Abstract
The rapamycin-sensitive mTOR complex 1 (mTORC1) promotes protein synthesis, cell growth, and cell proliferation in response to growth factors and nutritional cues. To elucidate the poorly defined mechanisms underlying mTORC1 regulation, we have studied the phosphorylation of raptor, an mTOR-interacting partner. We have identified six raptor phosphorylation sites that lie in two centrally localized clusters (cluster 1, Ser696/Thr706 and cluster 2, Ser855/Ser859/Ser863/Ser877) using tandem mass spectrometry and generated phosphospecific antibodies for each of these sites. Here we focus primarily although not exclusively on raptor Ser863 phosphorylation. We report that insulin promotes mTORC1-associated phosphorylation of raptor Ser863 via the canonical PI3K/TSC/Rheb pathway in a rapamycin-sensitive manner. mTORC1 activation by other stimuli (e.g. amino acids, epidermal growth factor/MAPK signaling, and cellular energy) also promote raptor Ser863 phosphorylation. Rheb overexpression increases phosphorylation on raptor Ser863 as well as on the five other identified sites (e.g. Ser859, Ser855, Ser877, Ser696, and Thr706). Strikingly, raptor Ser863 phosphorylation is absolutely required for raptor Ser859 and Ser855 phosphorylation. These data suggest that mTORC1 activation leads to raptor multisite phosphorylation and that raptor Ser863 phosphorylation functions as a master biochemical switch that modulates hierarchical raptor phosphorylation (e.g. on Ser859 and Ser855). Importantly, mTORC1 containing phosphorylation site-defective raptor exhibits reduced in vitro kinase activity toward the substrate 4EBP1, with a multisite raptor 6A mutant more strongly defective that single-site raptor S863A. Taken together, these data suggest that complex raptor phosphorylation functions as a biochemical rheostat that modulates mTORC1 signaling in accordance with environmental cues.
Introduction
The evolutionarily conserved mammalian target of rapamycin (mTOR)3 protein kinase functions in at least two distinct multiprotein complexes (1). The immunosuppressive drug rapamycin acutely inhibits signaling by mTOR complex 1 (mTORC1) (2), which contains mTOR, raptor (known as KOG1 in budding yeast), mLST8/G-protein β-subunit-like protein (GβL), PRAS40, and deptor (3–10). Rapamycin fails to acutely inhibit signaling by mTOR complex 2 (mTORC2) (2), which contains mTOR, rictor, mLST8/GβL, mSin1, PRR5/Protor, and deptor (3, 7, 8, 11–17). mTORC2 mediates hydrophobic motif phosphorylation of the survival kinase Akt (also known as protein kinase B) (18, 19) and modulates the organization of the actin cytoskeleton (11, 12). mTORC1 functions as an environmental sensor to regulate a plethora of cellular biosynthetic processes including protein synthesis, cell growth, and cell proliferation (20–22). Growth factors/mitogens (e.g. insulin and epidermal growth factor (EGF)) and nutritional cues (e.g. amino acids and glucose) promote, whereas growth factor or nutrient deprivation and cell stress (e.g. hypoxia) inhibit mTORC1 signaling (3, 23–25). Emerging data indicate that aberrantly high mTORC1 signaling may contribute to several prevalent human diseases including cancer, insulin-resistant diabetes, and cardiovascular diseases (26–31). Elucidating the biochemical mechanisms underlying cellular mTORC1 regulation may thus enable the development of novel therapeutics to treat various mTORC1-associated pathologies.
Raptor, the regulatory associated protein of mTOR, interacts with mTOR as well as with mTORC1 substrates S6K1 (ribosomal protein S6 kinase 1) and 4EBP1 (eukaryotic initiation factor 4E (eIF4E)-binding protein 1) (4, 5, 7, 32, 33). S6K1 and 4EBP1 each contain a TOR signaling motif that mediates raptor interaction and their subsequent phosphorylation by mTOR (32–35). Thus, raptor functions as a scaffolding protein that facilitates the recruitment of substrates to the mTOR kinase. mTORC1-mediated phosphorylation of S6K1 on its hydrophobic motif site (Thr389) and 4EBP1 (on several sites) coordinately up-regulates protein synthesis and promotes cell growth and cell cycle progression (22, 35–37). mTORC1-mediated phosphorylation of S6K1 aids the assembly of the eIF3 translation initiation complex (38), whereas phosphorylation of 4EBP1, a translational repressor, induces the release of 4EBP1 from eIF4E, allowing eIF4E to associate with other factors (i.e. eIF4G and eIF4A) to initiate cap-dependent translation (22).
Work from many laboratories has focused on identifying upstream regulators of mTORC1. The tuberous sclerosis complex (TSC) functions as an upstream mTORC1 inhibitor (39). Mutational inactivation of either TSC1 or TSC2, whose protein products heterodimerize to form a tumor suppressor complex, results in constitutively high mTORC1 signaling, the development of benign tumors in diverse organ systems, and cellular insulin resistance (30, 39, 40). Insulin/PI3K signaling activates mTORC1 via Akt-mediated phosphorylation of both TSC2 and PRAS40 (another mTORC1 interactor), which relieves the inhibitory effect of these proteins on mTORC1 (9, 10, 41–43). TSC2 possesses a GTPase-activating protein domain that acts on Rheb, a Ras-like GTP-binding protein that activates mTORC1; thus, TSC null cells contain high Rheb-GTP levels that constitutively promote strong mTORC1 signaling (39). When bound to mTOR, Rheb-GTP activates mTORC1 via an incompletely understood mechanism (9, 44, 45). Ras-mediated signaling also activates mTORC1 in a PI3K-independent manner by MAPK and RSK-mediated phosphorylation of TSC2 (suppresses TSC action) and RSK-mediated phosphorylation of raptor (augments mTORC1 signaling) (46–49). Conversely, energy deprivation inactivates mTORC1 via AMPK-mediated phosphorylation of both TSC2 (augments TSC action) and raptor (suppresses mTORC1 signaling) (50, 51). Although the biochemical mechanisms linking amino acid sufficiency to mTORC1 remain poorly defined, recent data demonstrate that Rag family GTPases bind raptor during nutrient sufficiency and induce the subcellular re-localization of mTORC1 to an internal membrane compartment that contains Rheb (24, 25, 52, 53).
To elucidate biochemical mechanisms underlying mTORC1 regulation, we have investigated the phosphorylation of raptor. We have identified multiple raptor phosphorylation sites that lie in two centrally localized clusters (cluster 1, Ser696/Thr706 and cluster 2, Ser855/Ser859/Ser863/Ser877) by tandem mass spectrometry (MS/MS); moreover, we have generated phosphospecific antibodies for each of these sites. Our data reveal that diverse mTORC1-activating stimuli including growth factors (e.g. insulin and EGF), amino acids, and cellular energy promote rapamycin-sensitive raptor Ser863 phosphorylation. In addition to promoting raptor Ser863 phosphorylation, insulin/Rheb signaling promotes raptor phosphorylation on several other sites (e.g. Ser859, Ser855, Ser877, Ser696, and Thr706). Strikingly, raptor Ser863 phosphorylation is absolutely required for raptor Ser859 and Ser855 phosphorylation. These data indicate that mTORC1 activation drives multisite raptor phosphorylation, with phosphorylation occurring in a hierarchical, Ser(P)863-dependent manner on a subset of sites. As our data reveal that raptor phosphorylation is required for insulin-stimulated mTORC1 activation, we propose that complex raptor phosphorylation enables mTORC1 to receive and integrate diverse mTORC1-regulatory stimuli.
EXPERIMENTAL PROCEDURES
Materials
Reagents were obtained from the following sources: Protein A-Sepharose CL-4B and protein G-Sepharose Fast Flow from GE Healthcare; CHAPS was from Pierce; Immobilon-P polyvinylidene difluoride membrane (0.45 μm) was from Millipore; autoradiography film (HyBlot CL) was from Denville Scientific; reagents for enhanced chemiluminescence (ECL) were from Millipore (Immobilon Western Chemiluminescent horseradish peroxidase substrate); and all chemicals were from either Fisher Chemicals or Sigma.
Commercial Antibodies
AU1, Myc (9E10), and HA (HA.11) antibodies were from Covance. FLAG-M2 and P-MAPK (Thr202/Tyr204) were from Sigma. Donkey anti-rabbit horseradish peroxidase and sheep anti-mouse horseradish peroxidase antibodies were from GE Healthcare. The following antibodies were from Cell Signaling Technology: P-S6K1 (Thr389) (rabbit monoclonal 108D2), P-4EBP1 (Thr37/Thr46), P-Akt (Ser473), total Akt, P-AMPK (Thr172), total AMPK, total S6, and total TSC1.
