Abstract
Large conductance, calcium-activated K+ (BK) channels are important regulators of cell excitability and recognized targets of intracellular kinases. BK channel modulation by tyrosine kinases, including focal adhesion kinase and c-src, suggests their potential involvement in integrin signaling. Recently, we found that fibronectin, an endogenous α5β1 integrin ligand, enhances BK channel current through both Ca2+- and phosphorylation-dependent mechanisms in vascular smooth muscle. Here, we show that macroscopic currents from HEK 293 cells expressing murine BK channel α-subunits (mSlo) are acutely potentiated following α5β1 integrin activation. The effect occurs in a Ca2+-dependent manner, 1–3 min after integrin engagement. After integrin activation, normalized conductance-voltage relations for mSlo are left-shifted at free Ca2+ concentrations ≥1 μm. Overexpression of human c-src with mSlo, in the absence of integrin activation, leads to similar shifts in mSlo Ca2+ sensitivity, whereas overexpression of catalytically inactive c-src blocks integrin-induced potentiation. However, neither integrin activation nor c-src overexpression potentiates current in BK channels containing a point mutation at Tyr-766. Biochemical tests confirmed the critical importance of residue Tyr-766 in integrin-induced channel phosphorylation. Thus, BK channel activity is enhanced by α5β1 integrin activation, likely through an intracellular signaling pathway involving c-src phosphorylation of the channel α-subunit at Tyr-766. The net result is increased current amplitude, enhanced Ca2+ sensitivity, and rate of activation of the BK channel, which would collectively promote smooth muscle hyperpolarization in response to integrin-extracellular matrix interactions.
Keywords: Biophysics, Channels/Potassium, Extracellular Matrix, Extracellular Matrix/Collagen, Extracellular Matrix/Fibronectin, Extracellular Matrix/Integrin, Signal Transduction/Phosphoprotein Phosphatases, Signal Transduction/Phosphoprotein Phosphatases/Tyrosine
Introduction
Large conductance, Ca2+-activated K+ channels (BK,3 Maxi-K) represent a subset of the superfamily of K+-selective ion channels widely expressed in neurons, smooth muscle, and other tissues. In vascular smooth muscle (VSM), BK channels are important targets of endothelium-derived vasoactive factors, and their activation by depolarization and/or increases in intracellular calcium leads to smooth muscle hyperpolarization and vasodilation (1–4). This sequence of events serves as a negative feedback mechanism to limit Ca2+ entry by closure of voltage-dependent Ca2+ channels (5). The sensitivity of BK channels to intracellular Ca2+ can be modulated, directly or indirectly, through channel phosphorylation by serine-threonine kinases and tyrosine kinases (8, 28, 29, 56).
Phosphorylation of the BK channel by tyrosine kinases, including focal adhesion kinase (6) and c-src (7, 8), suggests a possible involvement of the channel in integrin signaling. Integrins are a widely expressed class of adhesion receptor whose interactions with extracellular matrix (ECM) proteins lead to activation of signaling cascades, including protein tyrosine phosphorylation (9). In blood vessels, VSM integrins play important roles in the acute regulation of vascular tone (10) and in long term vascular remodeling (11, 12). We have previously shown that activation of VSM α5β1 integrins leads to potentiation of L-type calcium channel current through the involvement of the integrin-associated tyrosine kinases c-src and focal adhesion kinase (13, 14). Other studies have reported that BK channel activity is enhanced following direct channel phosphorylation by other src family kinases and by Pyk2 (proline-rich tyrosine kinase 2) (7, 8). Recently, we found that the endogenous α5β1 integrin ligand fibronectin (FN) potentiates BK channel current in VSM cells (15). Potentiation of native BK channels in VSM by multivalent α5β1 integrin ligands is significant at 1 min, peaks at 3–4 min, and is sustained for >8 min. The effect is blocked by the c-src inhibitor PP2 (15). Collectively, these observations suggest that the BK channel in VSM may be regulated by tyrosine kinases present in integrin-associated focal adhesion complexes.
The goals of the present study were as follows: 1) determine whether potentiation of BK current by α5β1 integrin activation could be reproduced in a heterologous expression system, and if so, 2) test if the effect was mediated by c-src, and 3) identify critical tyrosine phosphorylation sites on the channel. We present evidence that the activation of endogenous α5β1 integrins in HEK 293 cells transiently expressing the mouse BK channel α-subunit, mSlo, increases BK current amplitude at intermediate to high Ca2+ concentrations, enhances the speed of BK current activation at elevated intracellular Ca2+ concentrations, and enhances the Ca2+ sensitivity of the channel. The mechanism of channel regulation requires phosphorylation of the BK α-subunit at residue Tyr-766 by c-src.
EXPERIMENTAL PROCEDURES
Construction of cDNA Plasmids
cDNAs encoding the murine brain BK channel α-subunit (mSlo) and green fluorescent protein (GFP) were separately subcloned into the polylinker region of the SV40 promoter-based mammalian expression plasmid SRα using standard techniques (7). A full-length cDNA encoding human c-src tyrosine kinase was subcloned into SRα using the EcoRI site. Site-directed mutagenesis of mSlo and c-src was conducted using the Transformer mutagenesis kit (Clontech). An enzymatically inactive form of c-src was prepared by a Lys to Met mutation at position 298 in the catalytic domain of the kinase (16). Three mSlo tyrosine mutants were made by substituting Phe for Tyr at positions 766, 935, or 1027 (7). Because of 4- and 27-amino acid splicing inserts present in our mSlo clone, these positions correspond to identical sequences flanking Tyr residues at 762, 904, and 996 on hSlo (17).
Cell Transfection
HEK 293 cells (tsA-201 line) were maintained at 37 °C in a 5% CO2 incubator in Dulbecco's modified Eagle's medium containing l-glutamine, 4.5 g/liter d-glucose, and 10% (v/v) fetal bovine serum (Invitrogen). Transient transfection of the cells at 50–60% confluency was carried out in 35-mm tissue culture dishes using a cationic, lipid-based lipofection technique. 6–8 μl of Lipofectamine was mixed with 1.2–1.5 μg of total plasmid cDNA (mSlo and GFP) in 1 ml of serum-free Dulbecco's modified Eagle's medium and placed on cells for 5–6 h at 37 °C in a humidified incubator containing 5% CO2. In some protocols, mSlo (0.8 μg) was co-expressed with either wild-type c-src (WT c-src; 0.6 μg) or catalytically inactive c-src (0.6 μg). DNA-containing medium was then aspirated and replaced with serum-containing medium. After 24–48 h, cells were detached using mechanical force or 0.025% trypsin, 0.5 mm EDTA in phosphate-buffered saline (PBS) and replated onto sterile glass coverslips coated with 0.0001% (w/v in PBS) poly-l-lysine in 35-mm culture dishes. Electrophysiological recordings were typically performed on days 2–4 following transfection. For Western blotting, cells were harvested on day 3 following transfection.
Electrophysiology
Standard patch clamp methods were used to record macroscopic currents from excised, inside-out membrane patches (18) of transfected HEK 293 cells. An EPC9 amplifier (HEKA, Germany) was used for current recording and controlled by a Dell XPS computer running Pulse + Pulse-fit software through an ITC-16 interface (Instrutech, Port Washington, NY). Igor Pro (WaveMetrics, Oswego, OR) and SigmaPlot (SPSS, Ashburn, VA) were used for data analysis. Currents were typically sampled at 10 kHz and filtered at 5 kHz. BK channel currents were activated by voltage clamp pulses from a negative holding potential to test potentials ranging from −180 to +240 mV; tail currents were recorded at +50, −80, or −120 mV, as indicated, depending on the level of free Ca2+. Leak subtraction was performed for lower Ca2+ solutions (0, 1, and 5 μm) using a P/5 protocol, with leak pulses of opposite polarities to the test pulse, from a holding potential of −120 mV (19, 20). Currents recorded in 10 and 100 μm Ca2+ did not have leak subtraction performed. Typically, at each Ca2+ concentration, two to three families of current traces were recorded consecutively and averaged before analysis (21). All experiments were carried out at room temperature.
