Abstract
Erythrocyte invasion is critical to the pathogenesis and survival of the malarial parasite, Plasmodium falciparum. This process is partly mediated by proteins that belong to the Duffy binding-like family, which are expressed on the merozoite surface. One of these proteins, BAEBL (also known as EBA-140), is thought to bind to glycophorin C in a sialic acid-dependent manner. In this report, by the binding assay between recombinant BAEBL protein and enzyme-treated erythrocytes, we show that the binding of BAEBL to erythrocytes is mediated primarily by sialic acid and partially through heparan sulfate (HS). Because BAEBL binds to several kinds of HS proteoglycans or purified HS, the BAEBL-HS binding was found to be independent of the HS proteoglycan peptide backbone and the presence of sialic acid moieties. Furthermore, both the sialic acid- and HS-dependent binding were disrupted by the addition of soluble heparin. This inhibition may be the result of binding between BAEBL and heparin. Invasion assays demonstrated that HS-dependent binding was related to the efficiency of merozoite invasion. These results suggest that HS functions as a factor that promotes the binding of BAEBL and merozoite invasion. Moreover, these findings may explain the invasion inhibition mechanisms observed following the addition of heparin and other sulfated glycoconjugates.
Keywords: Diseases/Blood, Glycoproteins/Surface, Organisms/Parasite, Organisms/Protozoan, Protein/Binding/Heparin, Receptors/Membrane, Receptors/Oligosaccharides, Tissue/Organ Systems/Erythrocyte
Introduction
Malaria caused by Plasmodium falciparum kills approximately 1 million people per year. After entering the human bloodstream, the parasite propagates asexually via repeated cycles of erythrocyte invasion, cell division, and cell rupture. The process by which the parasitic merozoites invade erythrocytes involves the following steps: attachment, apical reorientation, junction formation, and the formation of a protective parasitophorous vacuole (1).
The Duffy binding-like (DBL)2 family is composed of adhesion molecules that are critical for junction formation between the apical end of the merozoite and the erythrocyte surface. Proteins in this family, such as EBA-175 (erythrocyte-binding antigen-175), are homologous to Plasmodium vivax Duffy-binding protein. These proteins contain one or more DBL domains, which are composed of conserved cysteine residues and are associated with erythrocyte binding (2). EBA-175 is bound to glycophorin A on the erythrocyte surface in a sialic acid-dependent manner (3). However, erythrocytes that are treated with neuraminidase remain susceptible to the merozoites of some clones (4), indicating the existence of sialic acid-independent invasion pathways. These alternative pathways are thought to be mediated, at least in part, by other members of the DBL family (5).
Parasite growth is inhibited by the addition of heparin, some sulfated saccharide anions, and sulfated chemical compounds (6–11). Although the inhibition mechanisms remain unclear, these reports suggest the possibility that sulfated moieties play a role in merozoite invasion. In addition to invasion, heparan sulfate (HS) is believed to function as a receptor for PfEMP1 (P. falciparum erythrocyte membrane protein 1), which is expressed on parasite-infected erythrocytes (iRBC). Thus, HS mediates the binding of iRBC to vascular endothelial cells or other erythrocytes (12–14).
In the present study, we focused on BAEBL (also known as EBA-140), which belongs to the DBL family and is thought to mediate an alternative pathway. Although BAEBL was shown to bind to erythrocytes in a sialic acid-dependent manner, as previously reported (15–19), additionally, we showed here that BAEBL binds to erythrocytes in an HS-dependent manner. Moreover, heparin was demonstrated to inhibit both of these binding pathways. The results of invasion inhibition assays suggest that HS plays a role in merozoite invasion. Further research on the mechanisms of inhibition by heparin and the identification of novel factors involved in merozoite invasion will facilitate the development of new antimalarial drugs that inhibit invasion.
EXPERIMENTAL PROCEDURES
Cells
Spodoptera frugiperda Sf9 insect cells were maintained in SF900II SFM (Invitrogen) that was supplemented with 10% fetal calf serum. Trichoplusia ni Tn5 insect cells were maintained in EX-CELL 405 medium (SAFC Biosciences, Lenexa, KS). Plat-E cells generated from 293T cells (20) were maintained in Dulbecco's modified Eagle's medium (Nissui, Tokyo, Japan) supplemented with 10% fetal calf serum, 1 μg/ml puromycin, and 10 μg/ml blasticidin. Jurkat-EcoVRc cells (kindly provided by Dr. Masayuki Shimojima) were maintained in RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum. Jurkat-EcoVRc cells are Moloney murine leukemia virus-sensitive Jurkat human T cells that express the mouse cationic amino acid transporter and that were prepared using a self-inactivating human immunodeficiency virus vector (21).
P. falciparum Parasite
The P. falciparum clone HB3 was obtained from the Malaria Research and Reference Reagent Resource Center (American Type Culture Collection, Manassas, VA). B+ erythrocytes from a single individual were used in all of the cultures and experiments. The cells were washed twice in incomplete RPMI 1640 medium that contained 25 mm HEPES and 367 μm hypoxanthine and then stored at 50% hematocrit at 4 °C. Cultures were maintained at 3% hematocrit in complete medium that was composed of incomplete medium that contained 5 mg/ml AlbuMAX II (Invitrogen), 27 mm NaHCO3, and 10 μg/ml gentamicin (22).
Antibodies
An anti-BAEBL antibody was produced by immunization with the synthetic peptides CNKVNKDKKRNEESL and CSSRDSENGRGDTTS, which comprise amino acid residues 565–578 and 947–960, respectively, of the BAEBL protein (GenBankTM accession number AAK49521). Specifically, a rabbit was immunized intradermally with 0.3 mg of both synthetic peptides in Freund's complete adjuvant (CAPPEL, Aurora, OH) three times at weekly intervals and then intravascularly with 0.05 mg of the peptides three times at weekly intervals. Serum was obtained from the rabbit 1 week after the sixth immunization.
Plasmid Construction
The baculovirus expression vector pBSV-8His was kindly provided by Dr. Peter F. Zipfel (Hans Knöll Institute, Jena, Germany) (23). The Fc region of murine IgG2a was cloned from the mouse IgG2a-pcDNA3.1 plasmid (kindly provided by Dr. Yorihiro Nishimura, National Institute of Infectious Diseases, Tokyo, Japan) into the SphI/BamHI sites of the pMIB/V5-His B plasmid (Invitrogen). The resultant plasmid, designated mouse IgG2a-pMIB/V5, was used as a template for PCR using the following primers: 5′-CATGCTGCAGCCCAGAGGGCCCACAATC-3′ (PstI site underlined) and 5′-GATCCCGGGAGAATTCCACCACACTGGAC-3′ (SmaI site underlined). The amplified fragment was cloned into the PstI/SmaI sites of pBSV-8His, and the resultant plasmid was designated pBSV-Fc-8His. Four types of BAEBL region II construct were amplified by PCR using the BAEBL-HB3-T8, BAEBL-E12-T8, BAEBL-Dd2/Nm-T8, and BAEBL-PNG3-T8 plasmid (kindly provided by Dr. Louis H. Miller (National Institutes of Health, Bethesda, MD)) (24) as a template, respectively, and the following primers: 5′-GCGGATCCCAATATACGTTTATACAGAA-3′ (BamHI site underlined) and 5′-GCGAATTCATATCGTGTTTTGTTTTAGG-3′ (EcoRI site underlined). These amplified fragments were cloned into the BamHI/EcoRI sites of pBSV-Fc-8His. The resultant plasmid was designated pBSV-Fc-BaeblHB3-8His, pBSV-Fc-BaeblE12-8His, pBSV-Fc-BaeblDd2/Nm-8His, and pBSV-Fc-BaeblPNG3-8His, respectively. The fusion proteins expressed by these plasmids contained amino acids 141–755 of the BAEBL region II (GenBankTM accession number AAK49521).