Custom Antibodies
Polyclonal antibodies to raptor, P-raptor (Ser696, Thr706, Ser855, Ser859, Ser863, Ser877), mTOR, S6K1, and P-S6 were generated in rabbits against keyhole limpet hemocyanin-coupled peptides using the custom antibody service from Covance. To generate immunogen, peptides and P-peptides (70% pure; synthesized by Advanced Peptides, Inc., Boston, MA) were coupled via an N-terminal cysteine to maleimide-activated mariculture keyhole limpet hemocyanin (Pierce). Anti-peptide antibodies were affinity purified by positive selection on antigen peptide that was coupled to Affi-Gel matrix (Bio-Rad). Phosphopeptide antibodies were affinity purified by positive selection on antigen phosphopeptide followed by negative selection on cognate antigen non-phosphopeptide and irrelevant Ser/Thr phosphopeptide (either Ser(P) or Thr(P) Jak2 peptide). The following peptides were used to generate antibodies: 1) raptor (amino acids 885–901, human): CSSSLTNDVAKQPVSRDL; 2) P-Ser696-raptor (amino acids 691–701, human), CNYALP(pS)PATTE; 3) Thr(P)706-raptor (amino acids 701–711, human), CEGGSL(pT)PVRDS; 4) Ser(P)855-raptor (amino acids 850–858, human), CVLDTS(pS)LTQ; 5) Ser(P)859-raptor (amino acids 854–862, human), CSSLTQ(pS)APA; 6) Ser(P)863-raptor (amino acids 860–868, human), CAPA(pS)PTNKG; 7) Ser(P)877-raptor (amino acids 872–882), human), CHQAGG(pS)PPASS; 8) mTOR (amino acids 221–237, rat), CTQREPKEMQKPQWYRHT; 9) S6K1 (C-terminal 17 amino acids, 485–502 of the 70-kDa rat αII isoform), CKQAFPMISKRPEHLRMNL; 10) P-S6 (amino acids 232–249), CRRL(pS)(pS)LRA(pS)TSK(pS)EE(pS)QK; 11) Ser(P)-Jak2, CSDVQI(pS)PTLRQ; and 12) Thr(P)-Jak2, CSVKYA(pT)LVSND.
Plasmids, cDNA Mutagenesis, and Sequencing
The pRK5/Myc-raptor plasmid was provided by D. Sabatini (MIT, Boston, MA). The pcDNA3/AU1-mTOR wild type (WT), rapamycin-resistant (RR; S2035I), kinase-dead (KD; D2338A), and RR/KD (S2035I and D2338A) plasmids were originally from R. Abraham (Wyeth, Pearl River, NY). The pRK7/HA-S6K1, pKH3/HA-mLST8/GβL, and pRK7/FLAG-Rheb plasmids were from J. Blenis (Harvard Medical School, Boston, MA).
The following oligonucleotides were used to create the S863A and S863D point mutations in the human Myc-tagged raptor cDNA (accession number AY090663) using QuikChange II XL (Stratagene). Capital letters indicate mismatch, and the three underlined nucleotides represent the codon mutated: S863A, forward: 5′-ctc acg cag tcg gcc ccc gcc GCc ccc acc aac aag ggc gtg cac-3′; reverse: 5′-gtg cac gcc ctt gtt ggt ggg gGC ggc ggg ggc cga ctg cgt gag-3′; S863D: forward, 5′-ctc acg cag tcg gcc ccc gcc GAc ccc acc aac aag ggc gtg cac-3′; reverse, 5′-gtg cac gcc ctt gtt ggt ggg gTC ggc ggg ggc cga ctg cgt gag-3′. The mutated cDNAs were fully sequenced.
In-gel Digestion, Mass Spectrometry, and Data Analysis
HEK293 cells (seven 15-cm plates per condition; ∼15 × 106 cells/15-cm plate) were untransfected or transiently transfected with Myc-tagged raptor (7 μg) (and pRK7 vector (13 μg)) and cultured in Dulbecco's modified Eagle's medium (DMEM)/fetal bovine serum (FBS). After lysis in Buffer A/CHAPS (see below), whole cell lysate (WCL) was immunoprecipitated overnight with Myc antibodies, resolved on SDS-PAGE, and stained with Coomassie Blue R-250. The Myc-raptor protein band was excised from the gel and cut into 1-mm cubes. Gel cubes were rinsed with water; destained with 50% acetonitrile (MeCN), 50% ammonium bicarbonate; dehydrated with 100% MeCN; and subjected to in-gel digestion with 6 ng/μl of sequencing grade modified trypsin (Promega) in 50 mm ammonium bicarbonate for 16 h at 37 °C. Peptides were extracted once with 50% MeCN, 2.5% formic acid (FA), and once with 100% MeCN. Dried peptides were resuspended in 2.5% MeCN, 2.5% FA and loaded using a Micro AS autosampler (Thermo Electron) and a Surveyor MS Pump Plus (Thermo Electron) onto a nano-electrospray microcapillary column packed with 14 cm of reverse phase MagicC18 material (5 μm, 200 Å, Michrom Bioresources, Inc.). Elution was performed with a 5–35% MeCN (0.15% FA) gradient over 45 min, after a 15-min isocratic loading at 2.5% MeCN, 0.15% FA. Solvent A was 2.5% MeCN, 0.15% FA and Solvent B was 99.85% MeCN, 0.15% FA. Mass spectra were acquired in a LTQ linear ion trap mass spectrometer (Thermo Electron). Throughout the entire run, 10 data-dependent MS/MS scans were acquired on the most abundant ions from the preceding precursor survey (MS1) scan. MS/MS were subjected to dynamic exclusion (repeat count 3, duration 30 s). Mass spectral data were searched against a human raptor protein data base using Turbo SEQUEST (Thermo Electron, version 27, revision 12) requiring no enzyme specificity and a 2-Da precursor mass tolerance. Cysteine residues were required to have a static increase in 71.0 Da for acrylamide (C3H5ON) adduction. Differential modification of 16.0 Da on methionine and 80.0 Da on serine, threonine, and tyrosine was permitted.
Cell Culture, Drug Treatment, and Transfection
HEK293 cells and immortalized TSC1+/+ and TSC1−/− mouse embryonic fibroblasts (originally from David Kwiatkowski, Brigham and Women's Hospital, Boston, MA) were cultured in DMEM that contained high glucose (4.5 g/liter), glutamine (584 mg/liter), and sodium pyruvate (110 mg/liter) (Invitrogen) supplemented with 10% FBS (Hyclone). 3T3-L1 fibroblasts were cultured in DMEM containing 10% calf serum and differentiated into adipocytes by a standard protocol (54, 55). All cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. All cells were serum deprived via incubation in DMEM supplemented with 20 mm HEPES (pH 7.2) for ∼20 h. Insulin (100 nm) (Invitrogen), EGF (25 ng/ml) (Invitrogen), or phorbol 12-myristate 13-acetate (PMA) (100 ng/ml) (Sigma) was added to serum-deprived cells for 30 min unless indicated otherwise. For drug treatment, serum-deprived cells were pre-treated with rapamycin (20 ng/ml) (Calbiochem), wortmannin (100 nm) (Upstate/Millipore), or UO126 (5 μm) (Calbiochem) for 30 min prior to growth factor addition. To effect amino acid deprivation, cycling HEK293 were incubated in Dulbecco's PBS containing d-glucose (1 g/liter) and sodium pyruvate (36 mg/liter) (D-PBS/Glc) for 60 min. Cells were stimulated with amino acids either by re-feeding with DMEM as source of amino acids or with 5× a minimal essential medium amino acid mixture (50× stock) (Invitrogen) for 30 min. HEK293 cells on 60-mm plates were transfected using TransIT-LT1 (Mirus) using a total of 4–5 μg of DNA per plate; the specific amounts of experimental plasmid transfected are stated in the figure legends. Cells were lysed ∼24–48 h post-transfection.
Cell Lysis, Immunoprecipitation, and Immunoblotting
Cells were washed 2 times with ice-cold PBS and collected in ice-cold lysis Buffer A unless indicated otherwise (10 mm KPO4 (pH 7.2), 1 mm EDTA, 5 mm EGTA, 10 mm MgCl2, 50 mm β-glycerophosphate, 1 mm sodium orthovanadate (Na3VO4), 5 μg/ml of pepstatin A, 10 μg/ml of leupeptin, 40 μg/ml of phenylmethylsulfonyl fluoride), as originally described (36), containing the detergent CHAPS (0.3%) (to preserve the mTOR-raptor interaction upon lysis) (4, 5). In some experiments (indicated in the figure legends), cells were lysed in Buffer B (40 mm HEPES, pH 7.4, 120 mm NaCl, 1 mm EDTA, 10 mm pyrophosphate, 10 mm β-glycerophosphate, 50 mm sodium fluoride, 1 mm Na3VO4, 10 μg/ml of leupeptin, 5 μg/ml of pepstatin A, 40 μg/ml of phenylmethylsulfonyl fluoride), containing CHAPS (0.3%), as originally described (5) (to observe prominent insulin-induced destabilization of the mTOR-raptor interaction). Lysates were spun at 13,200 × g for 5 min at 4 °C, and the postnuclear supernatants were collected. Bradford assay was used to normalize protein levels for immunoprecipitation and immunoblot analyses. For immunoprecipitation, WCL was incubated with antibodies for ∼1.5 h at 4 °C, incubated with protein A- or G-Sepharose beads for 1 h, washed three times in lysis buffer, and resuspended in 1× sample buffer (50 mm Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 2% β-mercaptoethanol, 0.02% bromphenol blue). Samples were heated at 95 °C for 5 min, resolved on SDS-PAGE gels, and transferred to polyvinylidene difluoride membrane using Towbin transfer buffer (25 mm Tris, 192 mm glycine, 10% methanol, 0.02% SDS). Immunoblotting was performed by blocking polyvinylidene difluoride membranes in TBST (40 mm Tris-HCl, pH 7.5, 0.9% NaCl, 0.1% Tween 20) containing 3% nonfat milk and incubating the membranes in TBST with 2% bovine serum albumin containing primary antibodies or secondary horseradish peroxidase-conjugated antibodies. Blots were developed by enhanced chemiluminescence (ECL).