Micropipettes were pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) using a Sutter P-97 electrode puller and filled with a solution containing (in mm) 10 KCl, 130 KOH, 1 MgCl2, 1 CaCl2, and 10 HEPES, with pH adjusted to 7.3 with methanesulfonic acid; resistances were 2.0–3.5 megohms. The bath solution contained (in mm) 10 KCl, 130 KOH, 1 MgCl2, 2 EGTA, and 10 HEPES with pH adjusted to 7.2 using methanesulfonic acid; variable amounts of a 0.1 m CaCl2 solution were added to give the desired free [Ca2+] (1, 5, 10, and 100 μm). The bath solution without Ca2+ added is referred to as 0 Ca2+, in which the free [Ca2+] was estimated to be 1–10 nm (7). The levels of free Ca2+ in the other solutions were confirmed using a calcium electrode (Orion model 93-20, WPI, Sarasota, FL). 50 μm of the divalent cation chelator (+)-18-crown-6-tetracarboxylic acid was present in the bath solution to prevent divalent cation block at high voltages (21, 22). The recording chamber (∼0.3-ml volume) was perfused at a constant rate of ∼1 ml/min using a ValveLinkTM 16 solenoid bank (AutoMate Scientific, Inc., NY) to switch between various solutions. Individual cells expressing BK channels were identified visually by co-expression of GFP under epifluorescence illumination using 480 nm excitation and 510 nm emission filters.
Data Analysis
BK channel activity was expressed as relative conductance, G/Gmax, with conductance (G) values determined from tail currents and normalized to Gmax for each family of traces. To quantify the voltage dependence of currents under different conditions, plots of G/Gmax versus voltage were fitted with Boltzmann Equation 1,
where e is the elementary charge; z is the equivalent gating charge; F is Faraday's constant; R is the gas constant (8.3 V C/mol K); V0.5 is the voltage for half-maximal activation of the channel, and V is the experimental test potential. Time constants of current activation (τ) were derived from single exponential fits of current recordings over a 7–10-ms period beginning 0.25 ms after the start of the voltage clamp step (7). Statistical differences in the V0.5 values and slopes of G-V relations between groups were compared using two-way repeated measures analyses of variance followed by Tukey's post hoc tests for multiple comparisons, with p < 0.05 considered to be significant.
Immunofluorescence and Confocal Microscopy
HEK 293 cells were grown to a cell density of 70–80% using a Lab-Tek II coverglass system (Lab Tech, Rochester, NY). Cells were washed gently with PBS and fixed by incubation in pre-cooled 100% methanol at −20 °C for 10 min. After brief washing, cells were treated with PBS containing 2% bovine serum albumin, 5% goat serum and blocked using an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's protocol. The washed slides were first incubated with mouse anti-human integrin α5β1 monoclonal antibody (1:100) and rabbit anti-BK channel polyclonal antibody (1:100) for 60 min at room temperature. Cells were then washed with PBS and incubated for 45 min at room temperature with Alexa Fluor 488 goat anti-rabbit IgG antibody to detect BK channels and with Alexa Fluor 568 goat anti-mouse IgG to label α5β1 integrins. Cells were embedded in mounting medium with 4′,6-diamidino-2-phenylindole for nuclear staining (Vector Laboratories) and examined using a Zeiss LSM 510 two-photon confocal system. Negative controls were performed using the same procedure with normal rabbit immunoglobulin substituted for primary antibody.
Immunoprecipitation and Western Blotting
The procedures used for the immunoprecipitation and immunoblotting were essentially the same as those previously described by Ling et al. (8). For in vitro phosphorylation of expressed wild-type and mutant BK channels by human c-src tyrosine kinase, immunoprecipitated BK channels bound to protein A-Sepharose beads were resuspended in 10 mm HEPES, pH 7.6, and 1 mm dithiothreitol and then added to a reaction mixture containing (final) the following: 50 mm HEPES, 125 mm NaCl, 5 mm MgCl2, 1 mm dithiothreitol, 0.2 mm Na3VO4, 4 mg/ml p-nitrophenyl phosphate, and ∼15 units of partially purified recombinant human c-src-(UBI/Millipore), pH 7.6. The reaction was started by addition of Na2-ATP (50 μm final) and carried out for 30 min at 30 °C. Concentrated Laemmli sample buffer was then added to stop each reaction, and samples were heated at 70 °C for 20 min prior to resolution by SDS-PAGE. Phosphotyrosine-containing proteins were detected by Western blotting using the anti-phosphotyrosine mouse monoclonal antibody 4G10.
For detection of channel phosphorylation after adhesion to different substrates, transfected cells were reseeded onto plates coated with either porcine gelatin (∼1 mg/ml) or human fibronectin (catalogue no. 354403, BD BioCoat, BD Biosciences). After ∼2 h, culture medium was aspirated, and cells were lysed directly on the coated dishes by addition of 0.3–0.4 ml of buffer containing the following: 25 mm Tris-HCl, pH 7.4, 130 mm NaCl, 1% (v/v) Triton X-100, 1 mm Na3VO4, 10 mm NaF, 1 mm EDTA, 1 mm EGTA, 1 mm benzamidine, 0.5 mm phenylmethylsulfonyl fluoride, and 5 μg/ml each of leupeptin, pepstatin A, and aprotinin. After centrifugation at ∼10,000 × g for 15 min at 4 °C, the soluble fractions were recovered and subjected to immunoprecipitation with an anti-BK channel antibody as described above.
For Western blotting, the supernatants or immunoprecipitates were mixed with Laemmli sample buffer containing 0.5% (v/v) β-mercaptoethanol, and the proteins were separated by SDS-PAGE. Proteins were electrotransferred to a nitrocellulose membrane (Bio-Rad) at 4 °C overnight in transfer buffer at constant voltage (18 V). Membranes were then briefly rinsed in Tris-buffered saline containing 0.1% Tween 20 (TTBS) and incubated at room temperature for 20–30 min in TTBS containing 5% skim milk powder to reduce nonspecific binding, followed by three rinses in TTBS for 5 min each. The nitrocellulose membrane was incubated with primary anti-src mouse monoclonal antibody 327 (1:200) in TTBS containing 1% skim milk powder for 1 h at room temperature, followed by 5-min washes with TTBS. Membranes were then incubated for 1 h with secondary antibody (goat anti-mouse IgG) diluted (1:4000) in TTBS containing 1% skim milk powder, followed by 5-min washes with TTBS. Blots were immediately developed by application of SuperSignal West Pico chemiluminescent substrate reagent (Pierce) for 2–3 min followed by exposure to x-ray film.
Reagents and Chemicals
Anti-human α5β1 integrin monoclonal antibody (Ab) was obtained from Chemicon/Millipore Corp. (Temecula, CA; MAB 1969). The c-src tyrosine kinase inhibitor PP2, anti-c-src mouse monoclonal Ab 327, and goat anti-mouse monoclonal IgG were purchased from Calbiochem. For immunofluorescence, immunoprecipitation, and Western blotting procedures, rabbit polyclonal anti-BK channel Ab (AB5228) was purchased from Chemicon. The mouse monoclonal anti-phosphotyrosine Ab (4G10) was obtained from Upstate Cell Signaling (Charlottesville, VA).
Secondary antibodies for immunofluorescence were obtained from Molecular Probes (Eugene, OR). Lipofectamine and high glucose-containing Dulbecco's modified Eagle's medium were purchased from Invitrogen. All other reagents, except as otherwise stated, were obtained from Sigma.