The expression vectors for four series of alanine mutant of BAEBL were constructed by the method described in the supplemental materials using primers shown in supplemental Table S1.
Constructs that contained the cDNAs of glypican 4, syndecan 1, and syndecan 4 were produced by reverse transcription-PCR using the total RNA of 293T cells as a template and the following primers: for glypican 4, 5′-CCCAAGCTTACCATGGCACGGTTCGGCTTG-3′ (HindIII site underlined) and 5′-CCGCTCGAGTTTGAGAATTATCTCCACTCTCTCTGC-3′ (XhoI site underlined); for syndecan 1, 5′-CCCAAGCTTCCGGTCCGGGCAGCATCAG-3′ (HindIII site underlined) and 5′-CCGCTCGAGCGTCAGGCATAGAATTCCTCCTG-3′ (XhoI site underlined); and for syndecan 4, 5′-CGGGATCCAGTCCGCGGTGGCCATGG-3′ (BamHI site underlined) and 5′-GGAATTCACGCGTAGAACTCATTGGTGG-3′ (EcoRI site underlined). The amplified fragments for glypican 4 and syndecan 1 were cloned into the HindIII/XhoI sites, and the fragment of syndecan 4 was cloned into the BamHI/EcoRI sites of pMX (25). The resultant plasmids were designated pMX-glypican4, pMX-syndecan1, and pMX-syndecan4, respectively.
Recombinant Baculovirus Expression
Each of the prepared plasmids (pBSV-Fc-8His, pBSV-Fc-BaeblHB3-8His, pBSV-Fc-BaeblE12-8His, pBSV-Fc-BaeblDd2/Nm-8His, and pBSV-Fc-BaeblPNG3-8His) and BaculoGold linearized baculovirus DNA (BD Biosciences) were co-transfected into Sf9 cells using the Cellfectin reagent (Invitrogen), as described previously (26). The five generated recombinant baculoviruses were designated Bac-Fc, Bac-Fc-BaeblHB3, Bac-Fc-BaeblE12, Bac-Fc-BaeblDd2/Nm, and Bac-Fc-BaeblPNG3, respectively. After three or four passages, the viruses were inoculated into Tn5 cells. At 72 h postinfection, the culture supernatants were collected. The recombinant proteins secreted into the medium were purified as previously described (23) and dialyzed in phosphate-buffered saline. These proteins were separated by 5–20% gradient SDS-PAGE (Atto, Tokyo, Japan) and visualized by silver staining.
Mammalian Expression Using a Retroviral Vector
To produce Jurkat cells that express glypican 1, glypican 4, syndecan 1, or syndecan 4, we transfected pMX-glypican1 (27), pMX-glypican4, pMX-syndecan1, and pMX-syndecan4 separately into Plat-E cells using Lipofectamine 2000 (Invitrogen). Jurkat-EcoVRc cells were incubated with the culture supernatant from the transfected Plat-E cells and analyzed 2–4 days postinfection.
Flow Cytometry
Erythrocytes (2.5 × 105) were treated with heparitinase from Flavobacterium heparinum (EC 4.2.2.8; Seikagaku Corp., Tokyo, Japan) or neuraminidase from Vibrio cholerae (EC 3.2.1.18; Sigma) for 1 h at 37 °C, and then washed in fluorescence-activated cell sorting buffer (2% fetal calf serum and 0.1% NaN3 in phosphate-buffered saline). The enzyme-treated or -untreated cells (1 × 105 cells) were resuspended in 27 nm solutions of the purified recombinant proteins and incubated for 1 h at 4 °C. The binding levels of the recombinant proteins were measured using an anti-mouse IgG antibody labeled with fluorescein isothiocyanate, as described previously (28), and quantified using a FACSCalibur (BD Biosciences) and the WinMDI 2.9 software (available from the Scripps Research Institute Web site). The values of the geometric mean fluorescence intensities were used to calculate the binding inhibition percentages. The percentage of binding inhibition was calculated by subtracting mean fluorescence intensity of mIgG2aFc from mean fluorescence intensity of BAEBL/Fc in the presence and absence of enzyme, respectively, dividing the former result by the latter result, multiplying the result by 100, and then subtracting the result from 100.
The effect of heparin, HS, and fetuin on the binding of protein was determined in the following experiment. The recombinant proteins were incubated with equivalent amounts of heparin from porcine intestinal mucosa (Hoechst Marion Roussel, Tokyo, Japan), HS from bovine kidney (Sigma), or fetuin from fetal calf serum (Sigma) at 4 °C for 1 h. These solutions were then mixed with erythrocytes (1 × 105) and incubated for 1 h at 4 °C. The binding levels of the recombinant proteins were detected and quantified as described above.
ELISA-based Binding Assay
The ELISA-based assay was performed as described previously (29). Briefly, ELISA plate wells were coated overnight at 4 °C with purified HS in coating buffer that contained 50 mm sodium bicarbonate (pH 9.6). The wells were then blocked with 3% skim milk at 37 °C for 1 h. Diluted recombinant proteins were added to the wells to allow binding. After a 1-h incubation at room temperature, the bound proteins were detected with a horseradish peroxidase-conjugated anti-mouse IgG F(ab′) fragment (1:5000 dilution; Amersham Biosciences) and the TMB microwell peroxidase substrate system (KPL, Gaithersburg, MD).
To inhibit binding, diluted heparin solution was added to the wells coated with HS and blocked with 3% skim milk immediately before the addition of equal amounts of BAEBL. After a 1-h incubation at room temperature, the bound proteins were detected as described above.
To measure the disassociation constant (Kd) for the binding of BAEBL to HS, various concentrations of BAEBL/Fc were added to ELISA plate wells coated with 10 μg/ml HS. Bound protein was detected as described above. The obtained data were analyzed by Scatchard plot analysis (30, 31).
Pull-down Assay
To collect the culture supernatant of P. falciparum, iRBC that was purified using Percoll-sorbitol was cultured for 20 h. The supernatant was collected by centrifugation at 100,000 × g for 10 min at 4 °C and stored at −80 °C until use. Pull-down assays were performed using heparin-agarose (Sigma), Ni2+-NTA-agarose (Qiagen, Hilden, Germany) or glutathione-Sepharose (GE Healthcare) beads. BAEBL was precipitated by the addition of a 50% suspension of each type of bead to a 5-fold dilution of the supernatant in NETT (150 mm NaCl, 5 mm EDTA, 50 mm Tris, pH 7.4, 0.5% Triton X-100, 0.02% NaN3) that contained 0.5% bovine serum albumin. After rotation for 3.5 h at 4 °C, the beads were washed twice with NETT containing 0.5% bovine serum albumin and three times with NETT. The beads were then boiled for 5 min in equal volumes of 2× sample buffer that contained 0.125 m Tris, pH 6.8, 4% SDS, 20% glycerol, and 10% 2-mercaptoethanol. The eluates were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and then probed with a rabbit anti-BAEBL polyclonal antibody, as described previously (26).