Treatment of Raptor with λ-Phosphatase
Following raptor immunoprecipitation, beads were washed 3 times in lysis buffer followed by two additional washes in ST buffer (50 mm Tris-HCl, pH 7.2, 150 mm NaCl). Beads were then resuspended in 1× phosphatase buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 2 mm dithiothreitol, 0.1 mm EGTA, 0.01% Brij35) that contained 2 mm MnCl2. Samples were incubated at 30 °C for 30 min in the absence or presence of λ-phosphatase (250 units) (New England Biolabs), and reactions were terminated by adding EDTA (pH 8.0) to 50 mm and sample buffer to 1× final.
mTORC1 in Vitro Kinase Assays
HEK293 cells on 10-cm plates were transiently transfected via the calcium phosphate method with Myc-raptor and AU1-mTOR plasmids. Twenty-four h post-transfection, cells were serum deprived for ∼24 h and then stimulated with insulin for 20 min. Cells were lysed in Buffer C/CHAPS (40 mm HEPES, pH 7.4, 2 mm EDTA, 10 mm pyrophosphate, 10 mm β-glycerophosphate, 0.3% CHAPS, protease inhibitors) (similar to Buffer B/CHAPS but lacking NaCl), and Myc-raptor was immunoprecipitated with 9E10 anti-Myc monoclonal antibody. The in vitro kinase activity of mTORC1 toward recombinant GST-4EBP1 was assayed using a modification of a protocol originally described in Sancak et al. (53). Briefly, anti-Myc immunoprecipitates were washed twice in Buffer C/CHAPS, twice in Buffer D/CHAPS (40 mm HEPES, pH 7.4, 2 mm EDTA, 150 mm NaCl, 10 mm pyrophosphate, 10 mm β-glycerophosphate, 0.3% CHAPS), and once in kinase buffer (25 mm HEPES, pH 7.4, 50 mm NaCl, 10 mm MnCl2, 50 mm β-glycerophosphate). Kinase assays were performed at 30 °C for 30 min in kinase buffer with 100 μm cold ATP, 3 μg of GST-4EBP1 and 10 μCi of [32P]ATP. Reactions were terminated with 2× sample buffer. 10% of the sample was used for a Thr(P)37/Thr(P)46-4EBP1 Western blot, and the rest was run on a separate gel, which was Coomassie stained, dried, autoradiographed using a phosphorous screen, and quantitated on a phosphorimager.
Image Editing
In some figures (indicated by a thin, vertical black line), irrelevant lanes were removed from a scanned autoradiograph and flanking lanes juxtaposed using Adobe Photoshop.
RESULTS
Insulin and Amino Acid Signaling Induce Raptor Phosphorylation in Intact Cells
Reversible protein phosphorylation regulates many signaling intermediates in the mTORC1 pathway. To determine whether mTORC1-activating stimuli modulate the phosphorylation state of the raptor, we first examined raptor electrophoretic mobility on SDS-PAGE. As shown in Fig. 1A, insulin stimulation of serum-deprived HEK293 cells retarded the mobility of raptor relative to untreated cells. Similar to the activation of mTORC1 signaling (as monitored by the phosphorylation of S6K1 and its substrate the ribosomal protein S6), insulin required the presence of amino acids to retard the mobility of raptor, and amino acids alone were insufficient. Thus, raptor supershift correlates with active mTORC1 signaling. To determine whether phosphorylation underlies the mobility shift of raptor, we treated raptor immunoprecipitates isolated from insulin-stimulated HEK293 cells with λ-phosphatase in vitro. Phosphatase treatment increased the mobility of raptor relative to that of insulin-stimulated but untreated cells (Fig. 1B). The striking mobility shift of such a large protein (150 kDa) suggests that raptor undergoes extensive phosphorylation. We also noted that overexpression of FLAG-Rheb induced a striking mobility shift of Myc-tagged raptor that was phosphatase-sensitive, indicating the Rheb induces raptor phosphorylation (Fig. 1C). Taken together, these data indicate that phosphorylation accounts in a significant way to insulin- and Rheb-stimulated raptor electrophoretic mobility shift.
FIGURE 1.
mTORC1-activating stimuli promote the phosphorylation of raptor. A, insulin retards the electrophoretic mobility of raptor on SDS-PAGE in an amino acid-dependent manner. In lanes 1 and 2, HEK293 cells were serum deprived and stimulated with insulin (30 min). In lanes 3–6, HEK293 cells were similarly serum deprived, incubated in D-PBS/glucose (60 min) to effect amino acid deprivation, followed by stimulation with DMEM alone (as source of amino acids), insulin alone, or both DMEM and insulin (30 min). WCL was resolved on SDS-PAGE and immunoblotted (IB) with the indicated antibodies. Note: to observe this degree of raptor mobility shift, lysate was resolved on 6% SDS-PAGE and run at 35 mA for ∼5 h. B, phosphorylation underlies insulin-stimulated raptor electrophoretic mobility shift. HEK293 cells were serum deprived and stimulated with insulin (lanes 2–3). WCL was immunoprecipitated (IP) with anti-raptor antibodies, and the immunoprecipitates were washed in phosphatase buffer (lanes 1–3), incubated in the absence (lanes 1 and 2) or presence (lane 3) of λ-phosphatase, and immunoblotted as indicated. Note: immunoprecipitates were resolved on 6% SDS-PAGE and run at 35 mA for ∼5 h. C, phosphorylation underlies Rheb-induced raptor electrophoretic mobility shift. HEK293 cells were co-transfected with Myc-raptor (0.5 μg) and AU1-mTOR (2.5 μg) with or without FLAG-Rheb (2.5 μg), as indicated, and serum deprived. WCL was immunoprecipitated with Myc antibodies. Myc immunoprecipitates were washed in phosphatase buffer (lanes 4 and 5) and incubated in the absence (lane 4) or presence (lane 5) of λ-phosphatase. WCL was also immunoblotted directly to confirm the expression of the various plasmids and the expected activation of mTORC1 signaling. Note: immunoprecipitates were resolved on 8% SDS-PAGE and run at 35 mA for 8 (long run) or 4 h (short run).
Identification of Multiple Raptor Phosphorylation Sites in Intact Cells by Tandem Mass Spectrometry
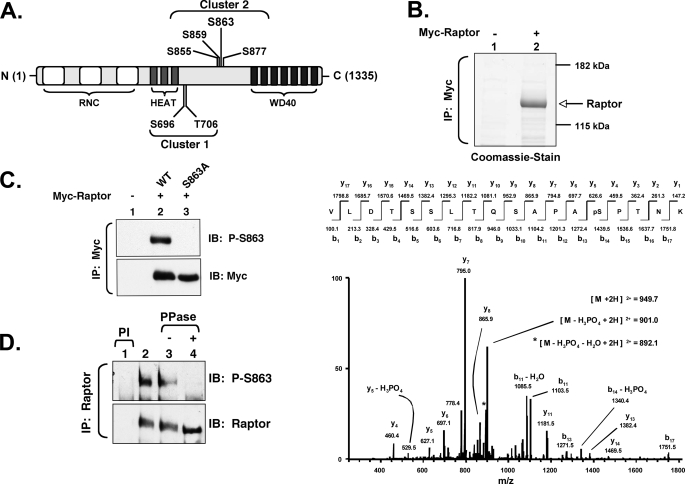
We thus sought to identify specific sites of raptor phosphorylation using liquid chromatography-tandem mass spectrometry. Immunoprecipitated Myc-raptor isolated from cycling HEK293 cells was resolved on SDS-PAGE, stained with Coomassie Blue, digested with trypsin, and analyzed by liquid chromatography-MS/MS using an LTQ linear ion trap mass spectrometer. Using this method, we identified six phosphorylation sites on raptor that lie in two centrally localized clusters: Ser696/Thr706 (cluster 1) and Ser855/Ser859/Ser863/Ser877 (cluster 2). These sites localize to a region of poor evolutionary conservation between the N-terminal HEAT repeats and the C-terminal WD repeats (Fig. 2A). Using the alignment algorithm Clustal V, the cluster 1 sites (e.g. Ser855/Ser859/Ser863/Ser877) display conservation from mammals (Homo sapiens, Rattus norvegicus, and Mus musculus) down to flies (Drosophila melanogaster), whereas cluster 2 sites (e.g. Ser696/Thr706) do not appear to be conserved in flies (supplemental Fig. S1). As this article focuses primarily on Ser863 phosphorylation (Ser(P)863), the MS/MS spectrum of the tryptic peptide harboring Ser(P)863 is shown (Fig. 2B), whereas the MS/MS spectra of the other identified sites (Ser696, Thr706, Ser855, Ser859, and Ser877) are not shown.
FIGURE 2.
Identification of Ser863 as a site of raptor phosphorylation in intact cells and generation of phosphospecific Ser863 antibodies. A, localization of cluster 1 (Ser696/Thr706) and cluster 2 (Ser855/Ser859/Ser863/Ser877) P-sites within the domain structure of raptor. The six P-sites identified map to a central, poorly conserved region of raptor that localizes between the N-terminal RNC (raptor N-terminal conserved) domains and HEAT repeats and the C-terminal WD40 repeats. B, low energy, collision-induced dissociation spectrum of the doubly charged raptor Ser(P)863 phosphopeptide. HEK293 cells were transfected with Myc-raptor, cultured in DMEM/FBS, and WCL was immunoprecipitated (IP) with Myc antibodies. A Coomassie-stained band of Myc-raptor (upper panel) was digested with trypsin after SDS-PAGE. Liquid chromatography-MS/MS and data analysis were conducted as described under “Experimental Procedures.” Note that the y4–y7 ion series clearly places the site of phosphorylation on Ser863. C, Ser(P)863 antibodies are site-specific. HEK293 cells were transfected with vector control (−) or with WT or S863A Myc-raptor alleles, as indicated. WCL was immunoprecipitated (IP) with Myc antibodies and immunoblotted (IB) with the indicated antibodies. D, Ser(P)863 antibodies are phosphospecific. HEK293 lysate was immunoprecipitated with preimmune (PI) antibodies (lane 1) or with anti-raptor antibodies (lanes 2–4). The immunoprecipitates were resuspended immediately in sample buffer (lanes 1 and 2) or washed in phosphatase (PPase) buffer and incubated in the absence (lane 3) or presence (lane 4) of λ-phosphatase. The immunoprecipitates were immunoblotted as indicated.