RESULTS
α5β1 Integrin Activation Enhances BK Current
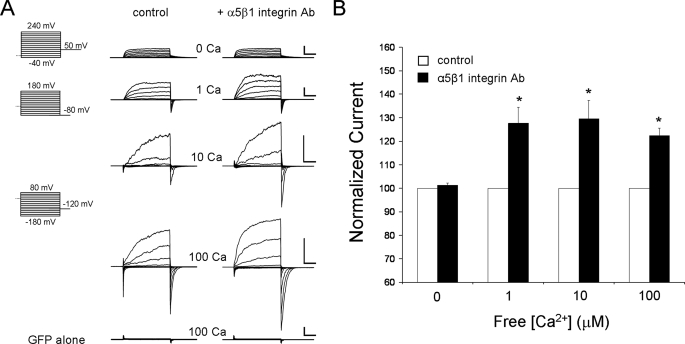
The activation of α5β1 integrin by multivalent ligands such as FN or insoluble α5β1 integrin antibody is associated with 30–50% enhancement in whole-cell BK current in VSM cells (15). BK current enhancement could be reproduced with heterologously expressed mSlo channels (Fig. 1) because HEK 293 cells endogenously express α5β1 integrin (supplemental Fig. 1); mSlo transfection did not alter α5β1 integrin expression. To activate α5β1 integrin by cross-linking, soluble anti-human α5β1 integrin monoclonal Ab (15 μg/ml) was applied to the macropatches using two different methods. In protocol A, each patch was used as its own control, and the integrin Ab was preloaded into the recording pipette before gigaseal formation; the tip was filled with standard pipette solution and then backfilled with the same solution containing integrin Ab. Preliminary tests indicated that the loading procedure permitted 3–5 min of control current recording before the Ab reached the cell surface (18). After gigaseal formation on a GFP-positive cell, a macropatch was excised (inside-out recording mode), and outwardly rectifying K+ currents were elicited by positive voltage steps. Fig. 1A shows sample recordings of mSlo current in macropatches before and after diffusion of α5β1 integrin Ab to the cell surface. Under these conditions, mSlo currents were significantly increased in amplitude after ∼5 min. Importantly, BK current enhancement did not occur over the same time course in patches exposed to the same backfilling procedure using vehicle or non-integrin Ab (soluble IgG) (supplemental Fig. 2). Membrane patches from cells transfected with GFP alone displayed only modest outward currents (i.e. <50 pA at +80 mV) in response to the same voltage protocols, even after exposure to 100 μm cytosolic free [Ca2+], and these small currents were not altered by integrin Ab application (Fig. 1A, bottom trace). The effect of α5β1 integrin Ab on BK current amplitude is summarized in Fig. 1B; after integrin activation, current amplitude was significantly increased at every Ca2+ concentration greater than zero. Qualitatively similar results were obtained using an alternative protocol (protocol B, described below) in which possible channel modification by kinases/phosphatases was permitted to occur prior to patch excision. Under those conditions, an increase in BK current was also consistently observed after application of α5β1 integrin Ab to the patch, between 62 and 102% potentiation of current depending on the Ca2+ concentration used in the recording. The larger effect on current amplitude compared with protocol A (Fig. 1B) is consistent with the probability that ATP supplies were available only for a limited amount of time in the patch microenvironment after excision into ATP-free solution (59, 60).
FIGURE 1.
Potentiation of mSlo current by α5β1 integrin activation. A, current traces recorded in symmetrical 140 mm K+ solutions from excised, inside-out membrane patches expressing mSlo before (left traces) and after (right traces) activation of α5β1 integrin with soluble α5β1 Ab, applied as described in protocol A. Activation of the integrin resulted in increases in the amplitude of current at all levels of cytosolic free Ca2+, except 0 Ca2+, compared with control currents in the same cells (one Ca2+ level per patch, except 0 and 1 μm Ca2+ traces are from the same patch). Outward currents were negligible (<50 pA at +80 mV, 100 μm Ca2+) in cells transfected with GFP but not mSlo cDNA (bottom traces). Scale bars are 2 nA, 5 ms for all current traces except bar for 10 μm Ca2+ trace is 1 nA, 5 ms. Voltage protocols varied with the free Ca2+ level. For 0 Ca2+, voltage steps were from −40 to +240 mV in 20-mV increments with tail currents measured at +50 mV. For 1 μm Ca2+, voltage steps were from −80 to +180 mV with tail currents measured at −80 mV. For 10 and 100 μm Ca2+, voltage steps were from −180 to +80 mV with tail currents measured at −120 mV. Except for the recordings in 0 and 1 μm Ca2+, the relative amplitudes of the currents are not comparable in the vertical direction, because of the use of different cells with different mSlo expression levels. B, peak mSlo current was measured at a common test potential for each [Ca2+]: +240 mV (0 Ca2+), +180 mV (1 μm Ca2+), and +80 mV (10 and 100 μm Ca2+). Except for 0 Ca2+, current was elevated ∼22–30% above the control level at each [Ca2+] in the presence of α5β1 integrin Ab. Asterisk indicates current values that are significantly different (p < 0.05), control versus integrin Ab treatment.
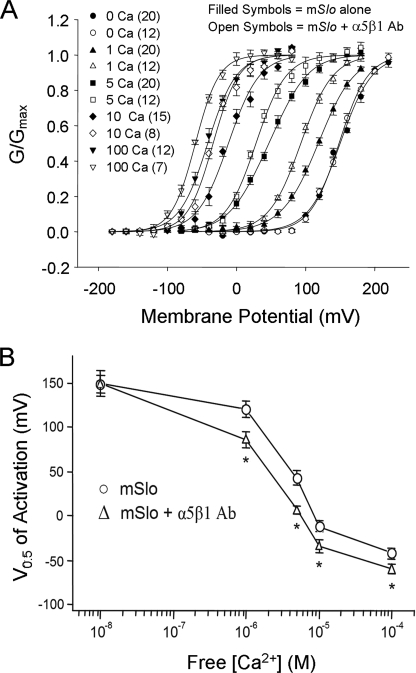
To test whether α5β1 integrin activation altered the calcium sensitivity of the BK channel, tail current analysis was used to determine the conductance-voltage (G-V) relations after exposure of the inner patch surface to different free [Ca2+] at a constant K+ driving force. Normalized G-V curves showed a progressive left-shift in channel gating along the voltage axis with increasing [Ca2+]. The increased time required for these protocols (to test 4–5 different calcium solutions on each patch) precluded the use of protocol A. Instead, we compared G-V relations in the five calcium solutions in macropatches exposed, or not exposed, to 3-min treatment with integrin Ab, where the entire recording pipette was loaded (or not) with Ab solution before gigaseal formation (protocol B). In macropatches exposed to α5β1 integrin Ab, BK currents showed enhanced Ca2+ sensitivity (Fig. 2A) at all Ca2+ concentrations (except 0 Ca2+), as evident from left-shifted G-V curves in the presence of the integrin Ab. Plots of the average V0.5 values versus free [Ca2+] for control patches and patches exposed to α5β1 integrin Ab are shown in Fig. 2B. The V0.5 values were significantly altered by α5β1 integrin activation at 1, 5, 10, and 100 μm Ca2+ but not at 0 Ca2+ (150 ± 15 mV after and 149 ± 16 mV before integrin activation), with the differences being greatest at 1 and 5 μm free Ca2+.
FIGURE 2.
α5β1 integrin activation increases BK channel calcium sensitivity. A, conductance-voltage (G-V) relationships were determined from tail current amplitudes measured 0.2–0.3 ms after the step to the tail potential and normalized to the peak tail current. Normalized data were fit with single Boltzmann functions. Comparison of G-V relations in excised patches expressing mSlo in the absence (filled symbols) or presence (open symbols) of α5β1 integrin Ab as applied using protocol B. The number of patches for each group is given in parentheses. B, V0.5 values plotted as a function of cytosolic free Ca2+ for cells with and without α5β1 integrin Ab. Consistent leftward shifts occurred following α5β1 integrin activation, except in 0 Ca2+. The value for the free [Ca2+] in 0 Ca2+ solution given here and in all subsequent plots of V0.5 versus [Ca2+] is an estimate (i.e. 10 nm) and likely overestimates the actual [Ca2+] of the 0 Ca2+ bath. Asterisk indicates V0.5 values that are significantly different (p < 0.05; determined by analysis of variance) from those of control patches (i.e. in the absence of integrin Ab).
