Abstract
We investigated whether the maturation of oocyte follicular epithelium of lizard is affected by d-aspartic acid (d-Asp). Our results demonstrated that d-Asp is endogenously present in the oocytes, and its distribution varies during the reproductive cycle and following intraperitoneal administration. At previtellogenesis, it is observed in the cytoplasm and nucleus of pyriform cells, in intermediate cells, in some small cells of the granulosa, in the ooplasm, and in some thecal elements. At vitellogenesis, d-Asp is localized in the proximity of the zona pellucida, in the theca, and in the ooplasm. Injected d-Asp is mainly captured by pyriform cells and ooplasm of previtellogenic oocytes, but a moderate accumulation is evident in the cytoplasm of some small granulosa cells and in the theca. d-Asp also increases the ovarian and plasmatic levels of 17β-estradiol and decreases those of testosterone. As a direct and/or indirect consequence of d-Asp, previtellogenic oocytes grow up and mature, resulting in a higher accumulation of carbohydrates in the granulosa, zona pellucida, and ooplasm, but also a reduction in the thickness of the granulosa layer and an increase of the theca stratum. Taken together, our results show that d-Asp may be related to the synchrony of reproduction, either enhancing the growth and maturation of follicular epithelium or influencing its endocrine functions. (J Histochem Cytochem 58:157–171, 2010)
Keywords: d-aspartic acid, oocyte, lizard, seasonal breeder, reproductive cycle
The female lizard Podarcis s. sicula, like most oviparous lizards inhabiting temperate zones, displays spring-confined activity of the genital apparatus (Filosa 1973). In early spring, the ovary resumes its growth, and several oocytes (two or three from each ovary) become increasingly yolky, to be ovulated in the first part of May. During the breeding period, three egg clutches can be laid by most females at 20-day intervals (Filosa 1973). Lizard follicular growth not only involves the growth and maturation of the oocyte, but also requires the storage of large quantities of yolk in the ooplasm (Filosa 1973; Filosa et al. 1979; Ciarcia et al. 1993). In the lizard P. sicula, growing oocytes have been classified into five different classes according to their diameter (ranging from 60 to 6000–9000 μm) and the structure of their follicular epithelium (Ghiara et al. 1968; Filosa 1973). The ovarian follicle is composed of a central oocyte surrounded by a bilayered, acellular membrane, the zona pellucida, which is bounded by follicular epithelium. In the lizard P. sicula, the follicular epithelium, the granulosa, is a highly dynamic structure that significantly changes in morphology and function in relation to oocyte growth. In primary follicles (40–100-μm diameter), it is composed of a single layer of small stem cells. In mid-previtellogenesis (150–1500-μm follicle diameter), some small cells differentiate into pyriform cells via intermediate cells, and the epithelium becomes multilayered (Andreuccetti et al. 1978; Andreuccetti 1992). As follicular development progresses, the zona pellucida is clearly subdivided into two distinct regions: an outer homogeneous layer adjacent to the granulosa and an inner, thicker striated layer (zona radiata) lying against the oolemma. During late previtellogenesis (1500–2000-μm follicle diameter) pyriform cells regress via apoptosis (Motta et al. 1996), and follicular epithelium gradually reorganizes and re-establishes a monomorphic monolayered condition in which the small cells persist as a unique component of the epithelium until ovulation (Filosa 1973). Surrounding the ovarian follicle are the thecal layers, the theca interna and theca externa, composed of connective tissue, blood vessels, and secretory cells.
The ovary produces a variety of steroids (Di Prisco et al. 1968; Chieffi and Botte 1970), which contribute to plasma sex hormone levels (Ciarcia et al. 1986). Typically, remarkable estrogen amounts are present in the blood during oocyte growth, whereas progesterone levels are higher during ovulation and as long as eggs are contained in the oviducts (Ciarcia et al. 1986).
Histochemical distribution of steroid dehydrogenases in gonadal tissues identifies several putative sites of sex hormone production, i.e., follicular walls (thecal and granulosa layers), peripheral ooplasma of young oocytes, postovulatory follicles, and atretic follicles (Botte and Delrio 1964). However, these findings provide generic information and do not allow any evaluation of the ovarian constituents actually implied in the synthesis of the sex hormones engaged in the induction of vitellogenin synthesis (17β-estradiol) (Ho 1987) and in the regulation of oviduct activity (progesterone, 17β-estradiol, and testosterone) (Botte et al. 1974,1976; Botte and Granata 1977).
In P. sicula (Andreuccetti et al. 2001) and in other squamate reptiles (Del Carmen et al. 1996; Uliano et al. 2001), the gradual modification of surface glycoprotein distribution over the follicular epithelium of oocytes depends on the stage of oocyte growth. In the lizard Ctenosaura pectinata, the zona pellucida has been reported to consist of a polysaccharide–protein complex, which exhibits positive periodic acid-Schiff (PAS) reaction and hyaluronidase labile metachromasia and alcianophilia (Del Carmen et al. 1996). Also, the follicular fluid has been found to exhibit positive PAS and metachromatic reactions (Del Carmen et al. 1996; Maurizii et al. 2004).
It is well know also that as in all vertebrates, in reptiles, the ovarian sex steroid output is directly linked to the hypothalamus–hypophysis axis and is under its control with positive and/or negative feedback. In fact, although the control of the endocrine activity of the vertebrate ovary has long been known to depend mainly on the gonadotropins secreted by the pituitary after hypothalamic GnRH stimulation, further observations have shown that this function is modulated in the gonad itself through several local agents that intervene in ovarian cell activity, acting as autocrine and/or paracrine mechanisms. In the past decade, several studies have shown that one of these molecules, directly involved in an endocrine action, could be d-aspartic acid (d-Asp). d-Asp is an endogenous amino acid occurring in the free form in several species of vertebrates and invertebrates, although it is present in much lower concentrations than is l-aspartate (l-Asp). The presence of endogenous d-Asp has been reported in neuroendocrine tissues in representatives of various animal phyla: molluscs, crustaceans, fish, amphibians, reptiles, chickens, rats, and humans (for review, see D'Aniello 2007). Well known for its neuroexcitatory activity, d-Asp is thought to play important roles in neurotransmission and neurosecretion within the central nervous system, as well as in the biosynthesis and/or secretion of hormones in endocrine glands, such as the pineal gland, hypothalamus, pituitary gland, adrenals, and gonads. Our research group has focused some studies on the role of d-Asp in the gonads of seasonal breeding vertebrates, i.e., the green frog, Rana esculenta, and the lizard P. sicula. These findings have clearly confirmed the role of d-Asp as a local regulator of sex hormone synthesis (Di Fiore et al. 1998; Assisi et al. 2001; Raucci et al. 2004,2005; Raucci and Di Fiore 2009). It has been proposed that d-Asp has different targets in the sex steroid production machinery, depending on the species and/or the sex; i.e., the amino acid operates differently, favoring androgen synthesis in males and estrogen production in females. To date, however, the majority of studies have addressed the evaluation of d-Asp's effect on steroidogenesis and the lack information concerning the role of this enantiomer in the morphology and maturation of ovarian components. Therefore, to extend the knowledge on characterization of ovarian activity, we have focused our attention on the presence of d-Asp in the ovary of P. sicula. Furthermore, for functional evidence of a biological role for this d-amino acid in the ovary, we have also described the histological, histochemical, and morphometric features of follicular epithelium as a consequence of d-Asp treatment. Finally, we have evaluated the uptake of d-Asp in the ovary and its role in sex steroid output. These observations have been carried out either throughout varied phases of the reproductive cycle and/or following in vivo experimental conditions.