To study the regulation of raptor phosphorylation in intact cells, we generated affinity-purified antibodies against raptor peptides phosphorylated on Ser863 as well as against peptides phosphorylated on Ser855, Ser859, Ser877, Ser696, and Thr706. As shown in Fig. 2C, the raptor Ser(P)863 antibody recognizes wild type Myc-raptor but not a Myc-raptor mutant bearing a phosphorylation site-defective, alanine substitution at Ser863 (S863A), thus confirming site specificity of this antibody. As shown in Fig. 2D, the raptor Ser(P)863 antibody recognizes raptor incubated in the absence but not presence of λ-phosphatase (see also Fig. 6C), thus confirming the phospho-specificity of this antibody.
FIGURE 6.
Rheb promotes multisite raptor phosphorylation in Ser(P)863-dependent and -independent manners. A, raptor Ser(P)863 is required for Rheb-induced supershift of Myc-raptor on SDS-PAGE. HEK293 cells were co-transfected with WT or S863A Myc-raptor (0.5 μg) and AU1-mTOR (2 μg) with or without FLAG-Rheb (2 μg). Cells were serum deprived, WCLs were immunoprecipitated (IP) with Myc antibodies, and immunoprecipitates were resolved and immunoblotted (IB) as indicated. WCL was also immunoblotted directly to confirm expression of the transfected proteins as well as the expected activation of mTORC1 signaling by FLAG-Rheb. Note, immunoprecipitates were resolved on 8% SDS-PAGE and run at 35 mA for 8 (long run) or 4 h (short run). B, Rheb promotes raptor phosphorylation on multiple sites, with Ser855 and Ser859 phosphorylation occurring in a Ser(P)863-dependent manner. HEK293 cells were transfected and analyzed as in A, employing WT, S863A, and S863D Myc-raptor alleles. Myc-raptor immunoprecipitates from serum-deprived cells were immunoblotted with our panel of raptor phosphospecific antibodies (e.g. Ser(P)863, Ser(P)859, Ser(P)855, Ser(P)696, Thr(P)706, and Ser(P)877) as well as with a commercially available phosphospecific antibody (e.g. Ser(P)792). Note: immunoprecipitates were resolved on 6% SDS-PAGE and run at 35 mA for ∼2.5 h. C, phospho-specificity of P-raptor antibodies. HEK293 cells were co-transfected, treated, and immunoprecipitated as in A. In lanes 4, 7, and 10, Myc-raptor immunoprecipitates were incubated with λ-phosphatase in vitro and immunoblotted as indicated. WCL was also immunoblotted directly to confirm expression of the transfected proteins as well as the expected activation of mTORC1 signaling by FLAG-Rheb.
The phospho-specificities of the raptor Ser(P)855, Ser(P)859, Ser(P)877, Ser(P)696, and Ser(P)706 antibodies were also confirmed, as shown in Fig. 6C. The phosphatase-sensitive mobility shift of raptor in response to mTORC1-activating signals, combined with the MS/MS and phospho-specific antibody data, indicate that raptor experiences extensive phosphorylation in vivo.
Insulin/PI3K and EGF/MAPK Signaling Promote mTORC1-associated Raptor Ser863 Phosphorylation
To investigate a role for the insulin pathway in regulation of site-specific raptor Ser863 phosphorylation in intact cells, we first examined 3T3-L1 adipocytes, a highly insulin responsive cell type. We pre-treated serum-deprived 3T3-L1 adipocytes cells with or without wortmannin, an inhibitor of PI3K, and rapamycin, an inhibitor of mTORC1, prior to insulin stimulation. Raptor was then immunoprecipitated and analyzed with Ser(P)863 antibodies. As shown in Fig. 3A, acute insulin stimulation increased raptor Ser863 phosphorylation in a wortmannin- and rapamycin-sensitive manner. These data indicate that insulin signals via PI3K to promote raptor Ser863 phosphorylation and suggest that active mTORC1 is required. To determine whether insulin-stimulated raptor Ser863 phosphorylation occurs as part of mTORC1, we immunoprecipitated mTOR from 3T3-L1 adipocytes and examined the Ser863 phosphorylation state of co-immunoprecipitated raptor. Similar to the results obtained upon direct raptor immunoprecipitation, we found that insulin promoted the wortmannin- and rapamycin-sensitive phosphorylation of mTOR-associated raptor Ser863. These data indicate that insulin-stimulated raptor Ser863 phosphorylation indeed occurs when raptor is part of mTORC1. We also examined Ser(P)863 regulation in HEK293 cells. As for 3T3-L1 adipocytes, acute insulin stimulation of growth factor-deprived cells increased raptor Ser863 phosphorylation in a wortmannin- and rapamycin-sensitive manner (Fig. 3B). We next examined the temporal relationship between insulin-stimulated raptor Ser863 phosphorylation and mTORC1 signaling (Fig. 3C). Serum-deprived HEK293 cells were stimulated with insulin for various amounts of time (5–60 min), and raptor immunoprecipitates were analyzed with Ser(P)863 antibodies. As shown in Fig. 3C, insulin treatment for 20 min promoted maximal raptor Ser863 phosphorylation, similar to the time course for insulin-stimulated S6K1 and S6 phosphorylation, with significant raptor Ser863 phosphorylation occurring at 5–10 min. These data indicate that insulin rapidly promotes raptor Ser863 phosphorylation, with a time course similar to the phosphorylation of downstream mTORC1 effectors.
FIGURE 3.
Insulin/PI3K signaling promotes rapamycin-sensitive raptor Ser863 phosphorylation in 3T3-L1 adipocytes and HEK293 cells. A, regulation of raptor Ser(P)863 in mTORC1 in 3T3-L1 adipocytes. Differentiated adipocytes were serum deprived, pre-treated with wortmannin (lane 4, left panel) or with rapamycin (lane 8, right panel), and stimulated with insulin. WCL was immunoprecipitated (IP) with preimmune sera (PI) (lanes 1 and 5), anti-raptor antibodies (upper panels, lanes 2–4 and 6–8), or anti-mTOR antibodies (middle panels, lanes 2–4 and 6–8) and immunoblotted (IB) with the indicated antibodies. WCL was also immunoblotted directly (lower panels) with the indicated antibodies to confirm the expected activation and/or inhibition of mTORC1 signaling. B, regulation of raptor Ser(P)863 in HEK293 cells. HEK293 cells were serum deprived, pre-treated with wortmannin (W, lane 4) or rapamycin (R, lane 5), and stimulated with insulin, as indicated for the adipocyte experiment. WCL was immunoprecipitated with preimmune sera (PI) (lane 1) or anti-raptor antibodies (lanes 2–5), and immunoprecipitates were immunoblotted with the indicated antibodies. WCL was also immunoblotted directly with the indicated antibodies to confirm the expected activation and/or inhibition of mTORC1 signaling by the various treatments. C, time course for insulin-stimulated raptor Ser(P)863. HEK293 cells were serum deprived and stimulated with insulin for various amounts of time (5–60 min). WCL was immunoprecipitated with anti-raptor antibodies and immunoblotted with the indicated antibodies. WCL was also immunoblotted directly with the indicated antibodies to confirm the expected activation and/or inhibition of mTORC1 signaling by the various treatments. Note: the asterisk indicates the 70-kDa S6K1 αII species; double asterisks indicate the 85-kDa S6K1 αI species. If not indicated otherwise, all S6K1 immunoblots in this article represent the better studied S6K1 αII species. D, EGF and PMA promote raptor Ser(P)863 independently of PI3K. HEK293 cells were serum deprived and stimulated with 10% FBS (lane 2), insulin (lane 3), EGF (lane 5), or PMA (lane 6). WCL was immunoprecipitated with anti-raptor antibodies and immunoblotted with the indicated antibodies. WCL was also immunoblotted directly with the indicated antibodies to confirm the expected modulation of PI3K (P-Akt), MAPK (P-MAPK (Thr202/TyrY204), and mTORC1 (P-S6) signaling by the various growth factors/mitogens. Note: p44Mapk and p42Mapk are also known as ERK1 and ERK2, respectively. E, MEK/MAPK signaling mediates EGF-stimulated raptor Ser(P)863. Myc-raptor-transfected HEK293 cells (0.5 μg) were serum deprived, pre-treated with UO126 (lane 5), and then stimulated with PMA (lane 3) or EGF (lanes 4–5). WCL was immunoprecipitated with Myc antibodies and immunoblotted with the indicated antibodies. WCL was also immunoblotted to confirm the expected activation/inhibition of MAPK and mTORC1 signaling.
In addition to the canonical insulin/PI3K pathway, the Ras-regulated MAPK (also known as ERK) pathway activates mTORC1 signaling in a PI3K-independent manner (46–49). To examine a role for MAPK signaling in regulation of raptor Ser863 phosphorylation, we stimulated serum-deprived HEK293 cells transiently expressing Myc-raptor with EGF or PMA (a phorbol ester that activates protein kinase C), mitogens that activate MAPK independently of PI3K (46–49). We found that these agonists promoted Myc-raptor Ser863 phosphorylation to a degree similar to that observed following insulin stimulation (Fig. 3D). Importantly, control immunoblots confirmed that whereas insulin, EGF, and PMA all activated mTORC1 signaling (as ascertained by increased P-S6), only insulin activated PI3K (as ascertained by increased P-Akt), whereas only EGF and PMA activated MAPK (as ascertained by increased P-MAPK). Importantly, the MEK inhibitor UO126 reduced EGF-stimulated raptor Ser(P)863 as well as the phosphorylation of MAPK and S6 (Fig. 3E), confirming that MEK/MAPK signaling mediates EGF-stimulated raptor Ser863 phosphorylation. These data indicate that activation of mTORC1 signaling via multiple mitogenic signaling pathways, the insulin/PI3K and the EGF/MAPK pathways, promotes raptor Ser863 phosphorylation.