Another effect of integrin activation not evident in Figs. 1 and 2 was that the speed of channel activation after a depolarizing step was sometimes increased. The supplemental Fig. 3 shows examples of this effect, which is more clearly evident after normalization of the currents to account for differences in their magnitude. Increased speed of current activation was occasionally evident at 1 and 10 μm Ca2+, but the effect was only consistently recorded at 100 μm Ca2+. Collectively, these results suggest that activation of α5β1 integrin leads to enhancement of mSlo current amplitude in the presence of Ca2+, induces a leftward shift in the channel Ca2+ sensitivity when free Ca2+ exceeds 1 μm, and lowers the time constant of current activation at high Ca2+ levels.
c-Src Is Involved in the Enhancement of BK Channel Activity
Because the effects of integrin activation on ion channels have been shown in some systems to be mediated by c-src (14), we tested whether c-src was involved in the enhancement of BK channel activity. Either wild-type c-src (WT c-src) or inactive c-src was co-expressed transiently with mSlo, and Western blotting was performed to test the relative expression levels of c-src. The results confirmed expression of c-src (∼60 kDa) at levels substantially higher (Fig. 3A) than those observed in nontransfected cells. With standard 2-s film exposure (Fig. 3A, lanes 1–5), endogenous c-src was not apparent, but with longer exposure (15 s), a faint band at ∼60 kDa could be detected (lane 6).
FIGURE 3.
Potentiation of mSlo Ca2+ sensitivity by overexpression of active c-src but not inactive c-src. A, Western blots comparing relative levels of c-src in nontransfected cells and after transient transfection with WT c-src or catalytically inactive c-src in addition to mSlo. Immunoreactive bands at ∼60 kDa (2 s exposure) are prominent in lane 3 (co-expression of mSlo and WT c-src) and lane 4 (co-expression of mSlo and inactive c-src) but not in lane 1 (co-transfection of mSlo with empty vector), lane 2 (transfection of mSlo alone), and lane 5 (nontransfected cells). α-Tubulin mouse monoclonal IgM (∼53 kDa) was used as a loading control. With longer time exposure (15 s), a faint band consistent with endogenous expression of c-src was observed in nontransfected cells (lane 6). B, summary of G-V relations from patches expressing mSlo with or without co-expression of WT c-src. G-V curves were left-shifted after co-expression of WT c-src at all Ca2+ levels except 0 Ca2+. The largest shifts occurred at 1 and 5 μm cytosolic free Ca2+. C, calculated V0.5 values from B are plotted as a function of free Ca2+ and show that a significant enhancement in Ca2+ sensitivity occurs in patches excised from cells co-expressing WT c-src but not inactive c-src, compared with mSlo alone. Co-expression of inactive c-src blocked the G-V curve shifts induced by α5β1 integrin activation (compare curve with mSlo + α5β1 Ab in Fig. 2B).
Currents were then recorded from transfected HEK 293 cells co-expressing WT c-src with mSlo, and the G-V relations from excised macropatches were compared with those from patches excised from cells expressing mSlo alone (Fig. 3B). Co-expression of WT c-src was associated with similar degrees of mSlo current potentiation and shifts in Ca2+ sensitivity as observed when mSlo was expressed alone, followed by α5β1 integrin activation (Fig. 3B, compare graph to Fig. 2B). As before, no significant shift occurred in 0 Ca2+ (∼150 to ∼149 mV), and leftward shifts in G-V curves were greatest at 1 and 5 μm [Ca2+]. In patches from cells co-expressing mSlo and WT c-src, the plots of V0.5 versus free [Ca2+] were significantly shifted to lower [Ca2+] compared with curves from patches expressing mSlo alone (Fig. 3C).
To determine whether α5β1 integrin activation enhances mSlo current through c-src, we tested the effect of α5β1 integrin activation in cells co-expressing inactive c-src and mSlo. Overexpression of kinase-inactive c-src would be expected to compete with the interaction between endogenous c-src and mSlo. Indeed, overexpression of inactive c-src blocked the shift in Ca2+ sensitivity induced by α5β1 integrin activation (Fig. 3C). Additionally, in cells co-expressing mSlo and WT c-src, the G-V relationship was left-shifted as before, but no additional shift in the G-V relationship was detectable after α5β1 integrin activation at any Ca2+ concentration (Fig. 4A). These results point to a nonadditive enhancement of BK channel activity in the presence of α5β1 integrin activation and c-src co-expression, suggesting the possibility that both phenomena occur through a common mechanism.
FIGURE 4.
Enhanced mSlo Ca2+ sensitivity following α5β1 integrin activation is prevented by the src family kinase inhibitor PP2. A, V0.5 versus [Ca2+] plots from patches expressing mSlo alone, mSlo after exposure to α5β1 integrin Ab (protocol B), mSlo, and WT c-src, or mSlo and WT c-src after exposure to α5β1 integrin Ab. Expression of WT c-src shifted the curve to the left, as in Fig. 3B, but no further shift occurred after application of integrin Ab. B, mSlo-transfected cells were exposed or not exposed to PP2 (1 μm) in the bath, followed by current recordings from excised patches. PP2 prevented the enhancement of mSlo Ca2+ sensitivity induced by α5β1 integrin activation. Plots of V0.5 versus free Ca2+ showed no significant differences between the groups at any free [Ca2+].
Constitutive overexpression of c-src might up-regulate other signaling pathways and thereby alter mSlo activity indirectly. Therefore, we tested the effects of the relatively specific soluble Src family kinase inhibitor, PP2 (1 μm), added to the bath solution, and we compared the G-V curves under those conditions to G-V curves from patches expressing mSlo in the absence of PP2. PP2 had no significant effect on the G-V relationship for mSlo current at any free [Ca2+] (Fig. 4B), suggesting that endogenous c-src did not constitutively increase mSlo activity in the absence of α5β1 integrin activation. However, PP2 blocked the shift in mSlo Ca2+ sensitivity induced by α5β1 integrin activation (Fig. 4B). There were no significant differences in Ca2+ sensitivity between the three groups.
mSlo C-terminal Residue Tyr-766 Is Critical for Potentiation of BK Channel Activity Following Integrin Engagement
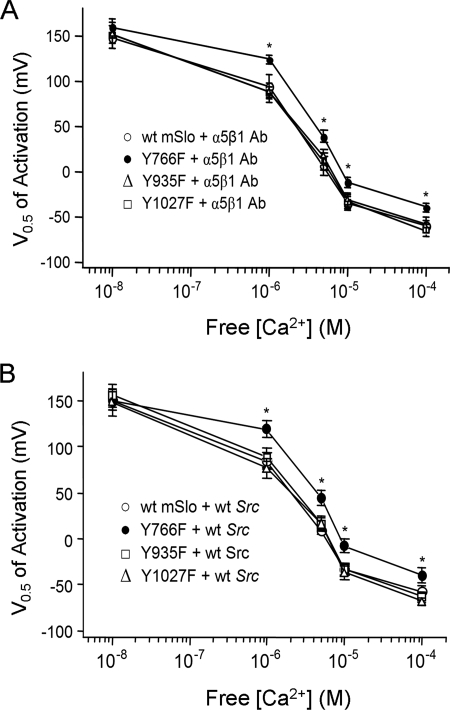
Ling et al. (7) reported that phosphorylation of the BK channel by c-src was prevented by a single Tyr substitution on the mSlo C terminus. To test if phosphorylation of the same site was critical for the enhancement of mSlo current by α5β1 integrin activation, we tested three different mSlo constructs, each containing a single tyrosine-to-phenylalanine mutation at three predicted c-src consensus phosphorylation sites, Y766F, Y935F, and Y1027F. Importantly, there were no significant differences in base-line current amplitude, voltage sensitivity, or Ca2+ sensitivity of the mSlo mutants compared with wild-type mSlo (supplemental Fig. 4), suggesting that these aspects of channel function were unaffected by the mutations. Macroscopic currents were subsequently recorded in excised patches from cells expressing each mSlo mutant in response to α5β1 integrin activation. A comparison of the G-V curves with those recorded from cells expressing WT mSlo indicated that only the Y766F mSlo mutant failed to exhibit a shift in Ca2+ sensitivity following α5β1 integrin activation (Fig. 5A); the Y935F and Y1027F mSlo mutants both showed the typical leftward shift in Ca2+ sensitivity observed with WT mSlo after α5β1 integrin activation. The results suggest that the Tyr-766 residue on mSlo is critical to the enhancement of BK channel Ca2+ sensitivity induced by α5β1 integrin activation.
