Abstract
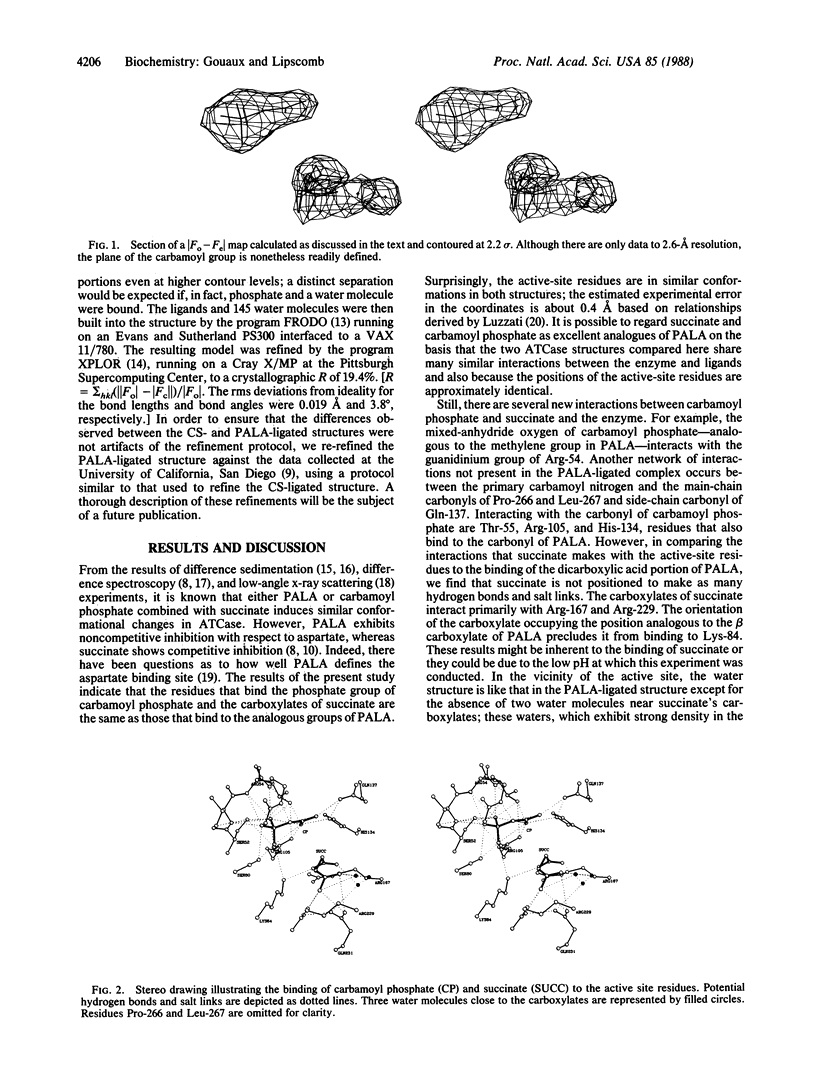
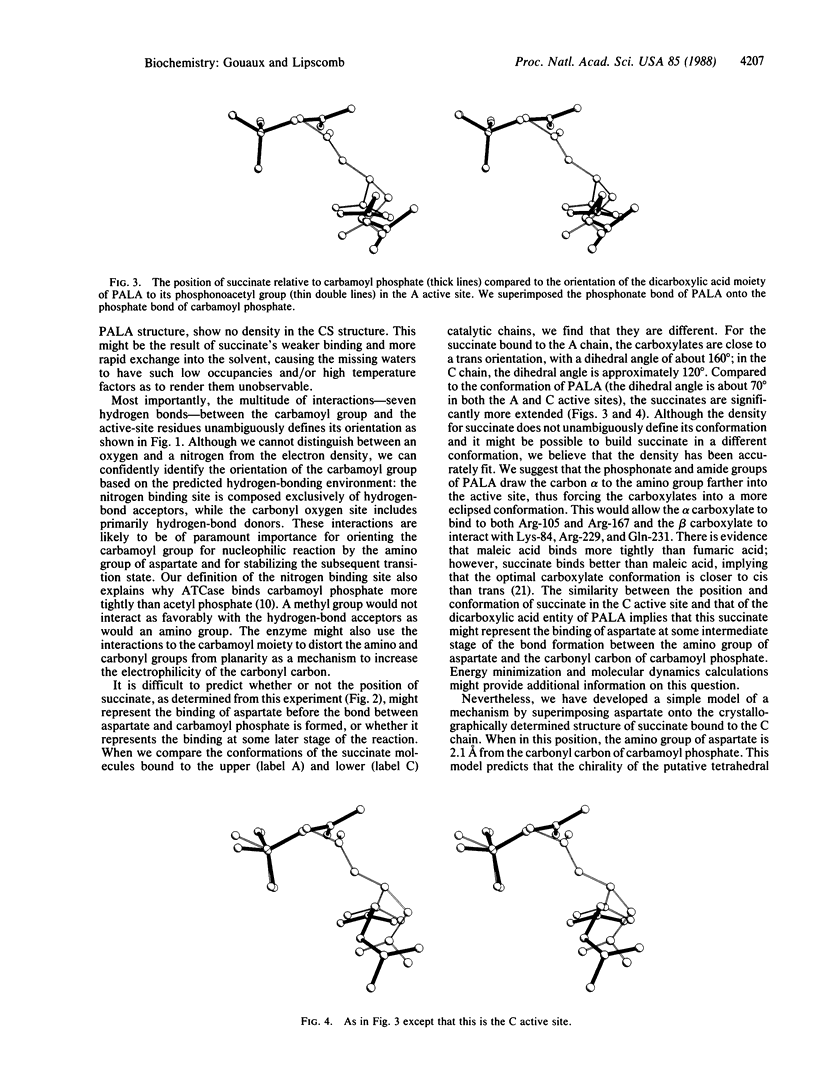
The three-dimensional structure of the ternary complex of carbamoyl phosphate, succinate, and aspartate carbamoyltransferase (EC 2.1.3.2) has been determined to 2.6-A resolution. The binding of the phosphate of carbamoyl phosphate is similar to the binding of the phosphonate of N-(phosphonoacetyl)-L-aspartate (PALA); interacting with the carboxylates of succinate are some of the same residues that interact with the carboxylates of PALA. The amino group of carbamoyl phosphate donates hydrogen bonds to the main-chain carbonyls of residues Pro-266 and Leu-267 and the side-chain carbonyl of Gln-137. In comparing the structure of the active sites in the PALA-enzyme complex to the active sites in the carbamoyl phosphate-succinate-enzyme complex, we find that they are similar.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Studies of the catalytic subunit by ultraviolet difference spectroscopy. J Biol Chem. 1969 Apr 10;244(7):1869–1877. [PubMed] [Google Scholar]
- Davies G. E., Vanaman T. C., Stark G. R. Aspartate transcarbamylase. Stereospecific restrictions on the binding site for L-aspartate. J Biol Chem. 1970 Mar 10;245(5):1175–1179. [PubMed] [Google Scholar]
- Dennis P. R., Krishna M. V., Di Gregorio M., Chan W. W. Ligand interactions at the active site of aspartate transcarbamoylase from Escherichia coli. Biochemistry. 1986 Apr 8;25(7):1605–1611. doi: 10.1021/bi00355a023. [DOI] [PubMed] [Google Scholar]