Materials and Methods
Animals
Adult female P. sicula lizards were caught by a local dealer in the area surrounding Naples (Italy). These animals were captured during the prereproductive (October–March), reproductive (April–July), and postreproductive (August–September) phases (Botte and Angelini 1980).
Soon after capture, five lizards were euthanized to provide basal data (time 0). Other lizards were reared in a laboratory terrarium maintained on a photothermal regimen consistent with the period of the year. The animals were given a regular supply of mealworms and fresh vegetables and were allowed to feed ad libitum. Mortality rates were low (<10%). These lizards were used for experiments in vivo.
Sample Collection
In relation to each period of the reproductive cycle, the animals were euthanized by exposure to cold. The ovulatory females were identified by abdominal palpation, maintained in individual boxes, and inspected each morning to record egg deposition to obtain postovulatory females.
Blood was collected by inserting a heparinized glass capillary tube into the heart. Then, the samples were centrifuged at 800 × g for 15 min, and the resulting plasma was stored at −20C for sex steroid analyses. The lizards were examined visually to determine the phase of development of their reproductive organs. The ovaries were manually excised and washed with cold isotonic saline solution [0.7% (w/v) NaCl] to eliminate any blood residue, weighed, frozen in liquid nitrogen, and later processed for d-Asp determination and steroid hormone assay. For microscopy studies, oocyte follicles were dissected from the ovary and divided according to their size: class A, between 40 and 100 μm in diameter; class B, 100–500 μm; class C, 500–1000 μm; class D, 1000–2000 μm; class E, ≥2000 to 9000 μm (Filosa 1973). Oocytes of class A–D are previtellogenic; those of class E are yellow vitellogenic oocytes. Oocytes were immediately immersed in fixative for light microscopy (either Bouin's solution or 4% paraformaldehyde/2% gluteraldehyde) and processed for histology, histochemistry, and d-Asp immunohistochemistry. At autopsy, the developmental stage of oviducts was also assessed.
The methods of capture and dissection and the captive rearing conditions were in accordance with Italian law (D. L.vo 116/92) and were authorized by the appropriate Italian government administrative office (Servizio Veterinario della A.S.L. 44, Prot. Vet. 22/95).
Short-term Experiments
This first set of experiments was conducted in vivo to determine the specificity and uptake time of d-Asp by ovary, and the effect of d-Asp injection and other d- and l-amino acids on the levels of ovarian and plasma steroid hormones. Twenty-five lizards, sorted into five groups of five animals each, were treated as follows: Lizards from Groups 1, 2, 3, and 4 received intraperitoneally 2.0 μmol/g body weight different amino acids (Sigma; Milan, Italy) dissolved in 100 μl reptilian physiological saline solution (0.7% NaCl) (Di Fiore et al. 1998). Group 1 was injected with d-Asp, Group 2 with l-Asp, Group 3 with d-alanine (d-Ala), and Group 4 with d-glutamate (d-Glu). The lizards from Group 5 were injected with vehicle alone (saline solution) and used as controls. Plasma and ovaries were collected at different times after the last injection (from 0 hr to 24 hr) and utilized as described above.
Long-term Experiments
Lizards were sorted into groups, each formed by five animals. The lizards were distributed into four groups, and the treatments were as follows: Lizards from Groups 1, 2, 3, and 4 received intraperitoneally 2.0 μmol/g body weight different amino acids dissolved in 0.25 ml of reptilian saline, every 2 days for 2 weeks; that is, Group 1 was injected with d-Asp, Group 2 with l-Asp, Group 3 with d-Ala, and Group 4 with d-Glu. The lizards of Group 5 were injected as above, but with solvent alone (physiological saline), and used as controls. Two days after the last injection, the animals were killed, and ovaries were fixed and processed for histology, histochemistry, and immunohistochemistry as reported below.
Sex Steroid Assays in Plasma and Ovary
Plasma sex steroid determinations were conducted utilizing enzyme immunoassay kits (Adaltis Italia S.P.A.; Bologna, Italy). The following limits of detection were observed: For testosterone, sensitivity was 50 pg/ml (intra-assay variability 4.0%, inter-assay variability 9.0%); for 17β-estradiol, sensitivity was 6 pg/ml (intra-assay variability 6.0%, inter-assay variability 7.5%). The addition of d-Asp and/or other d- and l-amino acids to the standard curve did not modify the assay sensitivity. Plasma samples (100 μl) were vortexed with ethyl-ether (1:10; v/v) for 5 min and centrifuged at 3000 × g for 10 min. The upper phase (ethyl-ether) was transferred to a glass tube. Two extractions were performed. The pooled ether phases were left to evaporate on a hot plate at 40–50C under a hood. The residue was dissolved in 0.5 ml sodium phosphate buffer (0.05 M, pH 7.5) containing BSA at a concentration of 10 mg/ml, and then utilized for the assay. Ovarian samples were homogenized 1:10 (w/v) with distilled water. The homogenate was then mixed vigorously with ethyl-ether (1:10, v/v) and the ether phase was withdrawn after centrifugation at 3000 × g for 10 min. Three extractions were performed. Pooled ether extracts were dried and then utilized for the enzyme immunoassays as previously reported (Di Fiore et al. 1998). Sex steroid recovery was 85% from plasma and 80% from tissues. Steroid recovery was assessed by parallel processing of tissue or plasma samples, to which known amounts of steroids had been added prior to extraction and assay.
Preparation of Samples for Amino Acid Determination
Ovarian samples were homogenized with 0.5 M perchloric acid in a 1:10 ratio and centrifuged at 30,000 × g for 20 min. The supernatant was brought to pH 7.5–8.5 by the addition of 5 M KOH and cooled for 30 min at 0C, and the potassium perchlorate precipitate was removed by centrifugation as in Di Fiore et al. (1998). The supernatant was adjusted to a pH of ∼2.5 with 1 M HCl, and the amino acids were purified on a cation exchange column (AG 50W-X8 resin, hydrogen ionic form, 200–400 mesh; BioRad, Milan, Italy). The sample was loaded on a column (1 × 3 cm) equilibrated with 0.01 M HCl, and, after washing with 10 ml of 0.01 M HCl, it was eluted with 8 ml of 4 M NH4OH. The eluates were dried by evaporation in small petri dishes on a hot plate at 40–60C under a hood. The dry eluates were dissolved in 1 ml of 0.01 M HCl. They were then purified by slowly passing through a Sep-pak C-18 cartridge (300 mg; Waters, Milan, Italy), which had been previously activated with methanol or acetonitrile and washed with distilled water. To recover the amino acids from these eluates, the cartridge was eluted twice with 2 ml of 0.01 M HCl. The resulting eluates were combined and dried using a Savant centrifuge or left to evaporate in small petri dishes at 40–50C under the hood. The dry residues were then dissolved in 200 μl of 0.01 M HCl and analyzed for d-Asp content.
d-Asp Assay
The d-Asp was determined with an HPLC assay using the o-phthaldialdehyde/N-acetyl-l-cysteine method and using the d-Asp oxidase (EC 1.4.3.1), an oxidative enzyme that oxidizes d-Asp. This method was fully described in a previous study (Di Fiore et al. 1998).
Histology and Histochemistry
After dissection, ovaries were rapidly immersed in Bouin's fluid. Tissues were serially sectioned at 5 μm and stained with hematoxylin/eosin (H/E) and trichrome-Mallory. Polysaccharides were detected by toluidine blue in Walpole buffer at pH 4.2 (Gabe 1968), PAS reagent (Humason 1979), and alcian blue/PAS (AB/PAS) (Bancroft and Stevens 1990). The mercury bromophenol blue method was used for proteins (Mazia et al. 1953). Follicular stages were classified as described by Andreuccetti et al. (1990).