FIGURE 5.
Mutation of a single tyrosine residue prevents the enhancement of mSlo current by α5β1 integrin activation. A, V0.5 versus [Ca2+] plots from the Y766F, Y935F, and Y1027F mSlo mutants compared with WT mSlo, after integrin activation. Only the Y766F mSlo mutant failed to show a shift in its apparent Ca2+ sensitivity after integrin activation. B, V0.5 versus [Ca2+] plots for Y766F, Y935F, and Y1027F mSlo mutants, compared with WT mSlo, after co-expression with WT Src. Only the Y766F mSlo mutant failed to show an increase in Ca2+ sensitivity with WT c-src co-expression.
To further test the contribution of c-src and mSlo residue Tyr-766 to the enhancement of BK channel activity, WT c-src was co-expressed with each of the three mSlo mutants, and currents were recorded from excised macropatches. In response to increasing concentrations of free Ca2+, only the G-V relationship of the Y766F mSlo mutant, when co-expressed with WT c-src, failed to be left-shifted compared with that of WT mSlo (Fig. 5B), i.e. the G-V curves recorded from patches expressing WT c-src and Y935F mSlo or Y1027F mSlo were left-shifted at free [Ca2+]s from 1 to 100 μm, similar to the G-V curves recorded from patches expressing WT mSlo.
The slopes of the G-V curves, each representing the voltage sensitivity of the channel, from the various groups in Figs. 2–5 are listed in supplemental Tables 1–3. None of the slopes were significantly different (ranging from ∼19 to ∼25 mV/e-fold change in open probability), except for two values as indicated in supplemental Table 3. The results suggest that the voltage sensitivity of mSlo was unaffected by α5β1 integrin activation even though the Ca2+ sensitivity of the channel was enhanced.
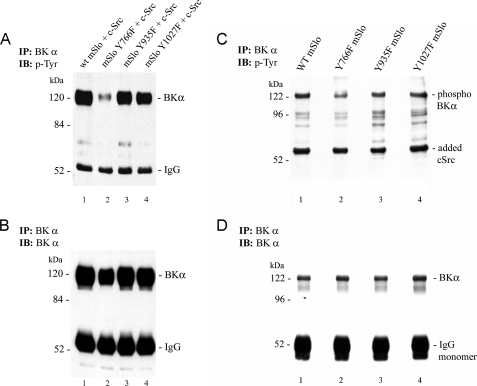
The electrophysiological results in Fig. 5 suggest that mSlo can be phosphorylated on residue Tyr-766, thereby enhancing the Ca2+ sensitivity of the channel. Fig. 6 shows two biochemical tests of this prediction. First, HEK 293 cells were transiently transfected with either WT or mutant mSlo, together with WT c-src. After cell lysis, the channel was immunoprecipitated, resolved by SDS-PAGE, and probed with an anti-phosphotyrosine (anti-Tyr(P)) antibody (Fig. 6A), and the amount of Tyr(P) detected in the Y766F mSlo mutant (lane 2) was substantially lower than that detected for the other two mSlo mutants or WT mSlo (lanes 1, 3, and 4). Fig. 6B shows nearly equal recovery of the WT and mutant BK channels after the membrane was stripped and reprobed with an anti-BKα antibody. The relative Tyr(P) signal was reduced to 40 ± 7% of control in the Y766F mutant channel.
FIGURE 6.
Mutation of Tyr-766 to Phe reduces direct tyrosine phosphorylation of the BK channel α-subunit in the presence of c-src. HEK 293 cells were transiently transfected with either WT or mutant forms of mSlo, together with WT human c-src cDNA. A, expressed BK channels were first isolated by IP and then detected by immunoblotting (IB) using anti-Tyr(P) Ab. The Y766F mutation in mSlo substantially reduced the level of BK channel Tyr phosphorylation observed in HEK 293 cells co-expressing c-src, whereas mutations at positions Tyr-935 and Tyr-1027 had little effect. B, immunodetection of the BK channel protein for the same nitrocellulose membrane in A after re-probing with anti-BK α antibody. Following correction for the total amount of immunoprecipitated BK α-subunit detected under each condition, the relative amount of Tyr(P) in each mutant mSlo channel was normalized to the level detected in WT mSlo (set as 1). For the Y776F, Y935F, and Y1027F mutants, the relative amounts of phosphotyrosine were 0.40 ± 0.07, 0.84 ± 0.06, and 0.77 ± 0.04, respectively (mean ± S.E.; n = 3 for each group). Statistical significance (p < 0.05) for the relative amount of phosphotyrosine-containing BK α-subunit was only noted between the WT and Y766F forms of the channel. The electrophoretic sizes (in kDa) and positions of molecular weight markers are denoted on the left-hand sides of the blots. C, in another set of experiments, WT or mutant mSlo channels were expressed in HEK 293 cells, followed by IP of each channel type. IP channels were then subjected to in vitro phosphorylation in the presence of purified human c-src and Mg-ATP. Proteins were resolved by SDS-PAGE, followed by immunoblotting (IB) using anti-Tyr(P) Ab. A prominent phosphotyrosine-containing band at the appropriate molecular weight for mSlo was detected in the samples containing either wild-type channel (lane 1), the Y935F mutant (lane 3), or the Y1027F mutant (lane 4); however, a much weaker signal was observed for the equivalent band in the sample containing the Y766F mutant channel (lane 2). The prominent Tyr(P)-containing band detected in each lane at ∼60 kDa represents the added purified c-src following its normal autophosphorylation. D, same nitrocellulose membrane depicted in C is shown after chemical stripping and re-probing with anti-BK α-subunit antibody to assess similar protein loading of the WT and mutant forms of the channel. The average Tyr(P) signal intensities for the mutant channel forms were calculated as the ratio of Tyr(P) signal in C to BK immunoreactivity in D and then normalized to the same ratio for the WT mSlo. The values for Y766F, Y935F, and Y1027F mSlo were 0.54 ± 0.11, 1.06 ± 0.21, and 0.92 ± 0.16 (mean ± S.E.), respectively, with WT mSlo set to 1.0.
This result was subsequently confirmed using an in vitro assay (Fig. 6, C and D). After transfection of cells with WT or mutant mSlo (without c-src co-expression), the cells were lysed, and mSlo was immunoprecipitated (7). BK channel immunoprecipitates were then incubated in vitro with purified, recombinant human c-src tyrosine kinase in the presence of Mg-ATP. The reaction mixture for each channel was resolved by SDS-PAGE, followed by electrotransfer to nitrocellulose. The membrane was subsequently probed with an anti-Tyr(P) antibody. Fig. 6C shows a prominent Tyr(P) band at the appropriate molecular weight for the BK channel α-subunit but with a substantially reduced amount of Tyr(P) detected in the Y766F mSlo mutant (lane 2) compared with that detected for WT mSlo (lane 1) or for the other mutants (lanes 3 and 4). Fig. 6D shows the same membrane after stripping and reprobing with the BKα antibody to document that there were approximately equal amounts of either WT or mutant immunoprecipitated BK channel protein present in each phosphorylation reaction mixture. The relative Tyr(P) signal was reduced to 54 ± 11% of control in the Y766F mutant channel in contrast to the relative Tyr(P) signals for other two mutant channels, which were not significantly different from that of the WT channel.