Invasion Assay
Invasion assays were performed as described previously (32, 33). Briefly, 1 × 107 target erythrocytes were treated with heparitinase or neuraminidase or incubated with the recombinant protein for 2 h at 37 °C. After they were washed with phosphate-buffered saline, the treated erythrocytes were mixed with 2 × 105 iRBC, predominantly early schizont, which were enriched by the Percoll-sorbitol method at 25 h after synchronization with the 5% d-sorbitol method (33). After 20 h, parasitemia of the ring stage parasite was evaluated using Giemsa-stained culture smears. The percentage of invasion inhibition was calculated by dividing the parasitemia of test cultures by that of control cultures, multiplying the result by 100, and then subtracting the result from 100.
The effects of heparin on merozoite invasion were determined by the addition of a diluted heparin solution to each culture immediately after the addition of iRBC. Invasion inhibition was detected and quantified as described above.
RESULTS
Recombinant Region II of BAEBL Protein Expressed as Fc Fusion Protein
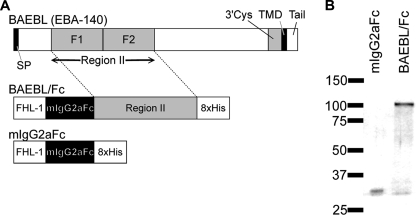
To analyze the binding of BAEBL, we prepared a recombinant BAEBL protein. Region II of BAEBL, which was derived from the P. falciparum HB3 clone, was fused with the Fc region of murine IgG2a at the N terminus and an octahistidine tag at the C terminus. The recombinant protein was expressed using a baculovirus expression system and designated BAEBL/Fc (Fig. 1A). Similarly, a recombinant protein of Fc region alone, designated mIgG2aFc, was prepared as a negative control. These recombinant proteins were purified on Ni2+-NTA-agarose. The protein purities were confirmed by SDS-PAGE with silver staining (Fig. 1B). Because a single band appeared at the expected molecular mass for each fusion protein, the prepared proteins were considered to be pure.
FIGURE 1.
Recombinant region II of BAEBL protein expressed as Fc fusion protein. A, schematic representations of P. falciparum BAEBL and the recombinant fusion proteins. BAEBL is composed of a signal peptide (SP), a region II, a 3′ cysteine-rich region (3′Cys), a transmembrane domain (TMD), and a cytoplasmic tail (Tail). The region II of BAEBL was cloned into the pBSV-Fc-His8 vector, which contains a secretion signal sequence (FHL-1), the Fc region of mouse IgG2a (mIgG2aFc), and an octahistidine C-terminal tag (8xHis). B, recombinant proteins expressed using the baculovirus expression system and purified on Ni2+-NTA-agarose. Each protein (25 ng) was separated by 5–20% gradient SDS-PAGE and subjected to silver staining. The molecular masses (kDa) are indicated on the left.
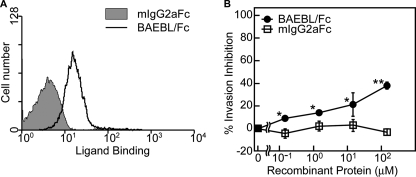
Flow cytometric analyses were performed to confirm binding between the recombinant proteins and erythrocytes (Fig. 2A). The binding affinity of BAEBL/Fc was compared with that of mIgG2aFc. Because BAEBL/Fc showed more potent affinity to erythrocyte than did mIgG2aFc, BAEBL/Fc was confirmed to be bound to erythrocytes.
FIGURE 2.
BAEBL/Fc binds to erythrocytes and inhibits the merozoite invasion. A, binding of recombinant proteins to erythrocytes. The cells were incubated with each of the recombinant proteins. Cell surface binding was detected using the FACSCalibur system. B, merozoite invasion inhibition by recombinant proteins. Erythrocytes were preincubated with buffer or a 0.15, 1.5, 15, or 150 nm concentration of each recombinant protein at 37 °C for 1 h and then mixed with iRBC. Parasitemia was assessed at 20 h postinoculation, as described under “Experimental Procedures.” Results are shown as the means of three independent experiments, and the error bars represent S.E. values. Asterisks and double asterisks indicate significant differences (p < 0.05 and p < 0.001, respectively), as determined by t test.
Using the invasion assay, we investigated whether the pretreatment of erythrocytes with the recombinant proteins inhibited merozoite invasion (Fig. 2B). BAEBL/Fc inhibited invasion in a dose-dependent manner and showed ∼38% invasion inhibition at the concentration of 150 μm. In contrast, mIgG2aFc showed no inhibitory effect. Because the observed inhibition rates are very similar to those observed in previous experiments using anti-BAEBL antibodies (18), it is reasonable to assume that our fusion proteins bind to a merozoite invasion receptor, thereby blocking invasion.
Recombinant BAEBL/Fc Binds to Erythrocytes via HS and Sialic Acid
In our previous study, we identified the interaction between BAEBL and glypican 1, a kind of heparan sulfate proteoglycan (HSPG), by retrovirus-mediated expression cloning (27). In addition, we found that seven potential glycosaminoglycan (GAG)-binding motifs, characterized by XBBXBX and XBBBXXBX (where B represents a basic residue, and X represents a hydropathic residue) (34), are predicted for BAEBL, and five of these motifs are located in region II (Fig. 3). These results enhance the possible binding of BAEBL mediated by HS.
FIGURE 3.
The amino acid sequence of P. falciparum BAEBL. The locations of potential GAG-binding motifs are shown in white letters on a black background. The region II expressed as the fusion protein in the present study is shaded in gray. These sequences are available from GenBankTM (accession number AAK49521).
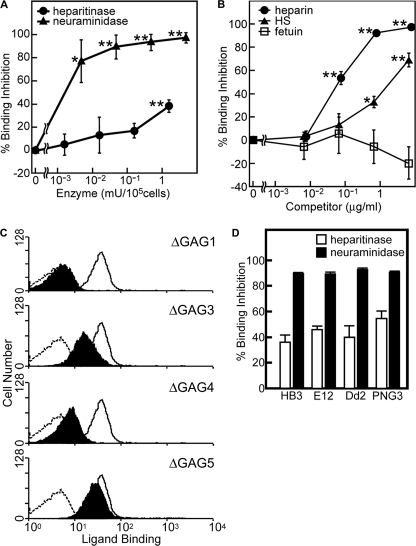
However, previous investigations have reported that BAEBL is bound to erythrocytes in a sialic acid-dependent manner (15–19). Therefore, to examine the binding properties of BAEBL, we selected neuraminidase, which is a sialic acid-specific lyase, and heparitinase, which is an HS-specific lyase, for enzymatic digestions of the erythrocytes. The binding of BAEBL to the enzyme-treated erythrocytes was quantified by flow cytometry (Fig. 4A). The binding efficiency of the heparitinase-treated erythrocytes dropped to ∼60–70%. In contrast, the binding efficiency of the neuraminidase-treated erythrocytes dropped to less than 10%. These results indicate that although BAEBL uses both sialic acid and HS moieties for binding to erythrocytes, a sialic acid moiety rather than an HS moiety is essential for binding.
FIGURE 4.