TSC Inhibits While Rheb Promotes Raptor Ser863 Phosphorylation
Moving downstream of PI3K in the insulin signaling pathway, we investigated whether the mTORC1-inhibitory TSC1-TSC2 complex suppresses raptor Ser863 phosphorylation. We found that immortalized mouse embryonic fibroblasts lacking TSC1 and thus functional TSC exhibited higher levels of raptor Ser863 phosphorylation compared with wild type, littermate-matched mouse embryonic fibroblasts upon serum deprivation (Fig. 4A). Importantly and as expected, serum-deprived TSC1 null cells exhibited elevated S6K1 and S6 phosphorylation compared with wild type fibroblasts. These data demonstrate that insulin/PI3K signaling promotes raptor Ser863 phosphorylation by suppressing TSC.
FIGURE 4.
TSC/Rheb signaling modulates raptor Ser863 phosphorylation. A, TSC suppresses raptor Ser(P)863. Littermate-matched, 3T3 immortalized mouse embryonic fibroblasts derived from TSC1+/+ or TSC−/− animals were serum deprived. Triplicate lysates were immunoprecipitated (IP) with anti-raptor antibodies and immunoblotted (IB) as indicated. WCL was also immunoblotted directly to confirm the absence of TSC1 and the expected activation of mTORC1 signaling. Note: we find that TSC1−/− fibroblasts express higher levels of total raptor protein when normalized for total protein content; thus, two-thirds of the immunoprecipitate from TSC1−/− cells was loaded relative to TSC1+/+ cells to normalize the amount of raptor between the two cell lines. WCL was loaded similarly. B, Rheb overexpression increases Myc-raptor Ser(P)863 and retards the mobility of Myc-raptor on SDS-PAGE. HEK293 cells were co-transfected with Myc-raptor (0.5 μg) and AU1-mTOR (2.5 μg) with or without FLAG-Rheb (2.5 μg), as indicated. The cells were serum deprived (lanes 1–5) and stimulated with insulin (lanes 6–8). WCL was immunoprecipitated with Myc antibodies and immunoblotted as indicated. WCL was also immunoblotted directly to confirm the expression of the various plasmids and the expected activation of mTORC1 signaling. C, rapamycin reduces Rheb-stimulated raptor Ser(P)863, performed as in B except that rapamycin was included in the culture medium during the entire ∼20 h serum deprivation period (lanes 6 and 7). D, Rheb-stimulated raptor Ser(P)863 requires active mTORC1. Performed as in B except that RR (lane 6) and RR/KD (lane 7) AU1-TOR alleles were also analyzed. At the time of serum deprivation, rapamycin was added to the culture medium (+ Rapa; lanes 5–7). ∼20 h later, cells were incubated in the absence or presence of insulin and lysed in Buffer B/CHAPS. WCL was immunoprecipitated with Myc antibodies and immunoblotted as indicated. WCL was also immunoblotted directly to confirm the expression of the various plasmids and the expected activation and/or inhibition of mTORC1 signaling by the various treatments.
Moving downstream of TSC1-TSC2 in the insulin signaling pathway, we investigated whether Rheb, the most direct mTORC1 activator identified to date, promotes raptor Ser863 phosphorylation. We therefore examined Ser863 phosphorylation on exogenously expressed Myc-raptor in cells co-transfected with AU1-mTOR in the absence and presence of co-transfected FLAG-Rheb. In HEK293 cells deprived of serum, FLAG-Rheb overexpression increased Myc-raptor Ser863 phosphorylation and markedly retarded the electrophoretic mobility of Myc-raptor on SDS-PAGE (Fig. 4B). Importantly, control immunoblots demonstrated the expected, constitutive activation of mTORC1 signaling conferred by FLAG-Rheb overexpression, as monitored by the phosphorylation of the mTORC1 effectors S6K1 and S6 in the absence of serum growth factors.
To determine whether Rheb overexpression increases raptor Ser863 phosphorylation in a manner that requires kinase active mTORC1, we analyzed the sensitivity of Rheb-induced Myc-raptor Ser863 phosphorylation to rapamycin treatment. Rapamycin reduced Rheb-induced phosphorylation of Myc-raptor Ser863 (Fig. 4C), indicating that Rheb overexpression promotes raptor Ser863 phosphorylation in an mTORC1-dependent manner. Last, we sought to eliminate the possibility that rapamycin inhibits Rheb-induced raptor Ser863 phosphorylation by blocking signal reception (via steric hindrance or allosteric conformational change) rather than by inhibiting mTORC1 signaling. We thus took advantage of two mutant alleles of AU1-mTOR, one bearing a mutation (S2035I) in the FRB-domain that renders mTOR unable to bind rapamycin, thus creating a rapamycin-resistant (RR) mutant, and another in which RR-mTOR contains a mutation (D2338A) rendering it kinase-dead (RR/KD) (Fig. 4D). By expressing AU1-mTOR RR versus RR/KD together with Myc-raptor and FLAG-Rheb in serum-deprived and rapamycin-treated HEK293 cells, we could assay the ability of RR- versus RR/KD-mTOR to rescue the rapamycin-mediated inhibition of raptor Ser863 phosphorylation. If both RR- and RR/KD-mTOR were to rescue similarly, then this result would suggest that rapamycin binding to mTOR is required for inhibition of Ser863 phosphorylation and would favor the “blockade of signal reception” model. If RR/KD-mTOR were to display defective rescue compared with RR-mTOR, then this result would suggest that inhibition of mTORC1 signaling explains the mechanism by which rapamycin inhibits Ser863 phosphorylation, as neither RR- nor RR/KD-mTOR bind rapamycin. Although expression of RR-mTOR-rescued rapamycin inhibited raptor Ser863 phosphorylation and Rheb-induced raptor supershift, expression of RR/KD-mTOR failed to rescue either (Fig. 4D). Thus, inhibition of mTORC1 activity or signaling underlies rapamycin-mediated inhibition of raptor Ser863 phosphorylation.
Raptor Ser863 Phosphorylation Requires Sufficient Levels of Amino Acids and Energy
To extend our observation that amino acids are required for insulin-stimulated, phosphorylation-mediated reduction in raptor electrophoretic mobility on SDS-PAGE (Fig. 1A), we examined the role of amino acids in regulation of raptor Ser863 phosphorylation. HEK293 cells were serum deprived and then further deprived of amino acids via incubation in Dulbecco's PBS/glucose. Cells were then stimulated with amino acids alone (by adding back either DMEM or a 5× minimal essential medium amino acid mixture), insulin alone, or both amino acids and insulin; endogenous raptor was immunoprecipitated and immunoblotted with Ser(P)863 antibodies. Insulin or amino acids alone failed to promote raptor Ser863 phosphorylation; when added together, however, these stimuli promoted raptor Ser(P)863 in a rapamycin-sensitive manner (Fig. 5A). As expected, the phosphorylation of mTORC1 effectors (e.g. S6K1 and S6) but not Akt required the presence of both amino acids and insulin. We next asked how withdrawal of amino acids from cycling HEK293 cells would affect raptor Ser863 phosphorylation. We found that incubation in amino-free medium that still contained serum growth factors for 60 min resulted in robust dephosphorylation of raptor Ser863, and acute re-stimulation with amino acid- and serum-replete medium increased raptor Ser863 phosphorylation in a rapamycin-sensitive manner (Fig. 5B). Consistent with the data obtained in HEK293 cells, we observed a similar amino acid requirement for insulin-stimulated Ser863 phosphorylation in 3T3-L1 adipocytes (Fig. 5C). We next asked whether induction of acute energy stress, which down-regulates mTORC1 signaling by increasing AMPK activity and thus enhancing TSC function, modulates raptor Ser863 phosphorylation. We thus added 2-deoxyglucose, a glycolytic inhibitor, to the culture medium of cycling HEK293 cells and found that 2-deoxyglucose treatment resulted in raptor Ser863 dephosphorylation (Fig. 5D). Importantly and as expected, 2-deoxyglucose mediated increased phosphorylation of AMPK (on Thr172) and decreased phosphorylation of S6 (50). These data demonstrate that sufficient levels of ATP/energy are required to maintain raptor Ser863 phosphorylation as well as the phosphorylation of mTORC1 substrates.
FIGURE 5.