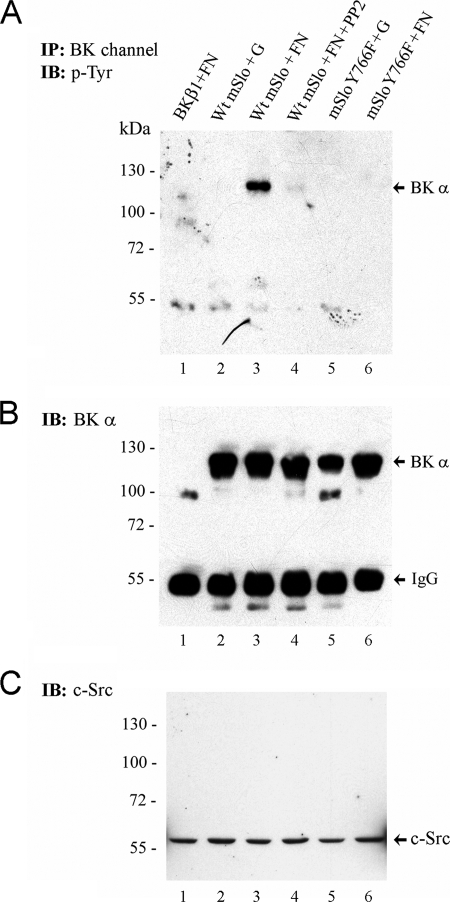
To test if integrin engagement led to Tyr phosphorylation of the BK channel α-subunit at residue Tyr-766, cells expressing WT or mutant mSlo were plated for 2 h on gelatin or FN and then subjected to immunoprecipitation with anti-BKα antibody followed by Western blotting for Tyr(P)-containing proteins and BK channel α-subunit. Fig. 7A shows a prominent Tyr-phosphorylated band at the same molecular weight as BKα when the cells adhere to FN (lane 3) but not to gelatin (lane 2). Reprobing the blot with BK channel Ab confirmed that the channel was detected at this molecular weight (Fig. 7B). Importantly, treatment of the cells with PP2 blocked FN-induced Tyr phosphorylation of the same protein (Fig. 7B, lane 4), consistent with involvement of c-src in phosphorylation of the channel. Comparison of Fig. 7B, lanes 3 and 6, suggests that Y766F mSlo does not become tyrosine-phosphorylated when the cells are plated on FN, further showing that this particular residue is critical for integrin-induced phosphorylation of the channel. It is important to note that channel phosphorylation occurred with endogenous levels of c-src in this experiment (in contrast to Fig. 6). Indeed, detection of c-src in whole-cell lysates (Fig. 7C) confirmed equivalent levels of endogenous c-src expression across the different treatment groups.
FIGURE 7.
Fibronectin induces tyrosine phosphorylation of BK channels in intact cells. Following transient expression of either WT mSlo or Y766F mSlo in HEK 293T cells, transfected cells were seeded onto tissue culture dishes coated with either porcine gelatin (G) or human FN. After 90–120 min, cells were lysed, and BK channel protein was isolated by immunoprecipitation. A, Western blot of BK channel immunoprecipitates probed with 4G10 anti-Tyr(P) Ab. B, same nitrocellulose membrane from A probed with a polyclonal antibody recognizing the BK α-subunit. Transfection of cells with cDNA encoding the BK β1-subunit served as a negative control (lane 1). In lane 4, the src kinase inhibitor PP2 (1 μm final) was added to WT mSlo-transfected cells seeded on FN-coated dishes. The positions of the monomeric BK α-subunit and IgG heavy chain are shown on the right-hand side. C, detection of endogenous c-src tyrosine kinase protein in whole lysates from transfected HEK 293T cells seeded under the same conditions as in A. An equal amount of cell lysate protein from each sample (∼40 μg) was loaded per lane. The electrophoretic positions of pre-stained molecular mass markers (size in kDa) are indicated on the left-hand side of each blot. Data are representative of two similar experiments. IB, immunoblot.
DISCUSSION
The results presented here significantly advance our understanding of how BK channel activity is potentiated by α5β1 integrin activation. The potentiation manifests as an increased current amplitude at free [Ca2+] >0, enhanced Ca2+ sensitivity of the channel at intermediate Ca2+ levels (1–100 μm), and increased speed of channel activation at high [Ca2+] (100 μm). Co-expression of wild-type human c-src resulted in comparable enhancement of current and Ca2+ sensitivity as produced by integrin activation. Both effects were absent in a mutant channel with a single amino acid substitution at Tyr-766 but were unaffected by similar substitutions at two other Tyr residues within the C terminus of the channel. In addition, overexpression of catalytically inactive c-src to compete with endogenous c-src or application of the soluble c-src inhibitor PP2 blocked the effects of integrin activation. These results strongly suggest that signaling through the ECM-integrin-src axis plays a central role in the regulation of mSlo gating, mediated by phosphorylation of the channel at residue Tyr-766. Physiologically, the enhancement of BK channel activity would be expected to limit excitability in smooth muscle and neurons by turning off Ca2+ influx, because L-type Ca2+ channels in these tissues are also potentiated following α5β1 integrin activation (13, 14). In nonexcitable tissues such as endothelium, activation of the BK channel, when expressed (23), through integrin-ECM interactions (24), is predicted to induce membrane hyperpolarization and drive production of endothelium-dependent vasodilators through an increase in the passive driving force for external Ca2+ entry through nonvoltage-regulated channels (e.g. store-operated calcium entry). Whether integrin activation modulates the activity of intermediate and/or small conductance Ca2+-activated K+ channels remains to be determined.
BK Channel Regulation by α5β1 Integrin and c-Src
It is well established that integrin interactions with the ECM result in the transduction of signals to the cytoskeleton and subsequent activation of intracellular kinases such as extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK), protein kinase A (25, 26), and c-src (14, 27). Several of the downstream targets for these kinases are ion channels, including a number of K+ channels (11, 31–33). Tyr(P)-dependent regulation of the BK channel can also occur in other situations (28).
The results of such studies are consistent with our findings that α5β1 integrin activation or co-expression with WT c-src produced comparable potentiation of mSlo current and enhancement in Ca2+ sensitivity. The effect on mSlo gating was greatest at 1 and 5 μm Ca2+ and negligible at Ca2+ < 1 μm. No additional enhancement in channel sensitivity by α5β1 integrin stimulation was observed in cells overexpressing WT c-src, suggesting that α5β1 integrin activation and c-src share a common pathway in regulating mSlo activity. Apparently, the levels of endogenous c-src were sufficient for activation of mSlo by α5β1 integrin. The observation that c-src overexpression did not produce a larger shift in mSlo Ca2+ sensitivity than integrin activation suggests that the modest level of endogenous c-src was not limiting the effect of integrin activation on mSlo. Importantly, overexpression of catalytically inactive c-src in our study blocked the effect of integrin activation on mSlo Ca2+ sensitivity, which is consistent with competition between inactive c-src and endogenous c-src for interaction with mSlo.
In apparent contrast to our finding that c-src overexpression enhances the Ca2+ sensitivity of mSlo, the data of Alioua et al. (29) suggest that BK channel phosphorylation by endogenous c-src leads to inhibition of BK channel activity in VSM. Although different isoforms of the BK channel (mSlo and hSlo) were used in our respective studies, the two channels have ∼96% predicted amino acid identity (GenBankTM accession numbers U09383 and U11058) with differences occurring only at two splice insert sites that do not appear to be critical for c-src phosphorylation. However, hSlo and mSlo channels exhibit several notable functional differences, including differences in their gating currents (30, 31), differences in their voltage sensitivities in the absence of Ca2+ (30, 32, 33), and an ∼4-fold lower sensitivity of hSlo to charybdotoxin (34, 35). Alioua et al. (29) found that c-src directly phosphorylated heterologously expressed hSlo and produced a rightward shift in the G-V relationship (29). A more likely difference between our results and those of Alioua et al. (29) could be related to the degree of BK channel phosphorylation induced by c-src co-expression under both experimental conditions. As shown in Fig. 6, co-expression of c-src both in situ and in vitro results in phosphorylation primarily at a single residue (Tyr-766) of the BK α-subunit. This result was confirmed in Fig. 7, where integrin engagement resulted in BK channel phosphorylation at Tyr-766 in the presence of endogenous levels of c-src. However, it is possible that different and/or multiple Tyr residues on hSlo are phosphorylated by c-src, resulting in channel inhibition rather than enhancement. The presence of the β1 BK channel subunit may also alter the functional effect of c-src-mediated BK channel phosphorylation (36). Although we expressed only the BK channel α-subunit in this study, we found similar degrees of mSlo current potentiation and integrin-induced increased Ca2+ sensitivity with or without co-expression of the β1 BK channel subunit (15); however, we did not test the role of c-src under those conditions.