Immunohistochemistry
After dissection, ovaries were rapidly immersed in fixative solution (4% paraformaldehyde, 2% gluteraldehyde in phosphate buffer at pH 7.4). To inactivate the residual glutaraldehyde, deparaffinated sections were pretreated for 20 min with 0.5% NaBH4 in phosphate buffer at pH 7.4 and then incubated in normal goat serum (1:20; Sigma). Next, they were incubated again overnight at 4C with rabbit anti-d-Asp polyclonal antibody, prepared as described by Schell et al. (1997), at 1:50 dilution in antibody diluent (0.1 M PBS, pH 7.4, with 0.1% BSA). After washing with PBS, the sections were incubated for 1 hr at room temperature with biotinylated goat anti-rabbit immunoglobulin (DAKO; Glostrup, Denmark) at 1:500 dilution. Then they were incubated again for 1 hr with streptavidin peroxidase (Boehringer; Ingelheim, Germany) at 1:200 dilution. Finally, they were stained with 3-3′-diaminobenzidine tetrahydrochloride (DAKO) and 0.3% H2O2 in PBS solution. The specificity of the primary antibody was determined by liquid-phase preabsorption, performed for 18 hr at 4C with d-Asp (1 mM; Sigma).
Morphometry
For morphometric evaluation, five random photomicrographs of ovaries (H/E and trichrome-Mallory stained) for each animal of each experimental group were analyzed by National Institutes of Health Image 1.6 program (free access at http://rsb.info.nih.gov/nih-image). The following parameters were determined: area pyriform cells (μm2), thickness of ovarian epithelium (μm), and thickness of connectival theca (μm).
Statistical Analysis
Data were compared by ANOVA followed by Duncan's test for multi-group comparison and Student's t-test for between-groups comparison. All data were expressed as mean ± SD. The level of significance was taken at p<0.01 and p<0.05. In addition, the correlation coefficients (r) between d-Asp content in the ovary and both ovarian and plasma concentrations of steroid hormones were calculated.
Results
Ovarian Morphology
The present observations confirmed those already reported in the literature for this species. The ovaries of P. sicula undergo an annual cycle of growth and developmental maturation with three to four ovulatory waves between April and July (reproductive period). From August to September, a refractory stage occurs. During this phase, only oocytes of classes A, B, and C (see Materials and Methods section) are present in the ovary (Romano and Limatola 2000), followed by a prereproductive period (October to March), during which the oocytes undergo slow growth and prepare themselves for vitellogenesis and ovulation. In the reproductive period (April–July), only a certain number of oocytes start growing, giving rise to a follicular hierarchy. The following results refer to the lizard captured during the prereproductive phase of the sexual cycle.
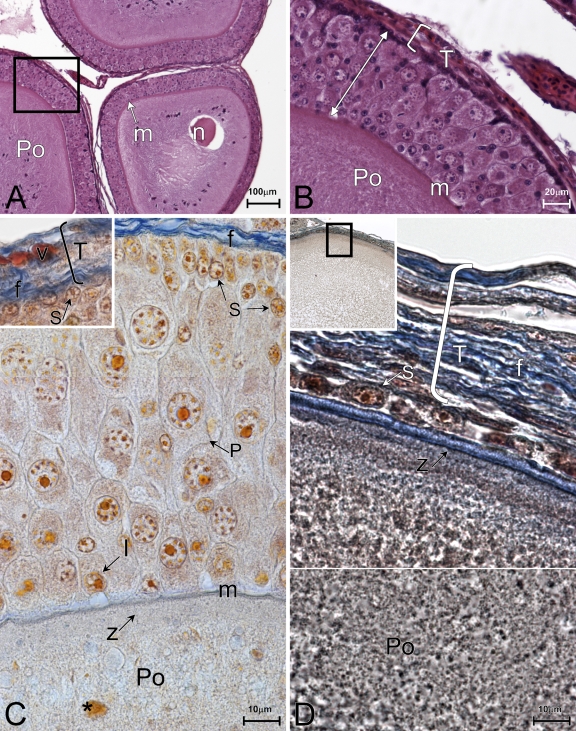
Growing previtellogenic oocytes (Figure 1A) are surrounded by a follicular epithelium, which is composed of multilayered and polymorphic cells (Figure 1B). The basement membrane separates the surface of the epithelium from the underlying stroma (Figures 1B and 1C, m). The ooplasm stains moderately with eosin, and a distinct nucleus is localized nearly to the center (Figure 1A). The ooplasm contains an abundance of fibrillar basophilic granules mainly located in the periphery of the ooplasm. In contrast, the center exhibits finely granular cytoplasm (Figure 1A). The ovarian stroma is composed of connective tissue surrounding ovarian follicles (Figure 1C, inset, f), and the capillaries are also observed (Figure 1C, inset, v).
Figure 1.
Morphology. (A–C) Previtellogenesis. Oocytes contain basophilic bundles of fibers in the ooplasm (A). The granulosa completely surrounds the oocytes (A,B) and appears multilayered and polymorphic with small cells (C, S), pyriform cells (C, P), and intermediate cells (C, I). The zona pellucida is slightly distinguishable (C, z), and the theca (C, inset, bracket, T) shows many fibers (C, inset, f). (D) Vitellogenesis. Oocyte size increases (D, inset), and the ooplasm shows an alveolar appearance (Po). The granulosa is a monolayer consisting of small cells (D, s). The zona pellucida (z) and theca (bracket, T) are well defined. (A,B) Hematoxylin/eosin (H/E) staining. (C,D) Mallory staining. (D) Horizontal white line, image collage.
Different cell types are detected in the follicular epithelium: small cells, pyriform cells, and intermediate cells (Figure 1C). Small cells are ovoid elements more abundant in the apical boundary of the granulosa, the region closest to the oocyte (Figure 1C). At early previtellogenesis, small cells form a continuous layer under the stroma. The cytoplasm of these cells is finely granulated, with a round nucleus and a dense nucleolus. Follicular epithelium of previtellogenic oocytes is mainly represented by pyriform cells, which are mostly located in the inner region of the granulosa. Pyriform cells are characteristically flask-shaped, with a large and basal nucleus containing clumps of heterocromatin and a dense nucleolus. The cytoplasm contains basophilic granules, vacuoles, and fibers (Figure 1C). Intermediate cells are mostly observed on the basal side of the follicular epithelium. These cells are spherical and have a round nucleus and lightly granular cytoplasm (Figure 1C). At this stage, the zona pellucida is slightly distinguishable from the cytoplasm of the oocyte (Figure 1C, z).
At vitellogenesis, many oocytes of class E are found (Figure 1D, inset). They appear noticeably increased in volume, whereas the granulosa layer diminishes in width as the pyriform cells become reduced in size and flattened in shape. The prolongations that extend to the zona pellucida, characteristic of pyriform cells at earlier stages of folliculogenesis, are not seen at this stage (cf. Figures 1C and 1D). At this stage, the follicular epithelium of vitellogenic oocytes appears fully remodeled, as compared with previtellogenesis (Figures 1D vs 1C). It is mainly composed of small cells arranged in a monolayer. Notable is the thickness of the zona pellucida.