Insulin-stimulated raptor Ser863 phosphorylation requires sufficient levels of amino acids and cellular energy. A, effect of amino acid and insulin stimulation alone and in combination on raptor Ser(P)863. HEK293 cells were serum deprived followed by incubation in D-PBS/glucose (60 min) to effect amino acid deprivation (lanes 5–14). Amino acid-deprived cells were then pre-treated with rapamycin (R) (lanes 9 and 14), and stimulated with amino acids alone, insulin alone, or both amino acids and insulin, as indicated. In lanes 6, 8, and 9, DMEM was used as source of amino acids, whereas in lanes 11, 13, and 14, 5× minimal essential medium amino acids were used. In lanes 1–4, the cells were not amino acid deprived, they were simply serum deprived and stimulated with insulin (30 min). WCL was immunoprecipitated (IP) with preimmune (PI) or raptor antibodies and immunoblotted (IB) as indicated. WCL was also immunoblotted directly to confirm the expected activation and/or inhibition of mTORC1 signaling by the various treatments. B, amino acid (AA) withdrawal decreases, whereas amino acid re-addition increases raptor Ser(P)863 in a rapamycin-sensitive manner. HEK293 cells cultured in DMEM/FBS were incubated in D-PBS/glucose containing 10% dialyzed FBS (60 min) to effect amino acid deprivation in the continued presence of serum growth factors (lanes 3–8). Amino acid-deprived cells were pre-treated with rapamycin (lanes 7 and 8) and stimulated with DMEM/FBS (30 min) (lanes 5–8). WCL was immunoprecipitated with preimmune (PI) or raptor antibodies and immunoblotted as indicated. WCL was also immunoblotted directly to confirm the expected activation and/or inhibition of mTORC1 signaling. C, insulin-stimulated raptor Ser(P)863 requires amino acids in 3T3-L1 adipocytes. Differentiated adipocytes were serum deprived and then amino acid deprived (60 min). The cells were then pre-treated with rapamycin (R; lane 6) or wortmannin (W; lane 7) and stimulated with amino acids alone (using DMEM), insulin alone, or both amino acids and insulin (30 min) as indicated. WCL was immunoprecipitated with preimmune or raptor antibodies and immunoblotted as indicated. WCL was also immunoblotted directly to confirm the expected activation and/or inhibition of mTORC1 signaling by the various treatments. D, energy stress mediates raptor Ser863 de-phosphorylation. HEK293 cells were transfected with Myc-raptor (0.5 μg). Cycling cells were re-fed with DMEM/FBS that lacked (lanes 2 and 3) or contained (lanes 4 and 5) 2-deoxyglucose (2DG) (25 mm) and incubated 15 min. WCL was immunoprecipitated with Myc antibodies and immunoblotted as indicated. WCL was also immunoblotted directly to confirm the expected down-regulation of mTORC1 signaling and up-regulation of AMPK in response to energy stress.
Raptor Ser863 Phosphorylation Is Required for Rheb-induced Phosphorylation of Raptor on at Least Two Sites
As the phosphorylation of many highly phosphorylated proteins occurs in an ordered, hierarchical manner (e.g. 4EBP1) (56) and as FLAG-Rheb expression induces a strong, phosphorylation-dependent decrease in raptor electrophoretic mobility, we asked whether Ser863 phosphorylation controls the phosphorylation of other raptor P-sites first using electrophoretic mobility shift assay. We immunoprecipitated WT or S863A Myc-raptor from serum-deprived HEK293 cells that had been co-transfected with AU1-mTOR and FLAG-Rheb and analyzed Myc-raptor mobility on SDS-PAGE. We found that S863A Myc-raptor failed to undergo the striking reduction in mobility that occurs with WT Myc raptor in response to Rheb overexpression (Fig. 6A). These data are consistent with the idea that Ser863 phosphorylation primes raptor for phosphorylation on other sites. To confirm this idea, we employed our panel of raptor phospho-specific antibodies. We found that Rheb induced not only the phosphorylation of Ser863 but the phosphorylation of all the cluster 1 P-sites (e.g. Ser855/Ser859/Ser877) and all the cluster 2 sites (e.g. Ser696/Thr706) (Fig. 6B). Rheb failed to significantly increase phosphorylation on the AMPK site, Ser792 (51), indicating that not all raptor P-sites experience regulation via Rheb. Strikingly, Rheb failed to induce the phosphorylation of Ser859 and Ser855 on S863A Myc-raptor, indicating that raptor Ser863 phosphorylation is absolutely required for the phosphorylation of raptor Ser855 and Ser859. As Rheb also failed to induce raptor Ser855 and Ser859 phosphorylation on S863D Myc-raptor, this observation suggests that substitution of the “phospho-mimetic” amino acid Asp for Ser at Ser863 insufficiently mimics Ser863 phosphorylation to enable Rheb to induce Ser(P)855 and Ser(P)859. These data demonstrate that Rheb induces site-specific raptor phosphorylation on multiple but not all sites and that Ser(P)863 primes raptor for hierarchical phosphorylation (e.g. on Ser855 and Ser859). Importantly, we confirmed the phospho-specificities of the P-raptor antibodies directed against cluster 1 and cluster 2 P-sites by incubating Myc-raptor immunoprecipitates from FLAG-Rheb-overexpressing cells in the absence and presence of λ-phosphatase (Fig. 6C). Phosphatase treatment strongly reduced the immunoreactivity of the P-raptor antibodies directed against cluster 1 (Ser(P)855, Ser(P)859, Ser(P)863, and Ser(P)877) and cluster 2 (Ser(P)696 and Thr(P)706) P-sites, thus confirming the phospho-specificity of these antibodies. Taken together, these data demonstrate that Rheb-mediated signaling drives multisite raptor phosphorylation, with Ser863 phosphorylation functioning as a master biochemical switch that primes raptor for hierarchical phosphorylation.
To determine whether raptor phosphorylation modulates mTORC1 kinase activity, we assayed the ability of insulin-stimulated mTORC1 containing WT versus phosphorylation site-defective raptor to phosphorylate substrate in vitro. To perform these in vitro kinase assays, HEK293 cells were co-transfected with Myc-raptor alleles (WT, S863A, or raptor mutated at all six P-sites (6A)) together with AU1-mTOR alleles (WT or KD). Myc-raptor was immunoprecipitated to immunoisolate mTORC1, which was then incubated in vitro with ATP (both cold and radiolabeled) and recombinant GST-4EBP1. As previously reported (9, 49), mTORC1 containing WT Myc-raptor promoted 4EBP1 phosphorylation in vitro in response to insulin in a kinase-dependent manner (Fig. 7). mTORC1 containing S863A or 6A Myc-raptor, however, displayed reduced 4EBP1 phosphorylation relative to WT complexes, with 6A Myc-raptor complexes showing more defective kinase activity than S863A complexes. These data demonstrate an important regulatory role for raptor phosphorylation in promoting mTORC1 kinase activity.
FIGURE 7.
mTORC1 containing phosphorylation site-defective raptor exhibits reduced in vitro kinase activity toward GST-4EBP1. HEK293 cells on 10-cm plates were co-transfected with Myc-raptor alleles (2 μg) (WT, S863A, or 6A) together with AU1-mTOR alleles (8 μg) (WT or KD), serum deprived, and stimulated with insulin for 20 min. Myc-raptor was immunoprecipitated (IP) with Myc antibodies, and the in vitro kinase activity of co-precipitated AU1-mTOR (and endogenous mTOR) was assayed using recombinant GST-4EBP1 (isolated from bacteria) as substrate in the presence of both cold and hot [32P]ATP. In vitro kinase reactions were analyzed by Thr(P)37/Thr(P)46-4EBP1 Western blotting (IB) and by autoradiographic analysis of 32P incorporation into GST-4EBP1. The graph shows the level of 32P incorporated into GST-4EBP1 as determined using a phosphorimager.
DISCUSSION
To better understand the biochemical mechanisms controlling mTORC1 signaling as well as the molecular function of raptor, we have studied raptor phosphorylation in intact cells. We have identified six sites on raptor whose phosphorylation increases strongly upon overexpression of Rheb (Ser696, Thr706, Ser855, Ser859, Ser863, and Ser877), with a subset of sites exhibiting hierarchical phosphorylation. It is important to note that other groups have identified several of these raptor phosphorylation sites (P-sites) (Ser855, Ser859, Ser863, and Ser877) as well as other P-sites in large-scale, MS/MS-based phospho-proteomic screens (57–66). Notably, Ser696 and Thr706 were not identified in these other proteomic screens and thus represent novel sites of phosphorylation. Based on our data and that of others, we propose that raptor functions as a molecular sensor of the cellular environment, with complex phosphorylation allowing the integration of diverse mTORC1-regulatory stimuli.
Here we have focused primarily although not exclusively on Ser863 phosphorylation and demonstrate that insulin promotes rapamycin-sensitive Ser863 phosphorylation, consistent with the recent findings of Wang et al. (67). We demonstrate further that the canonical PI3K/TSC/Rheb pathway mediates insulin-stimulated raptor Ser863 phosphorylation. Growth factors/mitogens (e.g. EGF and PMA) that activate mTORC1 signaling via the MAPK pathway independently of PI3K also promote raptor Ser863 phosphorylation. In cycling cells, sufficient levels of amino acids and cellular energy are required to maintain raptor Ser863 phosphorylation. Thus, diverse mTORC1-activating stimuli promote raptor Ser(P)863. Our data that insulin-stimulated raptor Ser863 phosphorylation requires kinase-active mTORC1 and displays rapamycin sensitivity in intact cells, together with the data of Wang et al. (67) that mTOR phosphorylates raptor Ser863 in vitro, strongly suggest that mTOR itself mediates raptor Ser863 phosphorylation. Thus, raptor represents a novel mTOR substrate. Consistently, Ser863 lies within a “proline-directed” motif (pS-P), similar to the 4EBP1 sites phosphorylated by mTOR. The finding that mTOR directly phosphorylates an mTORC1 component is not without precedent, as mTOR directly phosphorylates PRAS40 (on Ser183, Ser212, and Ser221) upon insulin-stimulated mTORC1 activation (68, 69), which relieves the suppressive effect of PRAS40 on mTORC1 and thus contributes to activation of mTORC1 signaling.