- Farrington G. K., Kumar A., Wedler F. C. Design and synthesis of new transition-state analogue inhibitors of aspartate transcarbamylase. J Med Chem. 1985 Nov;28(11):1668–1673. doi: 10.1021/jm00149a022. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gouaux J. E., Krause K. L., Lipscomb W. N. The catalytic mechanism of Escherichia coli aspartate carbamoyltransferase: a molecular modelling study. Biochem Biophys Res Commun. 1987 Feb 13;142(3):893–897. doi: 10.1016/0006-291x(87)91497-5. [DOI] [PubMed] [Google Scholar]
- Hervé G., Moody M. F., Tauc P., Vachette P., Jones P. T. Quaternary structure changes in aspartate transcarbamylase studied by X-ray solution scattering. Signal transmission following effector binding. J Mol Biol. 1985 Sep 5;185(1):189–199. doi: 10.1016/0022-2836(85)90190-1. [DOI] [PubMed] [Google Scholar]
- Heyde E. A unifying concept for the active site region in aspartate transcarbamylase. Biochim Biophys Acta. 1976 Nov 8;452(1):81–88. doi: 10.1016/0005-2744(76)90059-0. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Changes in the sedimentation coefficient promoted by the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Biochemistry. 1977 Nov 15;16(23):5077–5083. doi: 10.1021/bi00642a021. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Inouye T., Goldin A., Stark G. R. Antitumor activity of N-(phosphonacetyl)-L-aspartic acid, a transition-state inhibitor of aspartate transcarbamylase. Cancer Res. 1976 Aug;36(8):2720–2725. [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Swyryd E. A., Seaver S. S., Stark G. R. N-(phosphonacetyl)-L-aspartate, a potent transition state analog inhibitor of aspartate transcarbamylase, blocks proliferation of mammalian cells in culture. J Biol Chem. 1974 Nov 10;249(21):6945–6950. [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]