d-Asp Immunolocalization
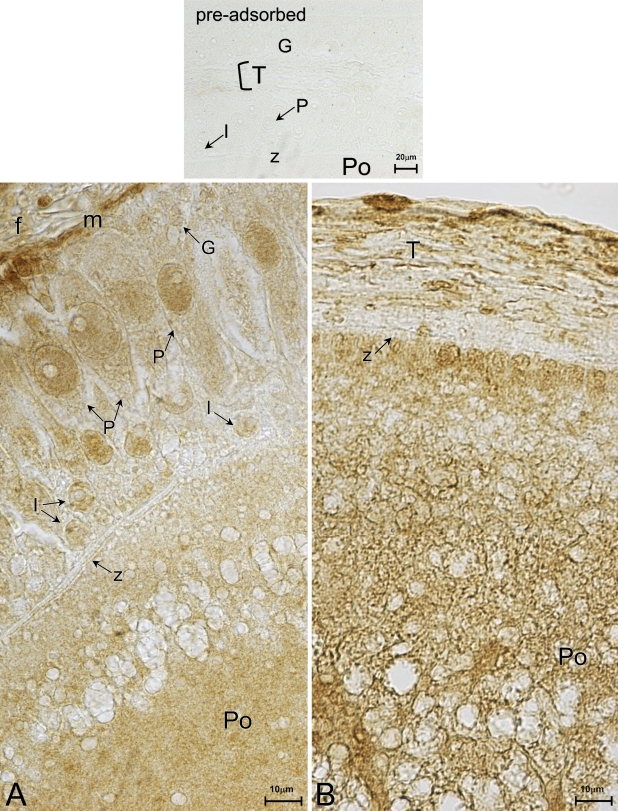
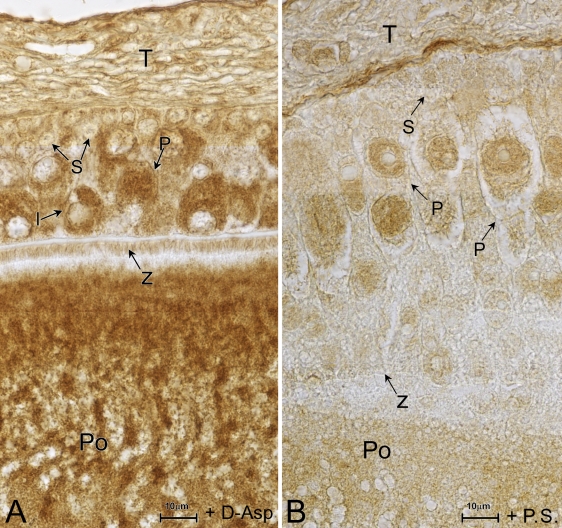
Immunohistochemical staining with polyclonal anti-d-Asp antibody reveals that endogenous d-Asp is specifically detected in the cytoplasm and in the nucleus of the pyriform cells of previtellogenic follicles (Figure 2A). A weak immuno-signal is also observed in the intermediate cells (Figure 2A, I). The basal membrane of the granulosa also is d-Asp positive (Figure 2A, m); instead, the theca appears weakly stained. Small cells and the area in proximity to the zona pellucida are negative to the antibody. d-Asp immunopositivity is distinguishable in the ooplasm, especially near the center (Figure 2A, Po). At vitellogenesis, as the granulosa is organized into a monolayer, d-Asp immunoreactivity is strongly found in the ooplasm of the oocytes (Figure 2A, Po) and is labeled in the ovarian cortex as well (Figure 2B). Additionally, noticeable immunopositivity is also detected in some elements of the theca (Figure 2B). No immunostaining is observed in the control (preadsorbed).
Figure 2.
d-aspartic acid (d-Asp) immunostaining. (A) Previtellogenic epithelium with polymorphic and multilayered granulosa. d-Asp immunoreactivity is evident in the cytoplasm and in the nucleus of pyriform cells (P) and lightly stains some intermediate cells (I). Notable label is detected in the ooplasm (Po). (B) Vitellogenesis. In the follicular epithelium, pyriform cells have disappeared. d-Asp positivity is present in the oocyte ooplasm (Po) and in proximity to the zona pellucida (z). Many elements stain in the theca (T). (A,B) Immunostaining; preadsorbed, control.
Histochemistry
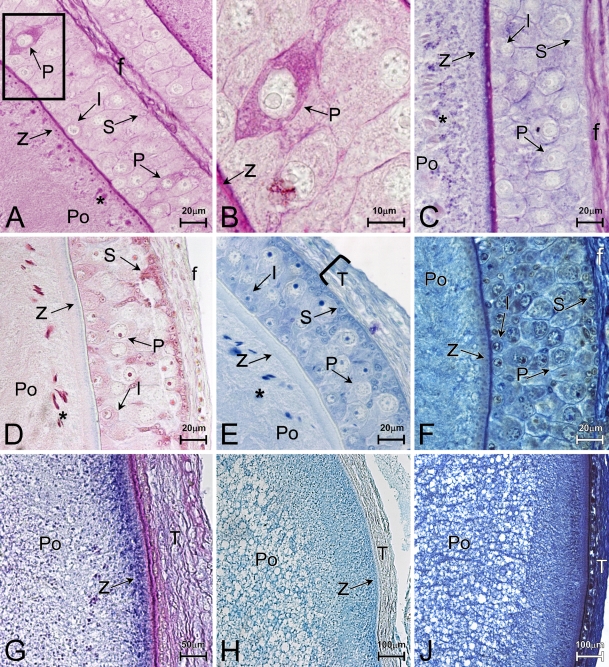
Small cells stain negatively for PAS (Figure 3A, S) and AB/PAS (Figure 3C, S). However, many of the small granulosa cells exhibited intense toluidine blue (Figure 3E) and safranine (Figure 3D) staining, especially those small cells in the apical region of the granulosa. Intermediate cells are negative for PAS (Figure 3A, I) and AB/PAS (Figure 3C, I) reactions, and weakly positive for safranine (Figure 3D, I).
Figure 3.
Histochemistry. (A–F) Previtellogenesis. Periodic acid-Schiff (PAS)-positive reaction in the cytoplasm of pyriform cells (A,B, P). Note: some PAS-positive granules in peripherical ooplasm (A, asterisk). (C) Alcian blue/PAS (AB/PAS) positivity in the pyriform cells (P) and ooplasm (Po). Small cells (S) are safranine-positive (D) and toluidine blue–positive (E), whereas the pyriform cells (P) are not. Notable alcianophilia is observed on the zona pellucida (C, z). (F) Bromophenol blue positivity is detectable throughout the whole follicle. (G,H) Vitellogenesis. Intense PAS-positive reaction at the level of the zona pellucida (z), whereas the ooplasm (Po) is alcian blue–positive (G,H). (J) Bromophenol blue throughout the whole oocyte. (A,B) PAS reaction. (C,G) AB/PAS. (D) AB/safranine. (E) Toluidine blue. (H) AB. (J) Bromophenol blue.
The cytoplasm of some pyriform cells contains granules that are stained intensely by the PAS reaction (Figures 3A and 3B, P). The PAS-positive reaction in the pyriform cells exhibits varying intensities and distributions; some cells exhibit a patchy or light distribution of stain in the cytoplasm, whereas other cells stain intensely throughout the cytoplasm (Figures 3A and 3B). Pyriform cells stain minimally with toluidine blue (Figure 3E, P); they are negative for safranine (Figure 3D, P), and appear moderately AB/PAS positive (Figure 3C, P). Bromophenol blue–positive reaction is also observed throughout the follicular epithelium, zona pellucida, and ooplasm (Figure 3F).
At this stage, the zona pellucida is intensely stained for PAS (Figure 3A, z) and AB/PAS (Figure 3B, z), whereas it is weakly positive for the AB (Figure 3C) and negative for safranine (Figure 3D, z) and for toluidine blue (Figures 3E and 3D, z). Moreover, the fine reticular granules at the extreme periphery of the ooplasm show staining for PAS (Figure 3A, asterisk), AB/PAS (Figure 3C, asterisk), and safranine (Figure 3D, asterisk). These granules appear orthochromatic for toluidine blue (Figure 3E, asterisk).