Recombinant BAEBL/Fc binds to erythrocytes via HS and sialic acid. A, binding inhibition of BAEBL/Fc to enzyme-treated erythrocytes. Erythrocytes (105 cells) were pretreated with buffer; 0.0016, 0.016, 0.16, or 1.6 milliunits of heparitinase; or 0.004, 0.04, 0.4, or 4 milliunits of neuraminidase and then incubated with BAEBL/Fc or mIgG2aFc. B, binding inhibition of BAEBL/Fc preincubated with soluble heparin. The recombinant protein was mixed with buffer, heparin (at a final concentration of 0.0077, 0.077, 0.77 or 7.7 μg/ml), HS, or fetuin (at a final concentration of 0.0067, 0.067, 0.67, or 6.7 mg/ml). The mixtures were incubated at 4 °C for 1 h and then added to erythrocytes. C, the binding of motif-deleted BAEBLs to erythrocytes. Erythrocytes (105 cells) were incubated with 24 nm mIgG2aFc (dashed line), BAEBL/Fc (solid line), BAEBL-ΔGAG1/Fc, BAEBL-ΔGAG3/Fc, BAEBL-ΔGAG4/Fc, or BAEBL-ΔGAG5/Fc. The bindings of motif-deleted BAEBLs are shaded in black. D, binding inhibition of BAEBL/Fc derived from four types of BAEBL variants to enzyme-treated erythrocytes. BAEBL/Fc from HB3 (VSKK), E12 (ISKK), Dd2/Nm (VSTK), and PNG3 (INRE) were incubated with erythrocytes pretreated with buffer, 1.6 milliunits of heparitinase, or 4 milliunits of neuraminidase. Bound protein was quantified, and percentage of binding inhibition was calculated as described in “Experimental Procedures.” Results are shown as the means of three independent experiments. Error bars, S.E. Asterisks and double asterisks indicate significant differences (p < 0.05 and p < 0.001, respectively) as determined by t test.
Moreover, the preincubation of BAEBL/Fc with HS or heparin, which is chemically similar to HS but more highly sulfated (35), inhibited the binding of BAEBL to erythrocytes (Fig. 4B). These results confirm the involvement of the HS moiety in the binding of BAEBL. In addition, considering that BAEBL-erythrocyte binding is primarily mediated by sialic acid, heparin treatment is suggested to inhibit the sialic acid-dependent binding of BAEBL.
On the other hand, in the presence of fetuin, which is a sialic acid-rich glycoprotein, the binding inhibition was not observed. This might suggest the importance of something other than sialic acid (e.g. a special conformation of the protein backbone) in the sialic acid-mediated binding of BAEBL.
To determine whether predicted GAG-binding motifs are key factors for the binding of BAEBL with HS or erythrocytes, the basic amino acids represented by “B” in four XBBXBX motifs (GAG1, GAG3, GAG4, and GAG5 in Fig. 3) were substituted by alanine. GAG2-deleted BAEBL was not made because of the different type of motif (XBBBXXBX). Amino acid residues 146QKRTHL151 were substituted by 146QAATAL151 in BAEBL-ΔGAG1/Fc, 366MKKSKT371 by 366MAASAT371 in BAEBL-ΔGAG3/Fc, 496LHRGHE501 by 496LAAGAE501 in BAEBL-ΔGAG4/Fc, and 633KRKEKI638 by 633KAAEAI638 in BAEBL-ΔGAG5/Fc. As compared with the wild type, BAEBL-ΔGAG1/Fc and -ΔGAG4/Fc showed greatly reduced binding to erythrocytes, and BAEBL-ΔGAG3/Fc showed slightly reduced binding, whereas BAEBL-ΔGAG5/Fc did not show apparent reduction (Fig. 4C). These results suggest the importance of the putative GAG-binding motifs, especially GAG1 and GAG4, for the binding of BAEBL to erythrocytes.
According to previous studies on BAEBL, polymorphisms in four particular amino acid positions of region II are suggested to determine its receptor specificity (24) or its affinity to erythrocytes (36). To verify the possibility that the HS-dependent binding of BAEBL is involved in such alterations determined by the polymorphisms, we prepared Fc fusion proteins of BAEBL derived from E12 (ISKK), Dd2/Nm (VSTK), and PNG3 (INRE) clones in addition to HB3 clone (VSKK). These proteins were tested for binding to the enzyme-treated erythrocytes (Fig. 4D). To the neuraminidase-treated erythrocytes, all types of BAEBL showed ∼90% binding inhibition. These results indicate that all types are bound to erythrocytes in a sialic acid-dependent manner. On the other hand, to the heparitinase-treated erythrocytes, each type showed 36–55% binding inhibition. The binding of BAEBL from PNG3 (VSKK) was inhibited at the highest level (∼55%), BAEBL from E12 (ISKK) showed the second highest inhibition (∼46%), and the other variants showed relatively lower inhibition (∼36–40%). As a result of the t test, there were no significant differences except for a significant difference between HB3 and PNG3 (p = 0.036). These results suggested the possibility that the polymorphisms in BAEBL slightly affect the HS-dependent binding.
Recombinant BAEBL/Fc Binds to HS and Heparin Independently of Its Peptide Backbone or Sialic Acid
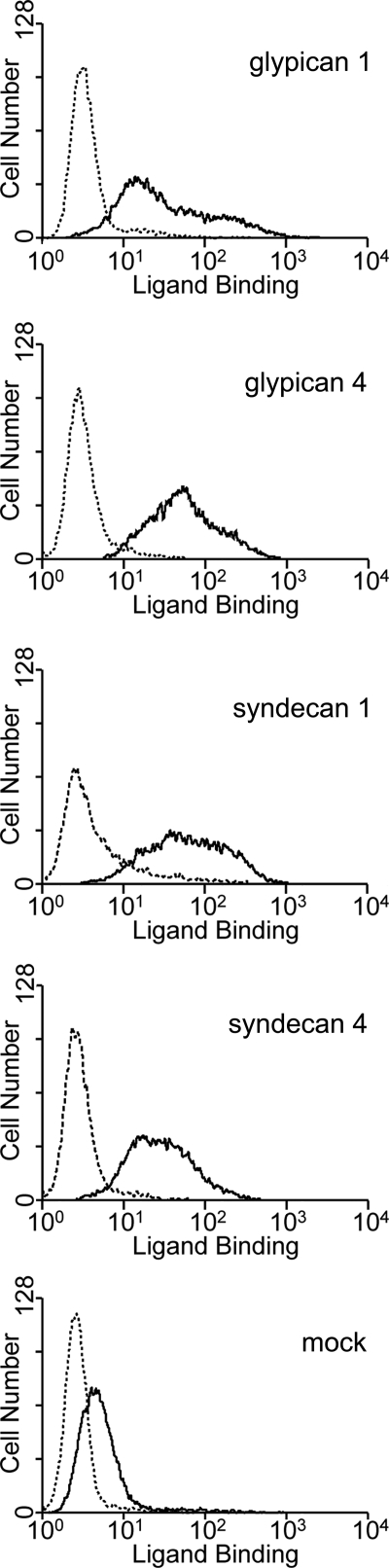
To investigate the HS-dependent binding of BAEBL in detail, HSPGs were expressed on Jurkat cells. Cell membrane-associated HSPGs generally comprise two different families: (i) the glypican family to which GPI-anchored HSPGs belong and (ii) the syndecan family to which transmembrane HSPGs belong (37). We chose glypican 1, glypican 4, syndecan 1, and syndecan 4 for expression using retrovirus vectors, because each of these molecules represents a subfamily into which the family is subdivided based on the amino acid sequences (37). Jurkat cells transduced with the HSPG expression retrovirus vectors showed higher affinity for BAEBL/Fc than those transduced with a mock vector (Fig. 5). These results suggest that HSPG expression on the cell surface enhances the binding affinity of BAEBL/Fc, irrespective of its core protein structure. Several potential N-glycosylation sequences together with GAG attachment sequences are predicted to be located in the core proteins of glypican 1, glypican 4, and syndecan 1, whereas no N-glycosylation sites are predicted for syndecan 4 (37). This indicates that the sialic acid and HS moieties do not need to be present in a single molecule to increase the binding of BAEBL/Fc.