Regulation of BK Channels via Membrane-delimited Signaling
Most of the protocols demonstrating potentiation of mSlo activity by integrin activation or c-src overexpression were performed after whole-cell activation of α5β1 integrin, followed by excision of inside-out macropatches. Because cell surface integrins were activated prior to gigaseal formation and patch excision, channel phosphorylation would have occurred before the electrophysiological recording was initiated. As such, there should be no need to expose the cytosolic surface of the excised patch to MgATP as no additional phosphorylation would be expected to occur following patch excision. These findings suggest that all of the components necessary for signaling through the ECM-integrin-c-src-mSlo complex are present in excised patches, i.e. that regulation occurs through a membrane-delimited pathway. This conclusion is consistent with other electrophysiological studies of the BK channel, where channel regulation by kinases has also been demonstrated in excised patches (7, 8, 29, 37–43). Our biochemical assays (Figs. 6 and 7) and confocal imaging results (supplemental Fig. 1) are consistent with the idea that integrins are part of a BK channel regulatory complex, in which integrin activation can regulate channel activity via c-src dependent channel phosphorylation even in the microenvironment of isolated membrane patches. Alioua et al. (29) previously showed that hSlo and c-src co-localize in both VSM and HEK 293 cells. Similar findings have been noted for BK (6, 56–58) and other K+ channels (44, 49).
Molecular Mechanism of BK Channel Regulation
Increased BK current amplitude following integrin activation could result from a number of different processes, for example, an increase in the number of active channels and/or increased stability of the Ca2+-dependent open state. Such possibilities might be resolved with single channel recording methods in low density patches. Another possibility is that new channels are inserted into the plasma membrane (45) upon integrin activation. Although insertion is likely to be a much slower process, it might still be achieved within the time course of BK current enhancement observed under our experimental conditions (1–3 min (15)). However, it is doubtful that all of the functional machinery (endoplasmic reticulum/Golgi) for channel trafficking and insertion would remain intact in excised membrane patches.
The increase in Ca2+ sensitivity following integrin activation likely reflects a phosphorylation-mediated increase in the efficiency of the coupling mechanism between Ca2+ binding and channel opening and/or an increase in the binding affinity for Ca2+. In this regard, Tyr-766 is predicted to reside within the putative RCK2 domain of the channel, which appears to interact functionally with the more proximal RCK1 domain to affect channel gating (46). The observation that V0.5 did not shift in 0 Ca2+ after integrin activation or with c-src overexpression is consistent with this idea and with the previous work of Ling et al. (7) who showed that c-src activity did not affect channel gating properties at very low (<0.9 μm) Ca2+ levels.
Pathophysiological Relevance
The effects of α5β1 integrin activation reported here are consistent with the participation of smooth muscle BK channels in the arteriolar dilation to soluble RGD peptides and proteolytic fragments of ECM (matricryptins) (47, 48). Matricryptins are produced in a number of conditions associated with matrix degradation (48) and can lead to alterations in vascular tone and reactivity through the activation/inhibition of ion channels (49). In addition, pathological conditions such as diabetes and hypertension are associated with altered ECM and/or integrin expression (50–55) that could change signaling through the ECM-integrin-src-BK channel axis in VSM. Increases in BK current due to increased expression of FN and/or vascular α5β1 integrin would promote VSM hyperpolarization, thereby decreasing Ca2+ entry through voltage-gated Ca2+ channels, and lead to vasodilation. At the same time, α5β1 integrin activation leads to potentiation of L-type Ca2+ current in VSM cells (in part through c-src (14)), Ca2+ entry, and vasoconstriction (13). Although the effects on the two channels might appear to cancel each other, the time courses are different, with the potentiation of BK channels being more sustained (15) and developing somewhat slower than the potentiation of L-type Ca2+ channels (13). Therefore, the net effect of α5β1 integrin activation in VSM is probably to produce a transient increase in Ca2+ influx that may subsequently trigger Ca2+ release and other Ca2+-dependent intracellular signaling mechanisms.
Supplementary Material
Acknowledgments
We are grateful for the technical assistance of David Durtschi, Judy Davidson, Luisa Sy, and Ni Ao. The cDNA sequence encoding human c-src tyrosine kinase was a gift of Dr. D. Fujita, University of Calgary.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-72989 and HL-71796 (to M. J. D.) and RR-017353 (to University of Missouri). This work was also supported by operating grant support (to A. P. B.) from the Canadian Institutes of Health Research and The Heart and Stroke Foundation of Alberta.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Tables 1–3.
- BK
- large conductance, Ca2+-activated K+ channel
- mSlo
- murine BK channel α-subunit
- hSlo
- human BK channel α-subunit
- Ab
- antibody
- HEK 293
- human embryonic kidney cell line
- VSM
- vascular smooth muscle
- ECM
- extracellular matrix
- PYK2
- proline-rich tyrosine kinase
- GFP
- green fluorescent protein
- PKA
- protein kinase A
- IP
- immunoprecipitation
- Tyr(P)
- phosphotyrosine
- WT
- wild type
- PBS
- phosphate-buffered saline
- FN
- fibronectin.
REFERENCES
- 1.Herrera G. M., Heppner T. J., Nelson M. T. (2000) Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R60–R68 [DOI] [PubMed] [Google Scholar]
- 2.Poolos N. P., Johnston D. (1999) J. Neurosci. 19, 5205–5212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurley B. R., Preiksaitis H. G., Sims S. M. (1999) Am. J. Physiol. 276, G843–G852 [DOI] [PubMed] [Google Scholar]
- 4.Brayden J. E., Nelson M. T. (1992) Science 256, 532–535 [DOI] [PubMed] [Google Scholar]
- 5.Nelson M. T., Cheng H., Rubart M., Santana L. F., Bonev A. D., Knot H. J., Lederer W. J. (1995) Science 270, 633–637 [DOI] [PubMed] [Google Scholar]
- 6.Rezzonico R., Cayatte C., Bourget-Ponzio I., Romey G., Belhacene N., Loubat A., Rocchi S., Van Obberghen E., Girault J. A., Rossi B., Schmid-Antomarchi H. (2003) J. Bone Miner. Res. 18, 1863–1871 [DOI] [PubMed] [Google Scholar]
- 7.Ling S., Woronuk G., Sy L., Lev S., Braun A. P. (2000) J. Biol. Chem. 275, 30683–30689 [DOI] [PubMed] [Google Scholar]
- 8.Ling S., Sheng J. Z., Braun A. P. (2004) Am. J. Physiol. Cell Physiol. 287, C698–C706 [DOI] [PubMed] [Google Scholar]
- 9.Laser M., Willey C. D., Jiang W., Cooper G., 4th, Menick D. R., Zile M. R., Kuppuswamy D. (2000) J. Biol. Chem. 275, 35624–35630 [DOI] [PubMed] [Google Scholar]
- 10.Platts S. H., Mogford J. E., Davis M. J., Meininger G. A. (1998) Am. J. Physiol. 275, H1449–H1454 [DOI] [PubMed] [Google Scholar]
