic, atropine-resistant autonomic neuromuscular transmission. Neuroscience. 1982 Apr;7(4):997–1006. doi: 10.1016/0306-4522(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Molgó J., Thesleff S. Studies on the mode of action of botulinum toxin type A at the frog neuromuscular junction. Brain Res. 1984 Apr 16;297(2):309–316. doi: 10.1016/0006-8993(84)90572-9. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E., Dreyer F. Intracellularly injected tetanus toxin inhibits exocytosis in bovine adrenal chromaffin cells. Nature. 1986 Nov 6;324(6092):76–78. doi: 10.1038/324076a0. [DOI] [PubMed] [Google Scholar]
- Poulain B., Baux G., Tauc L. Presynaptic transmitter content controls the number of quanta released at a neuro-neuronal cholinergic synapse. Proc Natl Acad Sci U S A. 1986 Jan;83(1):170–173. doi: 10.1073/pnas.83.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Prieto J., Sihra T. S., Evans D., Ashton A., Dolly J. O., Nicholls D. G. Botulinum toxin A blocks glutamate exocytosis from guinea-pig cerebral cortical synaptosomes. Eur J Biochem. 1987 Jun 15;165(3):675–681. doi: 10.1111/j.1432-1033.1987.tb11494.x. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985 Sep 5;260(19):10461–10466. [PubMed] [Google Scholar]
- Simonneau M., Tauc L., Baux G. Quantal release of acetylcholine examined by current fluctuation analysis at an identified neuro-neuronal synapse of Aplysia. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1661–1665. doi: 10.1073/pnas.77.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980 Jan;212(1):16–21. [PubMed] [Google Scholar]
- Simpson L. L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981 Sep;33(3):155–188. [PubMed] [Google Scholar]
- Tauc L., Hoffmann A., Tsuji S., Hinzen D. H., Faille L. Transmission abolished on a cholinergic synapse after injection of acetylcholinesterase into the presynaptic neurone. Nature. 1974 Aug 9;250(5466):496–498. doi: 10.1038/250496a0. [DOI] [PubMed] [Google Scholar]
- Tse C. K., Dolly J. O., Hambleton P., Wray D., Melling J. Preparation and characterisation of homogeneous neurotoxin type A from Clostridium botulinum. Its inhibitory action on neuronal release of acetylcholine in the absence and presence of beta-bungarotoxin. Eur J Biochem. 1982 Mar 1;122(3):493–500. [PubMed] [Google Scholar]
- Williams R. S., Tse C. K., Dolly J. O., Hambleton P., Melling J. Radioiodination of botulinum neurotoxin type A with retention of biological activity and its binding to brain synaptosomes. Eur J Biochem. 1983 Mar 15;131(2):437–445. doi: 10.1111/j.1432-1033.1983.tb07282.x. [DOI] [PubMed] [Google Scholar]