FIGURE 5.
Binding of BAEBL to four types of HSPGs expressed on Jurkat cells. Jurkat-EcoVRc cells transduced with a retrovirus vector from an empty plasmid (mock) or the vectors that carry the cDNA of glypican 1, glypican 4, syndecan 1, or syndecan 4 were incubated with BAEBL/Fc (solid line) or mIgG2aFc (dashed line). Bound protein was detected as described under “Experimental Procedures.”
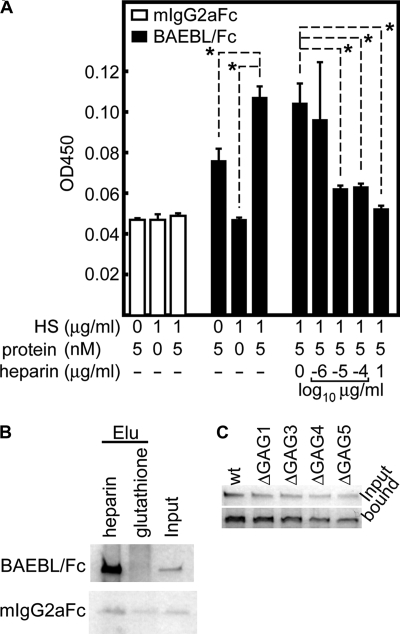
Using ELISA-based methods with purified HS as the coating antigen, the reactivity between BAEBL and HS under controlled conditions was estimated (Fig. 6A and supplemental Table S2). BAEBL/Fc, but not mIgG2aFc, bound to HS with a Kd of 8.18 ± 2.54 × 10−9 m. This binding was inhibited by the addition of soluble heparin. These results suggest that HS alone can mediate the binding of BAEBL.
FIGURE 6.
Recombinant BAEBL/Fc binds to purified HS and heparin. A, ELISA-based binding assays of BAEBL to HS. Purified HS was coated onto the wells of an ELISA plate and tested for binding efficiency to the recombinant proteins. The bound protein was detected with a horseradish peroxidase-conjugated anti-mouse IgG F(ab′) fragment. Binding inhibition was evaluated by the addition of buffer or 10−6, 10−5, 10−4, or 1 μg/ml soluble heparin to the HS-coated wells immediately before the addition of equal amounts of BAEBL. The optical densities at 450 nm (OD450) are shown as the means of three independent experiments. Error bars, S.E. The asterisks indicate a significant difference (p < 0.01) as determined by t test. B, heparin bead pull-down assays for recombinant proteins. A recombinant protein (500 ng) was incubated with one of two bead types (heparin-agarose and glutathione-Sepharose) for 3.5 h at 4 °C. Eluates (Elu) from the washed beads and 50 ng of untreated protein (Input) were separated by 5–20% gradient SDS-PAGE and subjected to silver staining. C, BAEBL/Fc (wild type (wt)) and four kinds of motif-deleted BAEBL/Fc (ΔGAG1–ΔGAG5) were incubated with heparin-agarose beads. After they were washed, the bound protein and 25 ng of untreated protein (input) were analyzed as described above.
Moreover, in a heparin bead pull-down assay, BAEBL/Fc was absorbed to a greater extent by heparin-agarose than by glutathione-Sepharose (Fig. 6B). In contrast, these two bead types were not significantly different with regard to the absorption of mIgG2aFc. These results showed that BAEBL/Fc binds to heparin, which in turn suggest that the binding of heparin to BAEBL/Fc induces the binding inhibition for erythrocytes.
Unexpectedly, all of the motif-deleted BAEBL proteins as well as wild type also bound to HS and heparin with relatively equal affinity (Fig. 6C and supplemental Table S2). This result contrasts with the apparent binding reduction of BAEBL-ΔGAG1/Fc and -ΔGAG4/Fc to erythrocytes (Fig. 4C). We speculate the possibility that the single motif deletion does not reduce the protein binding under artificial conditions because of the residual motifs, whereas some motifs (e.g. GAG1 or GAG4) function essentially under physiological condition.
BAEBL Secreted from P. falciparum Also Binds to Heparin
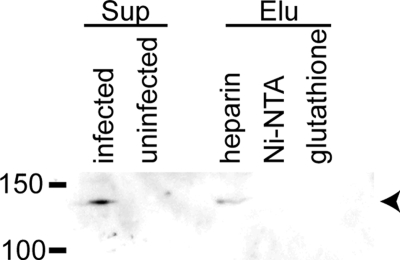
All of the analyses described above were performed using the recombinant fusion protein with the binding region of BAEBL. To elucidate whether BAEBL derived from a P. falciparum culture can bind to HS and to its analog heparin, we carried out pull-down assays with heparin-agarose beads. Beads incubated with the supernatant of P. falciparum iRBC were subjected to Western blotting using an anti-BAEBL polyclonal antibody (Fig. 7). As reported previously, this antibody detects a specific protein (140 kDa) (15–17) in the iRBC supernatant. Of the three bead types (heparin-agarose, Ni2+-NTA-agarose, and glutathione-Sepharose), only the extract from heparin-agarose included the BAEBL protein. These results indicated that BAEBL derived from P. falciparum cultures binds to heparin, raising the possibility that BAEBL interacts physiologically with HS. These observations are in agreement with the results of the recombinant fusion protein analyses.
FIGURE 7.
BAEBL secreted from P. falciparum also binds to heparin. Culture supernatants of the P. falciparum HB3 clone were incubated with heparin-agarose, Ni2+-NTA-agarose or glutathione-Sepharose for 3.5 h at 4 °C. The culture supernatants (Sup) of P. falciparum-infected and uninfected erythrocytes and the eluates (Elu) from the washed beads were separated on 8% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The specific band (arrowhead) for BAEBL was detected using a rabbit anti-BAEBL polyclonal antibody. The molecular masses (kDa) are indicated on the left.
HS-dependent Binding Is Involved in Merozoite Invasion
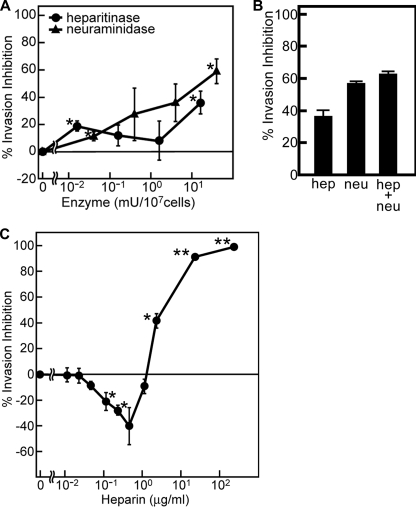
To determine whether HS-dependent binding is important for merozoite invasion, we performed an invasion assay using erythrocytes treated with heparitinase or neuraminidase (Fig. 8A). Consistent with previous studies, neuraminidase treatment partially inhibited merozoite invasion of the HB3 clone (58%) (4). In the case of heparitinase treatment, the maximal invasion inhibition is 35%. These data indicate that HS is at least partly associated with merozoite invasion. Simultaneous treatment with these two enzymes did not show a significant increase of the invasion inhibition (Fig. 8B). This result suggests that the invasion pathway via sialic acid and HS is virtually the same.
