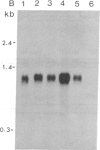
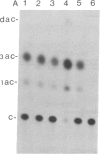
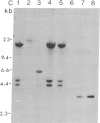
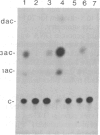
Abstract
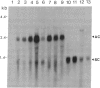
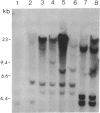
Tobacco plants expressing constitutive chloramphenicol acetyltransferase (CAT; EC 2.3.1.28) activity were obtained by transformation with a chimeric CAT gene driven by the cauliflower mosaic virus 19S promoter. Plants expressing different levels of CAT activity were retransformed with vectors containing CAT sequences transcriptionally fused in the antisense orientation between the coding region of the hygromycin-resistance gene and the 3′ end of the nopaline synthase gene. Several plants regenerated on high concentrations of hygromycin exhibited a loss of CAT activity, whereas plants retransformed with a vector conferring hygromycin resistance but lacking the antisense CAT sequence showed no reduction in CAT activity. RNA blot analysis revealed a strong correlation between the degree of CAT gene inactivation and the levels of stable antisense transcripts accumulated. The possibility that CAT gene inactivation was due to transferred DNA instability was discounted since a kanamycin-resistance gene contiguous with the CAT gene was expressed normally, and DNA blot analysis indicated no loss or rearrangements of the transferred DNA fragments. Thus, the imposed selection pressure enabled the selection of plants expressing high levels of stable bifunctional antisense transcripts that inhibited the activity of the targeted gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Alonso M. C., Johnston P., Phillips R. G., Lawrence P. A. Phenocopies induced with antisense RNA identify the wingless gene. Cell. 1987 Aug 14;50(4):659–663. doi: 10.1016/0092-8674(87)90039-0. [DOI] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Inhibition of Rous sarcoma virus replication by antisense RNA. J Virol. 1987 Mar;61(3):921–924. doi: 10.1128/jvi.61.3.921-924.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- De Benedetti A., Pytel B. A., Baglioni C. Loss of (2'-5')oligoadenylate synthetase activity by production of antisense RNA results in lack of protection by interferon from viral infections. Proc Natl Acad Sci U S A. 1987 Feb;84(3):658–662. doi: 10.1073/pnas.84.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garger S. J., Griffith O. M., Grill L. K. Rapid purification of plasmid DNA by a single centrifugation in a two-step cesium chloride-ethidium bromide gradient. Biochem Biophys Res Commun. 1983 Dec 28;117(3):835–842. doi: 10.1016/0006-291x(83)91672-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Guilley H., Dudley R. K., Jonard G., Balàzs E., Richards K. E. Transcription of Cauliflower mosaic virus DNA: detection of promoter sequences, and characterization of transcripts. Cell. 1982 Oct;30(3):763–773. doi: 10.1016/0092-8674(82)90281-1. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. Inhibition of heat shock protein synthesis by heat-inducible antisense RNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):399–403. doi: 10.1073/pnas.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Dimaio J., Strand M., Rice D. Stable and heritable inhibition of the expression of nopaline synthase in tobacco expressing antisense RNA. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8439–8443. doi: 10.1073/pnas.84.23.8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Lahners K. N., Lotstein R. J., Carozzi N. B., Jayne S. M., Rice D. A. Promoter cassettes, antibiotic-resistance genes, and vectors for plant transformation. Gene. 1987;53(2-3):153–161. doi: 10.1016/0378-1119(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timko M. P., Kausch A. P., Castresana C., Fassler J., Herrera-Estrella L., Van den Broeck G., Van Montagu M., Schell J., Cashmore A. R. Light regulation of plant gene expression by an upstream enhancer-like element. Nature. 1985 Dec 12;318(6046):579–582. doi: 10.1038/318579a0. [DOI] [PubMed] [Google Scholar]
- To R. Y., Booth S. C., Neiman P. E. Inhibition of retroviral replication by anti-sense RNA. Mol Cell Biol. 1986 Dec;6(12):4758–4762. doi: 10.1128/mcb.6.12.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington W. M. Stable repression of ribosomal protein L1 synthesis in Xenopus oocytes by microinjection of antisense RNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8639–8643. doi: 10.1073/pnas.83.22.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]