At vitellogenesis, the oocytes appear bigger and the granulosa diminishes in width, as the pyriform cells become reduced and flattened in shape (Filosa 1973). The yolk spherical platelets are alcian blue–positive/PAS positive (Figures 3G and 3H, Po). Bromophenol blue–positive reaction is strongly observed throughout the ooplasm (Figure 3J, Po). The majority of the yolk platelets form a dense and narrow concentric ooplasm ring near the periphery, but the region rich in yolk platelets is separated from the oolemma and the zona pellucida by a peripherical platelet-free zone (Figure 3G).
d-Asp Content in the Ovary and Sex Hormones (T and 17β-E2) Concentrations in the Plasma and Ovary of the Lizard, P. sicula, During the Main Phases of the Reproductive Cycle
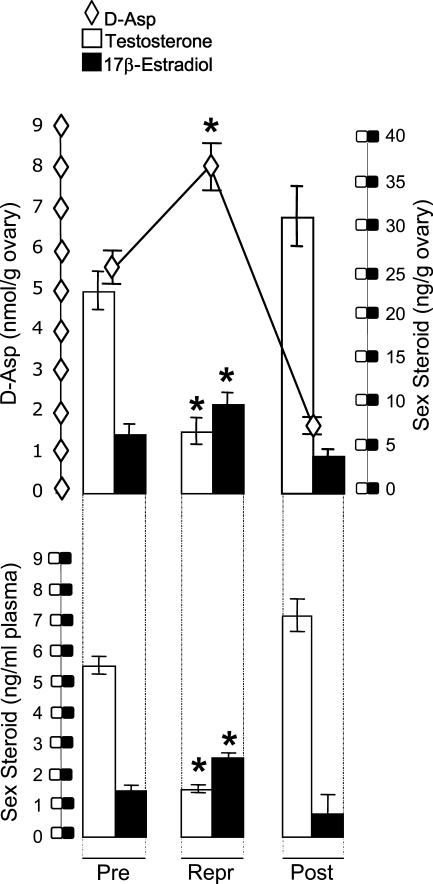
In the ovary, endogenous d-Asp content varied with the sexual cycle phase (Figure 4). In reproductive females, it was at the highest values, significantly lower in postreproductive females, and intermediate in females at the prereproductive phase. The differences in d-Asp concentrations at various phases of the reproductive cycle were significant: reproductive vs postreproductive, p<0.01; postreproductive vs prereproductive, p<0.01; and prereproductive vs reproductive, p<0.01.
Figure 4.
Concentration of d-Asp (rhomboids) and titres of 17β-estradiol (black columns) and testosterone (white columns) in the ovary (top) and plasma (bottom) of the lizard P. sicula during the reproductive cycle. Each point represents the mean value ± SD from five individual lizards. Pre, prereproductive period; Repr, reproductive period; Post, postreproductive period. *p<0.01.
In plasma and ovary, testosterone level was high in both pre- and postreproductive females and low in the reproductive phase of the cycle (p<0.01). Contrariwise, 17β-estradiol plasma and ovarian concentrations were low in pre- and postreproductive females and reached the highest value in reproductive females (p<0.01).Therefore, the d-Asp trend was inversely related to testosterone and directly parallel to 17β-estradiol patterns.
In fact, the analysis of correlation between d-Asp content and 17β-estradiol levels revealed a positive coefficient throughout the sexual cycle (r1 = 0.98, d-Asp in the ovary vs 17β-estradiol in the ovary; r2 = 0.99, d-Asp in the ovary vs 17β-estradiol in the plasma). In contrast, the comparison of d-Asp content with testosterone concentrations revealed a reverse correlation between the amino acid and the hormone (r3 = −0.93, d-Asp in the ovary vs testosterone in the ovary; r4 = −0.95, d-Asp in the ovary vs testosterone in the plasma).
d-Asp Immunolocalization After In Vivo Treatment
Immunohistochemically, exogenous d-Asp is highly accumulated by the ovary (Figure 5A) as compared with control ovary (vehicle alone). An increase of intensity of immunostaining is mainly concentrated in the cytoplasm of pyriform cells, whereas the nucleus was only slightly positive (Figures 5A vs 5B, P). A stronger d-Asp immunopositivity is also observed in some small cells (Figures 5A vs 5B, S) in proximity to the zona pellucida and in the ooplasm (Figures 5A vs 5B, z and Po, respectively), whereas the small cells appear weakly stained (Figures 5A vs 5B, S). d-Asp immunoreactivity is slightly increased in the theca (Figures 5A vs 5B, T).
Figure 5.
d-Asp immunolabeling in previtellogenic lizards in response to in vivo treatment. (A) In treated oocytes, exogenous d-Asp (+d-Asp) is clearly accumulated by ooplasm (Po) and pyriform cells (P). The label is also observed in intermediate (I) and small cells (S). Note: d-Asp immunoreactivity on the zona pellucida (z). (B) Control oocyte injected with physiological saline solution (+PS).
Histochemistry of Follicular Epithelium After d-Asp In Vivo Treatment
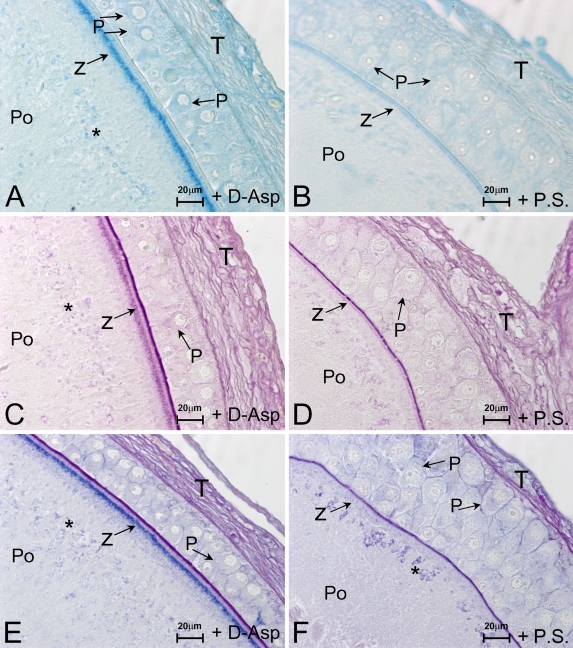
d-Asp treatment affects the histochemical properties of the granulosa and ooplasm. At the level of the follicular epithelium, a stronger AB staining is observed in the cytoplasm of pyriform cells (Figures 6A vs 6B; treated and control, respectively), whereas PAS reactivity is weakly increased through the granulosa (Figure 6C) as compared with control (Figure 6D). In addition, an intense alcianofilia and PAS positivity are observed in the proximity of the zona pellucida of treated lizard (Figure 6E, z), whereas the controls are moderately stained (Figure 6F, z).
Figure 6.
d-Asp histochemistry in previtellogenic oocytes in response to in vivo treatment. In the treated previtellogenic lizards (+d-Asp), an intense alcianophilia (A,E) and PAS-positive reaction (C,E) are localized in proximity to the zona pellucida (z) as compared with the control (B, D, and F, respectively). (A,B) AB. (C,D) PAS. (E,F) AB/PAS. Asterisks indicate yolk platelets.