FIGURE 8.
HS-dependent binding is involved in merozoite invasion. A, inhibition of merozoite invasion of enzyme-treated erythrocytes. Erythrocytes (107 cells) were treated with buffer, with 0.016, 0.16, 1.6, or 16 milliunits (mU) of heparitinase, or with 0.04, 0.4, 4, or 40 milliunits of neuraminidase at 37 °C for 2 h, followed by the invasion assay. B, inhibition of merozoite invasion of erythrocytes treated with heparitinase (16 milliunits) and neuraminidase (40 milliunits) simultaneously. In parallel, the treatment with either enzyme was performed. C, inhibition of merozoite invasion by the addition of soluble heparin. Erythrocytes were mixed with iRBC (2 × 105 cells), immediately before the addition of buffer or heparin at a final concentration of 0.01, 0.02, 0.05, 0.12, 0.23, 0.47, 1.17, 2.33, 23.3, or 233 μg/ml. In all experiments, parasitemia was assessed at 20 h post-inoculation, and percentage of invasion inhibition was calculated, as described under “Experimental Procedures.” Results are shown as the means of three independent experiments. Error bars, S.E. Asterisks and double asterisks indicate significant differences (p < 0.05 and p < 0.001, respectively) as determined by t test.
To examine further the relationship between invasion and HS, we examined the effects of heparin on merozoite invasion (Fig. 8C). The addition of high concentrations of heparin (2.33–233 μg/ml) potently inhibited merozoite invasion, as previously reported (7, 38). However, low concentrations of heparin (≤1.17 μg/ml) enhanced merozoite invasion. These results suggest that although appropriate concentrations of heparin functionally facilitate merozoite invasion, excessive levels of heparin cause inhibition.
DISCUSSION
In the present study, we show that BAEBL interacts with HS and heparin. This interaction appears to be independent of both the HSPG peptide backbone and the sialic acid moieties on the backbone. The treatment of erythrocytes with heparitinase decreased the binding rate of BAEBL to ∼36–55% and the merozoite invasion rate to ∼35%. These results suggest that HS on the surface of erythrocytes plays an important if not essential role in the binding of BAEBL and merozoite invasion.
BAEBL was predicted to contain seven potential GAG-binding motifs, five of which are located in region II. Two XBBXBX motifs, termed GAG1 and GAG4, are shown to play a critical role in the binding of BAEBL to erythrocytes. This suggests the possibility that these two motifs mediate not only the HS-dependent binding of BAEBL but also the sialic acid-dependent binding because sialic acid, but not HS, is essential for the binding. Blockage of these motifs by soluble heparin is thought to induce the binding inhibition of BAEBL. However, we cannot show the experimental evidence that these motifs mediate the binding to HS or heparin because of the insignificant difference between the binding of wild-type BAEBL and that of motif-deleted BAEBLs to HS or heparin. We speculate that, in the binding assay in vitro, each of the motifs could independently support the binding of BAEBL, resulting in nonphysiological redundancy. This redundancy might cause the relatively high affinity between BAEBL and HS (Kd of 8.18 ± 2.54 × 10−9 m). In the case of glycoprotein C of pseudorabies virus, because three GAG-binding motifs function independently, deletion of a single motif did not show the binding reduction (39), similar to our result. Moreover, they found that each motif recognizes the structural features of heparin selectively (e.g. a specific sulfate group or a minimum size of the oligosaccharide fragment) (40). In our case, HS or sialic acid containing a special structure recognized by GAG1 and GAG4 might be major on the erythrocyte surface.
In addition, sialic acid was found to be essential for BAEBL to be bound to erythrocytes. In contrast to HS, this binding requires something other than sialic acid moieties, such as a peptide backbone, because the addition of a sialic acid-rich protein, fetuin, did not show the binding inhibition of BAEBL. In the previous study of EBA-175, the amino acid sequence specific for glycophorin A is necessary for the binding (3). The authors speculated that the peptide may be involved in direct binding to EBA-175 or may contribute a unique conformation of the sialic acid residues. By a similar mechanism, BAEBL may also recognize a specific receptor, in agreement with previous reports demonstrating that BAEBL binds to glycophorin C in a sialic acid-dependent manner (17–19).
If glycophorin C is a sialic acid-mediated receptor for BAEBL, the question remains as to what roles HS plays. To understand the relationship between sialic acid and HS, we focus on a question raised by our results; although there is HS on the surface of the neuraminidase-treated erythrocytes, the binding of BAEBL to such erythrocytes was abolished almost completely. A possibility that we speculate upon is that the expression level of HS on erythrocytes is so low that the binding between BAEBL and HS on the neuraminidase-treated erythrocytes was hardly detected. Indeed, previous studies indicated the small amounts of HS on erythrocyte surface (13, 41). However, this speculation contradicts the findings that heparitinase treatment of erythrocytes reduced the binding of BAEBL by ∼36–55%.
To solve this discrepancy, we hypothesize that the small amount of HS supports the sialic acid-mediated binding of BAEBL and facilitates merozoite invasion by increasing the encounter rate and/or the affinity between BAEBL and a specific receptor during junction formation. The small amount of HS on erythrocytes also plays an important role as a receptor for PfEMP1 (12–14, 42). These facts raise the question of why P. falciparum uses HS, despite low level expression on the erythrocyte surface, for its survival or pathogenesis. Considering the previous report that the quantity of HSPGs seemed to decrease during the differentiation of the hematopoietic cell line into more mature erythrocytes (41), Vogt et al. (13) suggested the interesting possibility that quantity and quality of HSPGs would change over time, and the younger erythrocytes would carry more HS than the older ones. If this is the case, P. falciparum merozoites might tend to infect younger erythrocytes and utilize HS as a marker of young erythrocytes.
HS has been known as a co-receptor to regulate a broad variety of ligand-receptor encounters (43). For example, in the case of herpes simplex virus infections, HS plays important roles. Herpes simplex virus contains multiple structural glycoproteins on the envelope surface, which are designated gB, gC, gD, gH, and gL. Among these proteins, gB, gC, and gD bind to HS. gD is believed to have three alternative entry receptors: herpesvirus entry mediator, nectin 1, and specific O-sulfated moieties on HS (44). The deletion of amino acids 7–32 of gD eliminates the physical and functional interactions of gD with all of the receptors, with the exception of nectin-1. These results suggest that the binding interfaces for herpesvirus entry mediator and specific O-sulfated HS overlap (45). This is similar to our finding that BAEBL region II has the ability to bind to HS and sialic acid separately. Furthermore, herpes simplex virus gB and gC are believed to mediate binding of virion to HS on the cell surface (46–48). Similar to our findings, although this binding significantly enhances the efficiency of herpes simplex virus infection, it is not absolutely essential.