Using our panel of raptor phosphospecific antibodies, we find that Rheb not only promotes raptor Ser863 phosphorylation but also promotes raptor phosphorylation on at least five other sites (Ser696, Thr706, Ser855, Ser859, and Ser877). Strikingly, raptor Ser863 phosphorylation is absolutely required for Rheb-induced Ser859 phosphorylation, consistent with the data of Wang et al. (67), whose phosphopeptide mapping experiments noted the lack of Ser859 phosphorylation on a raptor S863A mutant. Additionally, our data show that raptor Ser855 phosphorylation also requires raptor Ser863 phosphorylation. As Ser863 phosphorylation potently modulates the electrophoretic mobility of raptor on SDS-PAGE, it is likely that Ser(P)863 controls the phosphorylation of other unmapped sites in addition to Ser855 and Ser859. Importantly, we find that raptor Ser863 phosphorylation is required for insulin-stimulated mTORC1 phosphorylation of 4EBP1 in vitro, similar to the findings of Wang et al. (67). Extending this work, we find that mTOR complexes containing 6A Myc-raptor are more defective than those containing S863A Myc-raptor, demonstrating that multisite raptor phosphorylation promotes mTORC1 kinase activity toward exogenous substrate. Based on the collective data, we propose a model (Fig. 8) whereby mTORC1 activation in response to diverse environmental cues (e.g. insulin, EGF, amino acids, and energy) (step 1) enables mTOR-mediated phosphorylation of raptor on Ser863 and possibly on other Pro-directed sites (e.g. Ser696, Thr706, and Ser877) (step 2). Ser863 phosphorylation functions to prime raptor for subsequent phosphorylation on Ser855/Ser859 and possibly on other unmapped sites by unknown kinases (step 3). Multiple raptor phosphorylation events then cooperate to regulate mTORC1 signaling (step 4) in response to environmental cues. MAPK signaling may promote Ser(P)863 by suppressing TSC function via TSC2 phosphorylation, as previously described (47, 48). Amino acids may promote raptor Ser(P)863 via RAG GTPase-mediated re-localization of mTORC1 to a Rab7-positive endomembrane compartment that contains its activator, Rheb, as proposed recently (53).
FIGURE 8.
Model-Complex raptor phosphorylation promotes mTORC1 signaling. mTORC1 activation by diverse environmental cues (e.g. insulin, EGF, amino acids, energy) (step 1) enables mTOR-mediated phosphorylation of raptor on Ser863 and possibly on other sites (e.g. Ser696, Thr706, and Ser877) (step 2) as well as mTOR-mediated phosphorylation of PRAS40. Ser863 phosphorylation then primes raptor for subsequent phosphorylation events on Ser859/Ser855 and possibly on other unmapped sites (step 3) by unknown kinases. Cooperative, multisite raptor phosphorylation promotes substrate phosphorylation and biochemical signaling (step 4). mTOR Ser1261 phosphorylation in response to insulin and amino acid signaling cooperates to promote mTORC1 signaling (73). For simplicity, not shown are the AMPK- and RSK-mediated phosphorylation events on raptor, which reportedly down-regulate or augment mTORC1 activity, respectively (49, 51).
Other work supports a functional role for raptor phosphorylation in regulation of mTORC1 signaling. Gwinn et al. (51) reported that upon induction of energy stress, AMPK phosphorylates raptor on two sites (Ser722 and Ser792), which is required for AICAR (an AMP mimetic that activates AMPK)-induced reduction in the in vitro kinase activity and in vivo signaling of mTORC1 (51). Additionally, Carriere et al. (49) reported that activation of MAPK signaling promotes RSK-mediated raptor phosphorylation on three sites (Ser719, Ser721, and Ser722), which is required for PMA-induced activation of the in vitro kinase activity of mTORC1. Thus, the collective data suggest that site-specific raptor phosphorylation by AMPK, RSK, and mTOR modulates mTORC1 activity, either negatively or positively.
We find that WT, S863A, and 6A Myc-raptor interact with mTOR (Fig. 7) and mTORC1 substrate 4EBP1 similarly under our assay conditions (data not shown), suggesting that raptor Ser863 and multisite phosphorylation contributes neither to mTOR-raptor interaction nor to the raptor-4EBP1 interaction. Although the function of raptor phosphorylation remains poorly defined, it seems reasonable to speculate that raptor phosphorylation may induce conformational changes in mTORC1 that allows raptor-bound substrates access to the kinase domain of mTOR. In the course of performing these co-immunoprecipitation experiments, we noted that insulin destabilizes the mTOR-raptor interaction, which is more prominent in certain lysis buffer conditions (e.g. Buffer C/CHAPS) (see Figs. 4D, 5C, and supplemental S2), similar to the nutrient-induced destabilization of the mTOR-raptor interaction reported by Kim et al. (5). These observations suggest that mTORC1-activating stimuli (e.g. insulin and nutrients) may induce allosteric conformational changes in mTORC1 components that modulate the way mTOR and its partners interact, which may contribute to mTORC1 function. Consistent with such a notion, nutrients and insulin weaken the raptor-PRAS40 interaction (10, 68, 70), and serum-stimulated phosphorylation of a novel mTORC1 inhibitor, deptor, was suggested recently to destabilize the mTOR-deptor interaction (8). To date, the biochemical mechanisms controlling the nutrient- and insulin-regulated mTOR-raptor interaction remain unknown.
We find that raptor phosphorylation occurs in a hierarchical manner, as raptor Ser859 and Ser855 phosphorylation absolutely requires priming phosphorylation on raptor Ser863. Sequence gazing reveals that the Ser855/Ser859/Ser863 cassette lies within a glycogen synthase kinase 3 consensus phosphorylation motif ((S/T)XXX(S/T)XXX(S/T)-(P)), raising the intriguing possibility that glycogen synthase kinase 3 mediates raptor Ser859 and Ser855 phosphorylation. Such a consensus directs glycogen synthase kinase 3-mediated phosphorylation of Ser/Thr residues located four amino acids N-terminal to a “primed” P-Ser/Thr residue (71, 72). It is enticing to speculate that raptor Ser859 and Ser855 phosphorylation may negatively regulate mTORC1, as glycogen synthase kinase 3 action generally opposes insulin effects. Future work will be required to elucidate the regulation and function of hierarchical raptor Ser859 and Ser855 phosphorylation.
The collective data suggest that complex raptor phosphorylation functions analogously to TSC1-TSC2 phosphorylation as a biochemical rheostat that integrates diverse mTORC1-regulatory signals (39). A challenge for the future will be to elucidate how complex raptor phosphorylation events cooperate to regulate mTORC1, both positively and negatively. We have determined recently that site-specific phosphorylation of mTOR on a novel site, Ser1261, in response to insulin/PI3K/TSC/Rheb signaling promotes mTORC1-mediated substrate phosphorylation and cell growth (73). The discovery of multiple, regulatory phosphorylation sites on raptor, PRAS40, and mTOR reveals reversible protein phosphorylation to be an important albeit complex biochemical mechanism that underlies the ability of mTORC1 to integrate signals from diverse environmental cues.
Supplementary Material
Acknowledgments
We thank all members of the laboratory as well as Martin G. Myers, Jr. for critical reading of the manuscript. We are indebted to J. Blenis for encouragement. We thank D. Sabatini, R. Abraham, N. Sonenberg, O. MacDougald, and D. Kwiatkowski for generously sharing reagents. This work utilized the Cell and Molecular Biology Core(s) of the Michigan Diabetes Research and Training Center funded by National Institutes of Health Grant 5P60 DK20572 from the NIDDK to the University of Michigan.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK078135 from the NIDDK (to D. C. F.) and P20 RR16462 to the Vermont Genetics Network. This work was also supported by the Institutional Development Award Networks of the Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources (to B. A. B.), a Jr. Faculty Award from the American Diabetes Association (to D. C. F.), the American Heart Association (to G. A. S. and B. E.), the Cole Foundation (to A. C.), Terry Fox Foundation through the Canadian Cancer Society Research Institute Grant 018311, and a Career Development Award from the Human Frontier Science Program (to P. P. R.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- mTOR
- mammalian target of rapamycin
- rictor
- rapamycin insensitive companion of mTOR
- GβL
- G-protein β-subunit-like protein
- mLST8
- lethal with sec13 protein 8
- PI3K
- phosphatidylinositol 3-kinase
- Rheb
- Ras homolog enriched in brain
- AMPK
- AMP-activated protein kinase
- PRAS40
- proline-rich Akt substrate of 40 kDa
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- RSK
- p90 ribosomal protein S6 kinase
- CHAPS
- 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate
- FA
- formic acid
- PMA
- phorbol 12-myristate 13-acetate
- EGF
- epidermal growth factor
- S6K1
- ribosomal protein S6 kinase 1
- 4EBP1
- eukaryotic initiation factor binding protein 1 4E
- eIF
- eukaryotic initiation factor
- TSC
- tuberous sclerosis complex
- MS
- mass spectrometry
- HA
- hemagglutinin
- WT
- wild type
- RR
- rapamycin-resistant
- KD
- kinase dead
- HEK
- human embryonic kidney
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- WCL
- whole cell lysate
- PBS
- phosphate-buffered saline
- GST
- glutathione S-transferase.