Morphometry
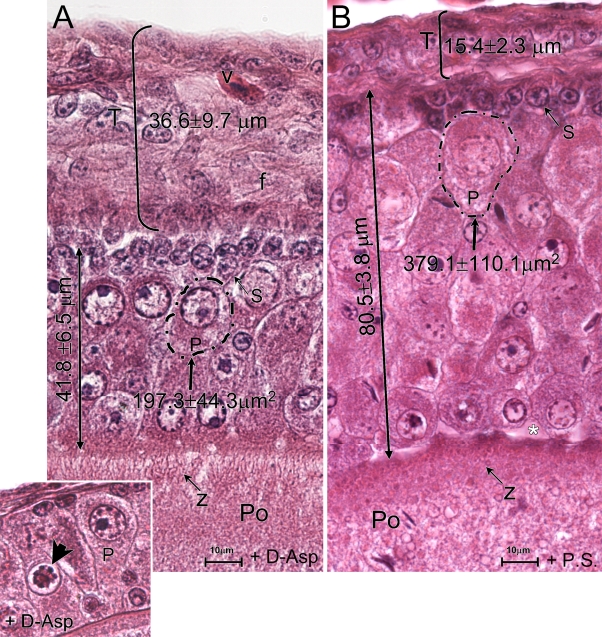
In the treated animals, d-Asp significantly increases the thickness of the theca layer of previtellogenic oocytes, from 15.4 ± 2.3 μm to 36.6 ± 9.7 μm (p<0.05; cf. Figures 7A and 7B; bracket, treated and control, respectively) and also decreases the thickness of the follicular epithelium from 80.5 ± 3.8 μm to 41.8 ± 6.5 μm (p<0.05; Figures 7A vs 7B, long arrow, treated and control, respectively). In treated lizard, the follicular theca of previtellogenic oocites appears more vascularized (Figure 7A) than the control (Figure 7B), and many fibers are detected between the cells forming the connectival layer surrounding the oocyte (Figure 7A, f). In the granulosa, the area of pyriform cells is lower as compared with the control (Figure 7A dashed line, 197 ± 44 μm2 vs Figure 7B, dashed line, 379.1 ± 110.1 μm2; p<0.05).
Figure 7.
Morphometric analysis of previtellogenic oocytes in response to d-Asp in vivo treatments. In d-Asp–treated lizards (A, +d-Asp) the theca layer (T) is clearly increased, whereas the thickness of the follicular epithelium and the volume are reduced, as compared with the control (B). Dashed line indicates area of pyriform cell. (Inset in A) An apoptotic nucleus of pyriform cells (arrowhead) in the follicular epithelium of treated lizards. (A,B) H/E.
Additionally, in d-Asp–treated animals, the zona pellucida is clearly distinct as a lighter band separating the epithelium from the ooplasm (Figure 7A, z). The ooplasmic fibers are dense clumps, smaller and thinner than those observed in the control (Figures 7A vs 7B, Po).
Additionally, significant morphological alteration can be induced in the follicular epithelium of the treated lizard, in which several pyriforms show clear evidence of death. In these cells, the nuclei are markedly irregular and contain cromatin and/or condensed chromatin and pale nucleoli (Figure 7A, inset).
Effect of In Vivo Administration of Different Amino Acids on Plasma and Ovarian Concentrations of Testosterone and 17β-Estradiol in Prereproductive Lizards
Table 1 shows the effects of treatment with different amino acids (l- and d-Asp, d-Ala, and d-Glu) on the plasmatic and ovarian concentration of sex hormones evaluated at 3 hr after administration. It can be seen that a specific effect is caused by d-Asp, whereas no significant effects were observed with the other amino acids tested. In both plasma and ovary of d-Asp–treated females, a decrease of testosterone (p<0.01 vs control) and an increase of 17β-estradiol (p<0.01 vs control) were induced.
Table 1.
Testosterone and 17β-estradiol concentrations in the plasma and ovary of Podarcis s. sicula evaluated at 3 hr after in vivo administration
| Plasma (ng/ml−1) |
Ovary (ng/g tissue−1) |
|||
|---|---|---|---|---|
| In vivo treatment | Testosterone | 17β-Estradiol | Testosterone | 17β-Estradiol |
| PS | 7.16 ± 0.61 | 0.75 ± 0.08 | 31.50 ± 2.76 | 4.20 ± 0.41 |
| d-Asp | 3.97* ± 0.29 | 4.08* ± 0.36 | 20.76* ± 1.95 | 14.20* ± 1.70 |
| l-Asp | 8.76 ± 0.77 | 0.87 ± 0.09 | 29.84 ± 2.62 | 3.10 ± 0.38 |
| d-Ala | 7.18 ± 0.65 | 1.02 ± 0.11 | 31.89 ± 3.05 | 3.06 ± 0.35 |
| d-Glu | 7.43 ± 0.81 | 1.20 ± 0.15 | 30.80 ± 2.97 | 3.12 ± 0.37 |
Each value represents the mean of five determinations ± SD. *p<0.01. PS, physiological saline; d-Asp, d-aspartic acid; l-Asp, l-aspartate; d-Ala, d-alanine; d-Glu, d-glutamate.
Although the administration of d-Asp to animals in the other two reproductive phases showed results similar to those seen in prereproductive animals, the effects were less noticeable during the reproductive period (data not shown).
In Vivo d-Asp Uptake in the Ovary After Its Short-term Treatment and Comparison With Sex Hormone Concentrations in the Ovary and Plasma
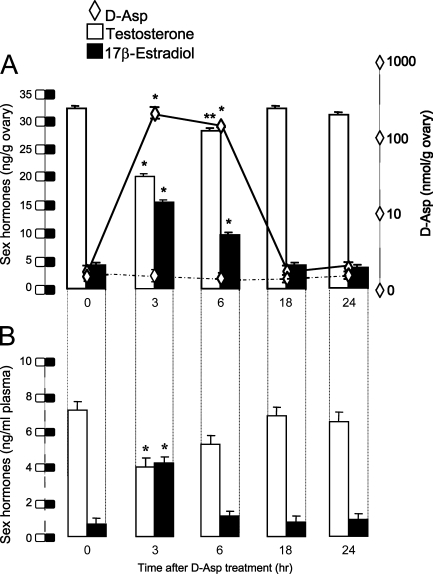
In the in vivo experiments, in which lizards were injected with d-Asp (2.0 μmol/g body weight), the administration of this amino acid was followed by its significant and rapid accumulation in the ovary. In fact, d-Asp was rapidly taken up by the ovary, so that 3 hr after injection, its concentration in the ovary was ∼10-fold greater than the values registered at time 0 (p<0.01). It remained considerably high at 6 hr (p<0.01) and then progressively decreased, reaching baseline values within 18–24 hr (Figure 8A).
Figure 8.
Uptake of d-Asp (rhomboids) and concentrations of steroid hormones [17β-estradiol (black columns) and testosterone (white columns), respectively] in the ovary (A) and plasma (B). Animals were injected at time 0 with either d-Asp or saline solution. Each value represents the mean ± SD of five determinations. *p<0.01 vs time 0. Dashed line, d-Asp control.
Regarding the effects of d-Asp on sex hormones, a significant decrease of testosterone and increase of 17β-estradiol was seen. This pattern occurred in both circulating hormones (Figure 8B, plasma) and in those present in the ovary (Figure 8A). However, this effect appeared to be reversible, because hormone levels were almost restored within 18 hr after the treatments.
Discussion
The ovarian follicles of P. sicula exhibit a clear seasonal cycle in morphology. At previtellogenesis (1500–2000-μm follicle diameter), the oocytes are surrounded by a granulosa composed of a single layer of cells. As folliculogenesis progresses, the granulosa becomes multilayered. Only after the vitellogenesis begins and active sequestration of yolk into ooplasm is initiated do the granulosa appear to be again composed of the single cell type. The granulosa cells are defined by their morphology in small, intermediate, and pyriform cells. The latter cells are nurse cells that produce and send organelles and RNA to the oocyte via an intercellular connecting bridge, thereby contributing to its growth (Taddei 1972; Andreuccetti et al. 1978; Motta et al. 1995).
Here, we have demonstrated the presence of naturally free d-Asp in ovarian follicles. At the morphological level, the labeling was detected in the ooplasm of the oocytes and in the cytoplasm and nucleus of pyriform cells of previtellogenic follicles. In late vitellogenesis, when the pyriform cells are regressed (Motta et al. 1996), d-Asp immunoreactivity was found in the oocyte ooplasm and labeled the zona pellucida as well. This differential pattern of d-Asp distribution suggests that the presence of d-Asp might characterize the follicles during the growth, maturation, and ovulation of oocytes.