The functional alterations derived from the polymorphisms in region II are necessary to understand functions of BAEBL. To date, two different proposals for the meaning of these polymorphisms were suggested. One proposal is that these polymorphisms determine the receptor specificity because the binding patterns of different BAEBL variants to neuraminidase- or trypsin-treated erythrocytes were drastically changed (24). The other is that these polymorphisms influence not the receptor specificity but the binding affinity to erythrocytes (36). Our results tend to support the latter possibility because the neuraminidase treatment equally inhibited the binding of all BAEBL variants. On the other hand, the heparitinase treatment showed the subtle differences in the binding inhibition of the BAEBL variants. A BAEBL variant from PNG3 (INRE) is inhibited at the highest level, E12 (ISKK) at the second highest level, and the other two forms (VSKK and VSTK) at relatively lower levels. In previous results reported by Maier et al. (36), INRE and ISKK forms were bound to erythrocytes at higher levels, but VSKK and VSTK forms were at lower levels. These correlations between our results, and those from the previous study may suggest that the HS-mediated binding of BAEBL is related to the binding affinity of BAEBL to erythrocytes. However, we did not observe the clear differences in the binding affinities of the BAEBL variants to erythrocytes (data not shown). We speculate that one reason for this was caused by the experimental system. In this study, purified recombinant region II of BAEBL was used for binding assays, whereas the previous study (36) used parasite culture supernatants. Region II alone might be insufficient for the complete binding of BAEBL in the former system; otherwise, contents in the supernatant other than BAEBL might affect the binding of BAEBL in the latter system. However, our system may largely explain properties of BAEBL because BAEBL/Fc binds to the erythrocyte surface molecule related to the merozoite invasion, judging from the invasion inhibition of the merozoite by BAEBL/Fc.
In the present study, the addition of soluble heparin and HS inhibited the binding of BAEBL and the merozoite invasion into erythrocytes. Several studies have already reported that heparin and other sulfated glycoconjugates inhibit the growth of malarial parasites (7–9, 11). In addition, some sulfated chemical compounds inhibit parasite entry into erythrocytes (6, 10). Our results showing the binding inhibition of BAEBL by heparin are not sufficient to explain the almost complete inhibition of the merozoite invasion by heparin because BAEBL is not an essential ligand for the invasion (18). The binding of full-length MSP-1 (merozoite surface protein-1), which is bound to erythrocytes in a sialic acid-dependent manner, was suggested to be inhibited by the addition of saccharide anions, including heparin (7). In addition, bindings of PfRH5 (P. falciparum reticulocyte-binding homologue 5) and EBA-175 to erythrocytes were also reported to be inhibited by the addition of heparin (49). Here we observed the same result with BAEBL, which suggests the possibility that binding inhibition of multiple ligands, including, at least, BAEBL, MSP1, EBA-175, and PfRH5, by heparin results in invasion inhibition. One potential mechanism for this inhibition, as proposed by Clark et al. (7), is that the saccharide anions, such as heparin, effectively mimic the anionic character of the erythrocyte surface receptor, thereby competitively inhibiting MSP-1 binding to erythrocytes. Our findings that soluble heparin inhibited both sialic acid- and HS-dependent bindings of BAEBL by the blockage of some critical motifs support the notion of competitive binding inhibition. However, PfRH5 does not fit this proposal because PfRH5 binds to erythrocytes in a sialic acid-independent manner (49).
In addition to the inhibitory effect, heparin oppositely showed the enhancement of the merozoite invasion at low concentrations. Because no binding enhancement of BAEBL was observed in this study, soluble heparin may affect invasion pathways independently of BAEBL.
Previous papers have suggested inhibitory effects of other sulfated glycoconjugates (e.g. dextran sulfate, pentosan polysulfate, fucoidan, and sulfatides) on merozoite invasion (7, 11). The binding of BAEBL to erythrocytes may also be inhibited by these glycoconjugates.
There are other reports on the role of HS in P. falciparum survival. In addition to the ligands mentioned above, binding of the PfEMP1 DBL1α domain to HS has been shown (12, 14, 42). A recent study reported that highly sulfated HSPGs on hepatocytes are involved in the cleavage of a malaria sporozoite protein, known as circumsporozoite protein. Circumsporozoite protein signals the transition from migration to invasion (50). Thrombospondin-related anonymous protein on the surface of sporozoites may interact with HSPGs on hepatocytes (51, 52). Additionally, the existence of HS in salivary glands and midguts of the mosquito has been reported (53). Because of the binding to circumsporozoite protein, this HS was suggested to mediate the transmission of the parasite. O-Glycosylated mosquito protein was also reported to have an important role in ookinete invasion of the midgut (54). Thus, HS and other GAGs are employed as receptors for various ligands that are expressed at different stages of the parasite, suggesting the importance of HS in the parasite life cycle. Interestingly, most of these reports suggest the possibility that the parasite ligand has multiple receptors, one of which is HS.
Further detailed analyses will elucidate heparin-mediated inhibition mechanisms and the significance of the binding to HS. This information will assist in the development of an effective antimalarial agent that inhibits the binding of various parasite molecules and has mild or no adverse effects as compared with heparin.
Supplementary Material
Acknowledgments
We are grateful to Dr. Peter F. Zipfel for providing the plasmid vector pBSV-8His; to Dr. Louis H. Miller for the plasmid vectors BAEBL-HB3-T8, BAEBL-E12-T8, BAEBL-Dd2/Nm-T8, and BAEBL-PNG3-T8; to Dr. Yorihiro Nishimura for the plasmid vector mouse IgG2a-pcDNA3.1; and to Dr. Masayuki Shimojima for the Jurkat-EcoVRc cells. We also thank Dr. Kiyoshi Kita and Dr. Takeshi Tanaka for assistance with the in vitro culturing of P. falciparum.
This work was supported by a grant-in-aid for young scientists from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Bio-Oriented Technology Research Advancement Institution.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- DBL
- Duffy binding-like
- HS
- heparan sulfate
- iRBC
- infected erythrocyte(s)
- HSPG
- heparan sulfate proteoglycan
- GAG
- glycosaminoglycan
- ELISA
- enzyme-linked immunosorbent assay
- NTA
- nitrilotriacetic acid.