- 11.Wang H. Q., Bai L., Shen B. R., Yan Z. Q., Jiang Z. L. (2007) Eur. J. Cell Biol. 86, 51–62 [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi M. M., Bender J. R. (2007) Trends Cardiovasc. Med. 17, 5–10 [DOI] [PubMed] [Google Scholar]
- 13.Wu X., Davis G. E., Meininger G. A., Wilson E., Davis M. J. (2001) J. Biol. Chem. 276, 30285–30292 [DOI] [PubMed] [Google Scholar]
- 14.Gui P., Wu X., Ling S., Stotz S. C., Winkfein R. J., Wilson E., Davis G. E., Braun A. P., Zamponi G. W., Davis M. J. (2006) J. Biol. Chem. 281, 14015–14025 [DOI] [PubMed] [Google Scholar]
- 15.Wu X., Yang Y., Gui P., Sohma Y., Meininger G. A., Davis G. E., Braun A. P., Davis M. J. (2008) J. Physiol. 586, 1699–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanks S. K., Quinn A. M. (1991) Methods Enzymol. 200, 38–62 [DOI] [PubMed] [Google Scholar]
- 17.Wallner M., Meera P., Ottolia M., Kaczorowski G. J., Latorre R., Garcia M. L., Stefani E., Toro L. (1995) Receptors Channels 3, 185–199 [PubMed] [Google Scholar]
- 18.Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Pflugers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 19.Cox D. H., Cui J., Aldrich R. W. (1997) J. Gen. Physiol. 109, 633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J., Cox D. H., Aldrich R. W. (1997) J. Gen. Physiol. 109, 647–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox D. H., Cui J., Aldrich R. W. (1997) J. Gen. Physiol. 110, 257–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horrigan F. T., Aldrich R. W. (2002) J. Gen. Physiol. 120, 267–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron A., Frieden M., Bény J. L. (1997) J. Physiol. 504, 537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawasaki J., Davis G. E., Davis M. J. (2004) J. Biol. Chem. 279, 12959–12966 [DOI] [PubMed] [Google Scholar]
- 25.Juliano R. L., Aplin A. E., Howe A. K., Short S., Lee J. W., Alahari S. (2001) Methods Enzymol. 333, 151–163 [DOI] [PubMed] [Google Scholar]
- 26.Lin T. H., Aplin A. E., Shen Y., Chen Q., Schaller M., Romer L., Aukhil I., Juliano R. L. (1997) J. Cell Biol. 136, 1385–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vulin A. I., Jacob K. K., Stanley F. M. (2005) Endocrinology 146, 3535–3546 [DOI] [PubMed] [Google Scholar]
- 28.Tian L., McClafferty H., Chen L., Shipston M. J. (2008) J. Biol. Chem. 283, 3067–3076 [DOI] [PubMed] [Google Scholar]
- 29.Alioua A., Mahajan A., Nishimaru K., Zarei M. M., Stefani E., Toro L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14560–14565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horrigan F. T., Aldrich R. W. (1999) J. Gen. Physiol. 114, 305–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefani E., Ottolia M., Noceti F., Olcese R., Wallner M., Latorre R., Toro L. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5427–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz F., Wallner M., Stefani E., Toro L., Latorre R. (1996) J. Gen. Physiol. 107, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meera P., Wallner M., Jiang Z., Toro L. (1996) FEBS Lett. 382, 84–88 [DOI] [PubMed] [Google Scholar]
- 34.Gribkoff V. K., Lum-Ragan J. T., Boissard C. G., Post-Munson D. J., Meanwell N. A., Starrett J. E., Jr., Kozlowski E. S., Romine J. L., Trojnacki J. T., Mckay M. C., Zhong J., Dworetzky S. I. (1996) Mol. Pharmacol. 50, 206–217 [PubMed] [Google Scholar]
- 35.Xu C. Q., Brône B., Wicher D., Bozkurt O., Lu W. Y., Huys I., Han Y. H., Tytgat J., Van Kerkhove E., Chi C. W. (2004) J. Biol. Chem. 279, 34562–34569 [DOI] [PubMed] [Google Scholar]
- 36.Tian L., Hammond M. S., Florance H., Antoni F. A., Shipston M. J. (2001) J. Physiol. 537, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis A., Peers C., Ashford M. L., Kemp P. J. (2002) J. Physiol. 540, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams S. E., Wootton P., Mason H. S., Bould J., Iles D. E., Riccardi D., Peers C., Kemp P. J. (2004) Science 306, 2093–2097 [DOI] [PubMed] [Google Scholar]
- 39.Zhou X. B., Wang G. X., Ruth P., Hüneke B., Korth M. (2000) Am. J. Physiol. Cell Physiol. 279, C1751–C1759 [DOI] [PubMed] [Google Scholar]
- 40.Barman S. A., Zhu S., Han G., White R. E. (2003) Am. J. Physiol. Lung Cell Mol. Physiol. 284, L1004–L1011 [DOI] [PubMed] [Google Scholar]
- 41.White R. E., Kryman J. P., El-Mowafy A. M., Han G., Carrier G. O. (2000) Circ. Res. 86, 897–905 [DOI] [PubMed] [Google Scholar]
- 42.Scornik F. S., Codina J., Birnbaumer L., Toro L. (1993) Am. J. Physiol. 265, H1460–H1465 [DOI] [PubMed] [Google Scholar]
- 43.Hall S. M., Redford E. J., Smith K. J. (2000) J. Neuroimmunol. 106, 130–136 [DOI] [PubMed] [Google Scholar]
- 44.Arcangeli A., Becchetti A., Cherubini A., Crociani O., Defilippi P., Guasti L., Hofmann G., Pillozzi S., Olivotto M., Wanke E. (2004) Biochem. Soc. Trans. 32, 826–827 [DOI] [PubMed] [Google Scholar]
- 45.Zarei M. M., Eghbali M., Alioua A., Song M., Knaus H. G., Stefani E., Toro L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10072–10077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H. J., Lim H. H., Rho S. H., Eom S. H., Park C. S. (2006) J. Biol. Chem. 281, 38573–38581 [DOI] [PubMed] [Google Scholar]
- 47.D'Angelo G., Mogford J. E., Davis G. E., Davis M. J., Meininger G. A. (1997) Am. J. Physiol. 272, H2065–H2070 [DOI] [PubMed] [Google Scholar]
- 48.Davis G. E., Bayless K. J., Davis M. J., Meininger G. A. (2000) Am. J. Pathol. 156, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis M. J., Wu X., Nurkiewicz T. R., Kawasaki J., Gui P., Hill M. A., Wilson E. (2002) Cell Biochem. Biophys. 36, 41–66 [DOI] [PubMed] [Google Scholar]
- 50.Upadhyay J., Aitken K. J., Damdar C., Bolduc S., Bagli D. J. (2003) J. Urol. 169, 750–755 [DOI] [PubMed] [Google Scholar]
- 51.Lindsey M. L., Mann D. L., Entman M. L., Spinale F. G. (2003) Ann. Med. 35, 316–326 [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Lemus L. A., Wu X., Wilson E., Hill M. A., Davis G. E., Davis M. J., Meininger G. A. (2003) J. Vasc. Res. 40, 211–233 [DOI] [PubMed] [Google Scholar]
- 53.Intengan H. D., Schiffrin E. L. (2000) Hypertension 36, 312–318 [DOI] [PubMed] [Google Scholar]
- 54.Serini G., Valdembri D., Bussolino F. (2006) Exp. Cell Res. 312, 651–658 [DOI] [PubMed] [Google Scholar]
- 55.Cordes N., Seidler J., Durzok R., Geinitz H., Brakebusch C. (2006) Oncogene 25, 1378–1390 [DOI] [PubMed] [Google Scholar]
- 56.Zhou R., Liu L., Hu D. (2005) Cardiovasc. Res. 68, 327–335 [DOI] [PubMed] [Google Scholar]
- 57.Rezzonico R., Schmid-Alliana A., Romey G., Bourget-Ponzio I., Breuil V., Breittmayer V., Tartare-Deckert S., Rossi B., Schmid-Antomarchi H. (2002) J. Bone Miner. Res. 17, 869–878 [DOI] [PubMed] [Google Scholar]
- 58.Lu R., Alioua A., Kumar Y., Eghbali M., Stefani E., Toro L. (2006) J. Physiol. 570, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeulin C., Seltzer V., Bailbé D., Andreau K., Marano F. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L489–L496 [DOI] [PubMed] [Google Scholar]
- 60.Toychiev A. H., Sabirov R. Z., Takahashi N., Ando-Akatsuka Y., Liu H., Shintani T., Noda M., Okada Y. (2009) Am. J. Physiol. Cell Physiol. 297, C990–C1000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.