Observations made throughout the reproductive cycle showed that d-Asp is present in the ovary and undergoes regular changes in its concentration. The level of d-Asp in the ovary was high in females engaged in active processes of vitellogenesis, and low in females at the postreproductive phase, whose gonads were in a quiescent state. Furthermore, the ovary shows a very high and relatively rapid ability to take up and accumulate exogenously administered d-Asp. At the epithelial level, administered d-Asp was mainly observed in the cytoplasm rather than in the nucleus of pyriform cells, in small cells, and in the theca of the oocyte. A noticeable increase of immunopositivity was also observed in the ooplasm. This differential immunostaining was well evidenced during the previtellogenesis, when the follicular epithelium shows a polymorphic and multilayered aspect (Filosa 1973; Filosa et al. 1979). As has already been demonstrated in the testis of mammals (D'Aniello et al. 1996,1998,2000; Sakai et al. 1998; Nagata et al. 1999), we also have reported the presence of d-Asp in the gonads and its implication in the endocrine activity in both sexes of amphibians (Di Fiore et al. 1998; Raucci et al. 2004) and reptiles (Assisi et al. 2001; Raucci et al. 2005; Raucci and Di Fiore 2009). Present data confirm that the administration of d-Asp causes a decrease in testosterone and an increase in 17β-estradiol levels, indicating an association between d-Asp and sex steroid hormones. Furthermore, this effect was specific to d-Asp, because the administration of other amino acids had no hormonal effect, either in the plasma or the ovary. In the female lizard, the physiological mechanism underlying the inverse and direct relationship between d-Asp and testosterone and 17β-estradiol, respectively, was elucidated in our previous in vitro studies (Assisi et al. 2001). These results showed that when d-Asp is added to an incubation medium containing testosterone and either homogenate or acetone powder of follicles, the aromatase enzyme was 4-fold greater in the presence of d-Asp than in its absence. However, we cannot exclude the possibility that d-Asp can enhance steroidogenesis by affecting cholesterol production, and future studies will be necessary to explore this hypothesis. Here we show a specific accumulation of exogenous d-Asp in both the theca and the granulosa, i.e., the sites where 17β-estradiol is synthesized by the ovary. As a direct consequence of d-Asp in vivo injection and/or an indirect effect of this amino acid on sex steroid production, the histochemical profile of the granulosa was modified. Physiologically, a histochemical differential staining has been observed when the cells of the granulosa were compared, i.e., the pyriform cells are stained by the PAS reaction or alcian blue but not by toluidine blue, whereas the small granulosa cells stain intensely with toluidine blue. d-Asp–treated previtellogenic oocytes show that many of the pyriform cells contained PAS-positive secretory granules similar to those seen in the cortical region of the ooplasm of vitellogenic oocytes. Likewise, some of the pyriform cells exhibited acidophilic granules in their cytoplasm, similar in staining appearance to the small yolk platelets observed in the ooplasm following the onset of vitellogenesis. Several authors have suggested that these cells are related to previtellogenic oocyte growth (Betz 1963; Goldberg 1970; Varma 1970; van Wyk 1984), whereas others have hypothesized that they assist with the transport of yolk precursors into the oocyte (Neaves 1971; Filosa and Taddei 1976). In this context, our observations suggest that endogenous d-Asp could enhance both processes, but further functional studies must be performed to determine the molecular mechanism through which this amino acid acts.
However, the effect of d-Asp on the maturation of follicular epithelium is also supported by our morphometric data, showing a reduction in the thickness of the granulosa layer and an increase of the theca stratum. The reduction in thickness of the granulosa layer is physiologically associated with a remodeling of follicular epithelium associated with programmed cell death (Motta et al. 1995,1996). Evidence shows that intermediate cells and pyriform cells are degenerated as vitellogenesis approaches. In fact, in follicles ranging between 1600 and 2000 μm in diameter, the epithelium gradually regresses to a homomorphic condition that persists throughout vitellogenesis (Filosa 1973). The regression of intermediate and pyriform cells is certainly under genetic control, as demonstrated by the occurrence of apoptosis, a genetically directed process, and by the persistence of RNA synthesis. In other systems, it was demonstrated that terminal differentiation and aging are also related to the expression of tissue transglutaminase (Tacher and Rice 1985; Thomázy and Fésüs 1989). In P. sicula, this enzyme is apparently expressed only during late previtellogenesis, as the finding of scaffolds suggests (Motta et al. 1996).
Finally, considering all of these results, we can sustain that d-Asp may be related to synchrony of reproduction. In fact, the seasonality and the distribution pattern of d-Asp in lizard ovarian tissues, together with the trends of sex steroid hormones, seem to enhance the maturation of the follicular epithelium of P. sicula, either promoting its growth or influencing its endocrine functions. An unresolved issue still remains to be elucidated: what is the mechanism of d-Asp action? This is an intriguing question that deserves further study, which we are preparing to undertake.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Andreuccetti P (1992) An ultrastructural study of differentiation of pyriform cells and their contribution to oocyte growth in representative squamata. J Morphol 212:1–11 [DOI] [PubMed] [Google Scholar]
- Andreuccetti P, Famularo C, Gualtieri R, Prisco M (2001) Pyriform cell differentiation in Podarcis sicula is accompanied by the appearance of surface glycoproteins bearing alpha-galNAc terminated chains. Anat Rec 263:1–9 [DOI] [PubMed] [Google Scholar]
- Andreuccetti P, Motta CM, Filosa S (1990) Regulation of oocyte number during oocyte differentiation in the lizard Podarcis sicula. Cell Differ Dev 29:129–141 [DOI] [PubMed] [Google Scholar]
- Andreuccetti P, Taddei C, Filosa S (1978) Intercellular bridges between follicle cells and oocyte during the differentiation of follicular epithelium in Lacerta sicula Raf. J Cell Sci 33:341–350 [DOI] [PubMed] [Google Scholar]
- Assisi L, Botte V, D'Aniello A, Di Fiore MM (2001) Enhancement of aromatase activity by D-aspartic acid in the ovary of the lizard Podarcis s. sicula. Reproduction 121:803–808 [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A (1990) Theory and Practice of Histological Techniques. 3rd ed. London, Churchill Livingstone
- Betz TW (1963) The ovarian histology of the diamond-backed water snake, Natriz rhombifera, during the reproductive cycle. J Morphol 123:245–255 [DOI] [PubMed] [Google Scholar]
- Botte V, Angelini F (1980) Endocrine control of reproduction in reptiles: the refractory period. In Delrio G, Brachet J, eds. Steroids and Their Mechanisms of Action in Nonmammalian Vertebrates. New York, Raven Press, 201–212