REFERENCES
- 1.Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. (1975) Science 187, 748–750 [DOI] [PubMed] [Google Scholar]
- 2.Adams J. H., Blair P. L., Kaneko O., Peterson D. S. (2001) Trends Parasitol. 17, 297–299 [DOI] [PubMed] [Google Scholar]
- 3.Sim B. K., Chitnis C. E., Wasniowska K., Hadley T. J., Miller L. H. (1994) Science 264, 1941–1944 [DOI] [PubMed] [Google Scholar]
- 4.Dolan S. A., Miller L. H., Wellems T. E. (1990) J. Clin. Invest. 86, 618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasvol G. (2003) Trends Parasitol. 19, 430–432 [DOI] [PubMed] [Google Scholar]
- 6.Kisilevsky R., Crandall I., Szarek W. A., Bhat S., Tan C., Boudreau L., Kain K. C. (2002) Antimicrob. Agents Chemother. 46, 2619–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark D. L., Su S., Davidson E. A. (1997) Glycoconj. J. 14, 473–479 [DOI] [PubMed] [Google Scholar]
- 8.Butcher G. A., Parish C. R., Cowden W. B. (1988) Trans. R. Soc. Trop. Med. Hyg. 82, 558–559 [DOI] [PubMed] [Google Scholar]
- 9.Kulane A., Ekre H. P., Perlmann P., Rombo L., Wahlgren M., Wahlin B. (1992) Am. J. Trop. Med. Hyg. 46, 589–594 [DOI] [PubMed] [Google Scholar]
- 10.Crandall I. E., Szarek W. A., Vlahakis J. Z., Xu Y., Vohra R., Sui J., Kisilevsky R. (2007) Biochem. Pharmacol. 73, 632–642 [DOI] [PubMed] [Google Scholar]
- 11.Xiao L., Yang C., Patterson P. S., Udhayakumar V., Lal A. A. (1996) Infect. Immun. 64, 1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogt A. M., Barragan A., Chen Q., Kironde F., Spillmann D., Wahlgren M. (2003) Blood 101, 2405–2411 [DOI] [PubMed] [Google Scholar]
- 13.Vogt A. M., Winter G., Wahlgren M., Spillmann D. (2004) Biochem. J. 381, 593–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barragan A., Fernandez V., Chen Q., von Euler A., Wahlgren M., Spillmann D. (2000) Blood 95, 3594–3599 [PubMed] [Google Scholar]
- 15.Thompson J. K., Triglia T., Reed M. B., Cowman A. F. (2001) Mol. Microbiol. 41, 47–58 [DOI] [PubMed] [Google Scholar]
- 16.Narum D. L., Fuhrmann S. R., Luu T., Sim B. K. (2002) Mol. Biochem. Parasitol. 119, 159–168 [DOI] [PubMed] [Google Scholar]
- 17.Mayer D. C., Kaneko O., Hudson-Taylor D. E., Reid M. E., Miller L. H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5222–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier A. G., Duraisingh M. T., Reeder J. C., Patel S. S., Kazura J. W., Zimmerman P. A., Cowman A. F. (2003) Nat. Med. 9, 87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobo C. A., Rodriguez M., Reid M., Lustigman S. (2003) Blood 101, 4628–4631 [DOI] [PubMed] [Google Scholar]
- 20.Morita S., Kojima T., Kitamura T. (2000) Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 21.Shimojima M., Ikeda Y., Kawaoka Y. (2007) J. Infect. Dis. 196, Suppl. 2, S259–S263 [DOI] [PubMed] [Google Scholar]
- 22.Trager W., Jensen J. B. (1976) Science 193, 673–675 [DOI] [PubMed] [Google Scholar]
- 23.Kühn S., Zipfel P. F. (1995) Gene 162, 225–229 [DOI] [PubMed] [Google Scholar]
- 24.Mayer D. C., Mu J. B., Feng X., Su X. Z., Miller L. H. (2002) J. Exp. Med. 196, 1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi M., Kinoshita S., Morikawa Y., Shibuya A., Phillips J., Lanier L. L., Gorman D. M., Nolan G. P., Miyajima A., Kitamura T. (1996) Exp. Hematol. 24, 324–329 [PubMed] [Google Scholar]
- 26.Kato K., Kawaguchi Y., Tanaka M., Igarashi M., Yokoyama A., Matsuda G., Kanamori M., Nakajima K., Nishimura Y., Shimojima M., Phung H. T., Takahashi E., Hirai K. (2001) J. Gen. Virol. 82, 1457–1463 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi K., Kato K., Sugi T., Yamane D., Shimojima M., Tohya Y., Akashi H. (2009) Anal. Biochem. 389, 80–82 [DOI] [PubMed] [Google Scholar]
- 28.Shimojima M., Miyazawa T., Ikeda Y., McMonagle E. L., Haining H., Akashi H., Takeuchi Y., Hosie M. J., Willett B. J. (2004) Science 303, 1192–1195 [DOI] [PubMed] [Google Scholar]
- 29.Hans D., Pattnaik P., Bhattacharyya A., Shakri A. R., Yazdani S. S., Sharma M., Choe H., Farzan M., Chitnis C. E. (2005) Mol. Microbiol. 55, 1423–1434 [DOI] [PubMed] [Google Scholar]
- 30.Horio T. (1995) Theory and Practice on Enzymes and Other Proteins, 2nd Ed., pp. 436–441, Nankoudo Co., Tokyo [Google Scholar]
- 31.Scatchard G. (1949) Ann. N.Y. Acad. Sci. 51, 660–672 [Google Scholar]
- 32.Kato K., Mayer D. C., Singh S., Reid M., Miller L. H. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 5552–5557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneko O., Soubes S. C., Miller L. H. (1999) Exp. Parasitol. 93, 116–119 [DOI] [PubMed] [Google Scholar]
- 34.Cardin A. D., Weintraub H. J. (1989) Arteriosclerosis 9, 21–32 [DOI] [PubMed] [Google Scholar]
- 35.Gallagher J. T., Walker A. (1985) Biochem. J. 230, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maier A. G., Baum J., Smith B., Conway D. J., Cowman A. F. (2009) Infect. Immun. 77, 1689–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 38.Vogt A. M., Pettersson F., Moll K., Jonsson C., Normark J., Ribacke U., Egwang T. G., Ekre H. P., Spillmann D., Chen Q., Wahlgren M. (2006) PLoS Pathog. 2, e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flynn S. J., Ryan P. (1996) J. Virol. 70, 1355–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trybala E., Bergström T., Spillmann D., Svennerholm B., Flynn S. J., Ryan P. (1998) J. Biol. Chem. 273, 5047–5052 [DOI] [PubMed] [Google Scholar]
- 41.Drzeniek Z., Stöcker G., Siebertz B., Just U., Schroeder T., Ostertag W., Haubeck H. D. (1999) Blood 93, 2884–2897 [PubMed] [Google Scholar]
- 42.Chen Q., Barragan A., Fernandez V., Sundström A., Schlichtherle M., Sahlén A., Carlson J., Datta S., Wahlgren M. (1998) J. Exp. Med. 187, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park P. W., Reizes O., Bernfield M. (2000) J. Biol. Chem. 275, 29923–29926 [DOI] [PubMed] [Google Scholar]
- 44.Spear P. G. (2004) Cell Microbiol. 6, 401–410 [DOI] [PubMed] [Google Scholar]
- 45.Yoon M., Zago A., Shukla D., Spear P. G. (2003) J. Virol. 77, 9221–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh M. T., WuDunn D., Montgomery R. I., Esko J. D., Spear P. G. (1992) J. Cell Biol. 116, 1273–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruenheid S., Gatzke L., Meadows H., Tufaro F. (1993) J. Virol. 67, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banfield B. W., Leduc Y., Esford L., Schubert K., Tufaro F. (1995) J. Virol. 69, 3290–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baum J., Chen L., Healer J., Lopaticki S., Boyle M., Triglia T., Ehlgen F., Ralph S. A., Beeson J. G., Cowman A. F. (2009) Int. J. Parasitol. 39, 371–380 [DOI] [PubMed] [Google Scholar]
- 50.Coppi A., Tewari R., Bishop J. R., Bennett B. L., Lawrence R., Esko J. D., Billker O., Sinnis P. (2007) Cell Host Microbe 2, 316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matuschewski K., Nunes A. C., Nussenzweig V., Ménard R. (2002) EMBO J. 21, 1597–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhouri R. R., Bhattacharyya A., Pattnaik P., Malhotra P., Sharma A. (2004) Biochem. J. 379, 815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinnis P., Coppi A., Toida T., Toyoda H., Kinoshita-Toyoda A., Xie J., Kemp M. M., Linhardt R. J. (2007) J. Biol. Chem. 282, 25376–25384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinglasan R. R., Kalume D. E., Kanzok S. M., Ghosh A. K., Muratova O., Pandey A., Jacobs-Lorena M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13461–13466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.