REFERENCES
- 1.Bhaskar P. T., Hay N. (2007) Dev. Cell 12, 487–502 [DOI] [PubMed] [Google Scholar]
- 2.Guertin D. A., Sabatini D. M. (2009) Sci. Signal. 2, pe24. [DOI] [PubMed] [Google Scholar]
- 3.Dunlop E. A., Tee A. R. (2009) Cell. Signal. 21, 827–835 [DOI] [PubMed] [Google Scholar]
- 4.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Cell 110, 177–189 [DOI] [PubMed] [Google Scholar]
- 5.Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 6.Kim D. H., Sarbassov D. D., Ali S. M., Latek R. R., Guntur K. V., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2003) Mol. Cell 11, 895–904 [DOI] [PubMed] [Google Scholar]
- 7.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Mol. Cell 10, 457–468 [DOI] [PubMed] [Google Scholar]
- 8.Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S., Sabatini D. M. (2009) Cell 137, 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007) Mol. Cell 25, 903–915 [DOI] [PubMed] [Google Scholar]
- 10.Vander Haar E., Lee S. I., Bandhakavi S., Griffin T. J., Kim D. H. (2007) Nat. Cell Biol. 9, 316–323 [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov D. D., Ali S. M., Kim D. H., Guertin D. A., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2004) Curr. Biol. 14, 1296–1302 [DOI] [PubMed] [Google Scholar]
- 12.Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 13.Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A., Sabatini D. M. (2006) Curr. Biol. 16, 1865–1870 [DOI] [PubMed] [Google Scholar]
- 14.Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 15.Yang Q., Inoki K., Ikenoue T., Guan K. L. (2006) Genes Dev. 20, 2820–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearce L. R., Huang X., Boudeau J., Pawłowski R., Wullschleger S., Deak M., Ibrahim A. F., Gourlay R., Magnuson M. A., Alessi D. R. (2007) Biochem. J. 405, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo S. Y., Kim D. H., Jun C. B., Kim Y. M., Haar E. V., Lee S. I., Hegg J. W., Bandhakavi S., Griffin T. J., Kim D. H. (2007) J. Biol. Chem. 282, 25604–25612 [DOI] [PubMed] [Google Scholar]
- 18.Hresko R. C., Mueckler M. (2005) J. Biol. Chem. 280, 40406–40416 [DOI] [PubMed] [Google Scholar]
- 19.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 20.Fingar D. C., Blenis J. (2004) Oncogene 23, 3151–3171 [DOI] [PubMed] [Google Scholar]
- 21.Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 22.Ma X. M., Blenis J. (2009) Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 23.Laplante M., Sabatini D. M. (2009) J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw R. J. (2008) Trends Biochem. Sci. 33, 565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinzalla V., Hall M. N. (2008) Nature 454, 287–288 [DOI] [PubMed] [Google Scholar]
- 26.Dann S. G., Selvaraj A., Thomas G. (2007) Trends Mol. Med. 13, 252–259 [DOI] [PubMed] [Google Scholar]
- 27.Guertin D. A., Sabatini D. M. (2007) Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 28.Inoki K., Corradetti M. N., Guan K. L. (2005) Nat. Genet. 37, 19–24 [DOI] [PubMed] [Google Scholar]
- 29.Tee A. R., Blenis J. (2005) Semin. Cell Dev. Biol. 16, 29–37 [DOI] [PubMed] [Google Scholar]
- 30.Harrington L. S., Findlay G. M., Lamb R. F. (2005) Trends Biochem. Sci. 30, 35–42 [DOI] [PubMed] [Google Scholar]
- 31.Polak P., Hall M. N. (2009) Curr. Opin. Cell Biol. 21, 209–218 [DOI] [PubMed] [Google Scholar]
- 32.Nojima H., Tokunaga C., Eguchi S., Oshiro N., Hidayat S., Yoshino K., Hara K., Tanaka N., Avruch J., Yonezawa K. (2003) J. Biol. Chem. 278, 15461–15464 [DOI] [PubMed] [Google Scholar]
- 33.Schalm S. S., Blenis J. (2002) Curr. Biol. 12, 632–639 [DOI] [PubMed] [Google Scholar]
- 34.Choi K. M., McMahon L. P., Lawrence J. C., Jr. (2003) J. Biol. Chem. 278, 19667–19673 [DOI] [PubMed] [Google Scholar]
- 35.Schalm S. S., Fingar D. C., Sabatini D. M., Blenis J. (2003) Curr. Biol. 13, 797–806 [DOI] [PubMed] [Google Scholar]
- 36.Fingar D. C., Salama S., Tsou C., Harlow E., Blenis J. (2002) Genes Dev. 16, 1472–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fingar D. C., Richardson C. J., Tee A. R., Cheatham L., Tsou C., Blenis J. (2004) Mol. Cell. Biol. 24, 200–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 39.Huang J., Manning B. D. (2008) Biochem. J. 412, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwiatkowski D. J., Manning B. D. (2005) Hum. Mol. Genet. 14, R251–R258 [DOI] [PubMed] [Google Scholar]
- 41.Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 42.Manning B. D., Tee A. R., Logsdon M. N., Blenis J., Cantley L. C. (2002) Mol. Cell 10, 151–162 [DOI] [PubMed] [Google Scholar]
- 43.Tee A. R., Fingar D. C., Manning B. D., Kwiatkowski D. J., Cantley L. C., Blenis J. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13571–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avruch J., Long X., Lin Y., Ortiz-Vega S., Rapley J., Papageorgiou A., Oshiro N., Kikkawa U. (2009) Biochem. Soc. Trans. 37, 223–226 [DOI] [PubMed] [Google Scholar]
- 45.Long X., Lin Y., Ortiz-Vega S., Yonezawa K., Avruch J. (2005) Curr. Biol. 15, 702–713 [DOI] [PubMed] [Google Scholar]
- 46.Tee A. R., Anjum R., Blenis J. (2003) J. Biol. Chem. 278, 37288–37296 [DOI] [PubMed] [Google Scholar]
- 47.Ma L., Chen Z., Erdjument-Bromage H., Tempst P., Pandolfi P. P. (2005) Cell 121, 179–193 [DOI] [PubMed] [Google Scholar]
- 48.Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 13489–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrière A., Cargnello M., Julien L. A., Gao H., Bonneil E., Thibault P., Roux P. P. (2008) Curr. Biol. 18, 1269–1277 [DOI] [PubMed] [Google Scholar]
- 50.Inoki K., Zhu T., Guan K. L. (2003) Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 51.Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008) Nat. Cell Biol. 10, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008) Science 320, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDougald O. A., Cornelius P., Liu R., Lane M. D. (1995) J. Biol. Chem. 270, 647–654 [DOI] [PubMed] [Google Scholar]
- 55.Student A. K., Hsu R. Y., Lane M. D. (1980) J. Biol. Chem. 255, 4745–4750 [PubMed] [Google Scholar]
- 56.Gingras A. C., Raught B., Gygi S. P., Niedzwiecka A., Miron M., Burley S. K., Polakiewicz R. D., Wyslouch-Cieszynska A., Aebersold R., Sonenberg N. (2001) Genes Dev. 15, 2852–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ballif B. A., Villén J., Beausoleil S. A., Schwartz D., Gygi S. P. (2004) Mol. Cell. Proteomics 3, 1093–1101 [DOI] [PubMed] [Google Scholar]
- 58.Beausoleil S. A., Jedrychowski M., Schwartz D., Elias J. E., Villén J., Li J., Cohn M. A., Cantley L. C., Gygi S. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12130–12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beausoleil S. A., Villén J., Gerber S. A., Rush J., Gygi S. P. (2006) Nat. Biotechnol. 24, 1285–1292 [DOI] [PubMed] [Google Scholar]
- 60.Daub H., Olsen J. V., Bairlein M., Gnad F., Oppermann F. S., Körner R., Greff Z., Kéri G., Stemmann O., Mann M. (2008) Mol. Cell 31, 438–448 [DOI] [PubMed] [Google Scholar]
- 61.Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imami K., Sugiyama N., Kyono Y., Tomita M., Ishihama Y. (2008) Anal. Sci. 24, 161–166 [DOI] [PubMed] [Google Scholar]
- 63.Munton R. P., Tweedie-Cullen R., Livingstone-Zatchej M., Weinandy F., Waidelich M., Longo D., Gehrig P., Potthast F., Rutishauser D., Gerrits B., Panse C., Schlapbach R., Mansuy I. M. (2007) Mol. Cell. Proteomics 6, 283–293 [DOI] [PubMed] [Google Scholar]
- 64.Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. (2006) Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 65.Sui S., Wang J., Yang B., Song L., Zhang J., Chen M., Liu J., Lu Z., Cai Y., Chen S., Bi W., Zhu Y., He F., Qian X. (2008) Proteomics 8, 2024–2034 [DOI] [PubMed] [Google Scholar]
- 66.Trinidad J. C., Thalhammer A., Specht C. G., Lynn A. J., Baker P. R., Schoepfer R., Burlingame A. L. (2008) Mol. Cell. Proteomics 7, 684–696 [DOI] [PubMed] [Google Scholar]
- 67.Wang L., Lawrence J. C., Jr., Sturgill T. W., Harris T. E. (2009) J. Biol. Chem. 284, 14693–14697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oshiro N., Takahashi R., Yoshino K., Tanimura K., Nakashima A., Eguchi S., Miyamoto T., Hara K., Takehana K., Avruch J., Kikkawa U., Yonezawa K. (2007) J. Biol. Chem. 282, 20329–20339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L., Harris T. E., Lawrence J. C., Jr. (2008) J. Biol. Chem. 283, 15619–15627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L., Harris T. E., Roth R. A., Lawrence J. C., Jr. (2007) J. Biol. Chem. 282, 20036–20044 [DOI] [PubMed] [Google Scholar]
- 71.Frame S., Cohen P. (2001) Biochem. J. 359, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen P., Goedert M. (2004) Nat. Rev. Drug. Discov. 3, 479–487 [DOI] [PubMed] [Google Scholar]
- 73.Acosta-Jaquez H. A., Keller J. A., Foster K. G., Ekim B., Soliman G. A., Feener E. P., Ballif B. A., Fingar D. C. (2009) Mol. Cell. Biol. 29, 4308–4324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.