- Botte V, Angelini F, Picariello O, Molino R (1976) The regulation of the reproductive cycle of the female lizard Lacerta sicula sicula Raf. Monit Zool Ital 10:119–133 [Google Scholar]
- Botte V, Delrio G (1964) Ricerche istochimiche sulla distribuzinone dei 3- e 17 cheto-steroidi e di alcuni enzimi della steroidogenase nell' ovario di Lacerta sicula. Boll Zool 32:191–195 [Google Scholar]
- Botte V, Granata G (1977) Induction of avidin synthesis by RNA obtained from lizard oviducts. J Endocrinol 73:535–536 [DOI] [PubMed] [Google Scholar]
- Botte V, Segal SJ, Koide SS (1974) Induction of avidin synthesis in the oviduct of lizard Lacerta sicula by sex hormones. Gen Comp Endocrinol 23:357–359 [DOI] [PubMed] [Google Scholar]
- Chieffi G, Botte V (1970) The problem of luteogenesis in non-mammalian vertebrates. Boll Zool 37:85–102 [Google Scholar]
- Ciarcia G, Lancieri M, Suzuki H, Manzo C, Vitale L, Tornese Buonamassa D, Botte V (1986) A specific nuclear protein and poly(ADPribose)transferase activity in lizard oviduct during the reproductive cycle. Mol Cell Endocrinol 47:235–241 [DOI] [PubMed] [Google Scholar]
- Ciarcia G, Paolucci M, Di Fiore MM (1993) Changes in ovarian follicles and in vitro sex hormone release in the lizard Podarcis sicula sicula. Mol Reprod Dev 35:257–260 [DOI] [PubMed] [Google Scholar]
- D'Aniello A (2007) D-aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Brain Res Rev 53:215–234 [DOI] [PubMed] [Google Scholar]
- D'Aniello A, Di Cosmo A, Di Cristo C, Annunziato L, Petruccelli L, Fisher G (1996) Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci 59:97–104 [DOI] [PubMed] [Google Scholar]
- D'Aniello A, Di Fiore MM, D'Aniello G, Colin FE, Lewis G, Setchell BP (1998) Secretion of D-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett 436:23–27 [DOI] [PubMed] [Google Scholar]
- D'Aniello A, Di Fiore MM, Fisher GH, Milone A, Seleni A, D'Aniello S, Perna AF, et al. (2000) Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J 14:699–714 [DOI] [PubMed] [Google Scholar]
- Del Carmen AM, Uribe A, Luz Portales GB, Guilliette LJ (1996) Ovarian folliculogenesis in the oviparous mexican lizard Ctenousaura pectinata. J Morphol 230:99–112 [DOI] [PubMed] [Google Scholar]
- Di Fiore MM, Assisi L, Botte V, D'Aniello A (1998) D-aspartic acid is implicated in the control of testosterone production by the vertebrate gonad. Studies on the female green frog Rana esculenta. J Endocrinol 157:199–207 [DOI] [PubMed] [Google Scholar]
- Di Prisco CL, Delrio G, Chieffi G (1968) Sex hormones in the ovaries of the lizard Lacerta sicula. Gen Comp Endocrinol 1:292–295 [DOI] [PubMed] [Google Scholar]
- Filosa S (1973) Biological and cytological aspects of the ovarian cycle in Lacerta sicula. Monit Zool Ital 7:151–165 [Google Scholar]
- Filosa S, Taddei C (1976) Intercellular bridges between germ cells during the first stages of oogenesis in adult lizard. In Muller-Berat N, ed. Progress in Differentiation Research. Amsterdam, North-Holland Publishing, 261–266
- Filosa S, Taddei C, Andreuccetti P (1979) The differentiation and proliferation of follicle cells during oocytes growth in Lacerta sicula. J Embryol Exp Morphol 54:5–15 [PubMed] [Google Scholar]
- Gabe M (1968) Detection de la basophilie dans un but histochimique. In Gabe M, ed. Tecniques Istologiques. Paris, Masson, 351–353
- Ghiara G, Limatola E, Filosa S (1968) Ultrastructural aspects of nutritive process in growing oocytes of lizard. In Bocciarelli DS, ed. Electron Microscopy. Roma, Tipografica Poliglotta, 331–332
- Goldberg SR (1970) Seasonal ovarian histology of the ovoviviparous iguanid lizard Sceloporus jarroui Cope. J Morphol 132:265–276 [DOI] [PubMed] [Google Scholar]
- Ho SM (1987) Endocrinology of vitellogenesis. In Norris DO, Jones RE, eds. Hormones and Reproduction in Fishes, Amphibians and Reptiles. New York, Plenum Press, 145–169
- Humason GL (1979) Animal Tissue Technique. 4th ed. San Francisco, Freeman and Company
- Maurizii MG, Alibardi L, Taddei C (2004) Alpha-tubulin and acetylated alpha-tubulin during ovarian follicle differentiation in the lizard Podarcis sicula Raf. J Exp Zool A Comp. Exp Biol 301:532–541 [DOI] [PubMed] [Google Scholar]
- Mazia D, Brewer PA, Alfert M (1953) The cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol Bull 104:56–67 [Google Scholar]
- Motta CM, Castriota Scanderberg M, Filosa S, Andreuccetti P (1995) Role of pyriform cells during the growth of oocytes in the lizard Podarcis sicula. J Exp Zool 273:247–256 [Google Scholar]
- Motta CM, Filosa S, Andreuccetti P (1996) Regression of the epithelium in late previtellogenic follicles of Podarcis s sicula: a case of apoptosis. J Exp Zool 276:233–241 [Google Scholar]
- Nagata Y, Homma H, Lee JA, Imai K (1999) D-aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett 444:160–164 [DOI] [PubMed] [Google Scholar]
- Neaves WB (1971) Intercellular bridges between follicle cells and oocyte in the lizard Anolis carolinensis. Anat Rec 170:285–302 [DOI] [PubMed] [Google Scholar]
- Raucci F, Assisi L, D'Aniello S, Spinelli P, Botte V, Di Fiore MM (2004) Testicular endocrine activity is upregulated by D-aspartic acid in the green frog, Rana esculenta. J Endocrinol 182:365–376 [DOI] [PubMed] [Google Scholar]
- Raucci F, D'Aniello S, Di Fiore MM (2005) Endocrine roles of D-aspartic acid in the testis of lizard Podarcis s. sicula. J Endocrinol 187:347–359 [DOI] [PubMed] [Google Scholar]
- Raucci F, Di Fiore MM (2009) The reproductive activity in the testis of Podarcis s. sicula involves D-aspartic acid: a study on c-kit receptor protein, tyrosine kinase activity and PCNA protein during annual sexual cycle. Gen Comp Endocrinol 161:373–383 [DOI] [PubMed] [Google Scholar]
- Romano M, Limatola E (2000) Oocyte plasma membrane proteins and the appearance of vitellogenin binding protein during oocyte growth in the lizard Podarcis sicula. Gen Comp Endocrinol 118:383–392 [DOI] [PubMed] [Google Scholar]
- Sakai K, Homma H, Lee JA, Fukushima T, Santa T, Tashiro K, Iwatsubo T, et al. (1998) Localization of D-aspartic acid in elongate spermatids in rat testis. Arch Biochem Biophys 351:96–105 [DOI] [PubMed] [Google Scholar]
- Schell MJ, Cooper OB, Snyder SH (1997) D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci USA 94:2013–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacher SM, Rice RH (1985) Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell 40:685–695 [DOI] [PubMed] [Google Scholar]
- Taddei C (1972) Significance of pyriform cells in ovarian follicle of Lacerta sicula. Exp Cell Res 72:562–566 [DOI] [PubMed] [Google Scholar]
- Thomázy V, Fésüs L (1989) Differential expression of tissue transglutaminase in human cells. An immunohistochemical study. Cell Tissue Res 255:215–224 [DOI] [PubMed] [Google Scholar]
- Uliano R, Ricchiari L, Prisco M, Andreuccetti P (2001) Surface glycoproteins bearing alpha-GalNAc terminated chains accompany pyriform cell differentiation in lizards. J Exp Zool 290:769–776 [DOI] [PubMed] [Google Scholar]
- van Wyk JH (1984) Ovarian morphological changes during the annual breeding cycle of the rock lizard Agama atra (Sauria: Agamidae). Navors nas Mus Bloemfontein 4:237–275 [Google Scholar]
- Varma SK (1970) Morphology of ovarian changes in the garden lizard, Calotes versicolor. J Morphol 131:195–209 [DOI] [PubMed] [Google Scholar]