Abstract
Extracellular matrix (ECM) underlies a complicated multicellular architecture that is subjected to significant forces from mechanical environment. Although various components of the ECM have been enumerated, mechanisms that evolve the sophisticated ECM architecture remain to be addressed. Here we show that periostin, a matricellular protein, promotes incorporation of tenascin-C into the ECM and organizes a meshwork architecture of the ECM. We found that both periostin null mice and tenascin-C null mice exhibited a similar phenotype, confined tibial periostitis, which possibly corresponds to medial tibial stress syndrome in human sports injuries. Periostin possessed adjacent domains that bind to tenascin-C and the other ECM protein: fibronectin and type I collagen, respectively. These adjacent domains functioned as a bridge between tenascin-C and the ECM, which increased deposition of tenascin-C on the ECM. The deposition of hexabrachions of tenascin-C may stabilize bifurcations of the ECM fibrils, which is integrated into the extracellular meshwork architecture. This study suggests a role for periostin in adaptation of the ECM architecture in the mechanical environment.
Introduction
The extracellular matrix (ECM)2 is a scaffold to maintain the tissue and organ structure, which regulates many aspects of cell behavior. Activation of cellular signaling by the ECM proteins has been investigated, whereas an importance of the ECM architecture has been underscored (1). It is quite certain that the diversity of three-dimensional configuration and connection of the ECM components invests the ECM architecture with multiple properties. Fibrillogenesis of the ECM takes place in the cell-matrix boundary, known as the matricellular space. The term “matricellular” was defined to denote a subset of ECM proteins whose properties could be distinguished from structural macromolecules and more bioactive proteins such as growth factors, cytokines, and proteases (2). Several secreted proteins concentrated in the matricellular space are thought to be involved in ECM fibrillogenesis (3) and have been designated as matricellular proteins (4).
At the beginning of this study, we focused on the myoseptum of zebrafish. The myoseptum, a connective tissue that transmits muscle contractile forces to bones and adjoining muscles, consists of collagen, fibronectin, tenascin-C, and periostin (5–7). Periostin is a secretory protein that is concentrated in the matricellular space (8). Our previous study showed that targeted disruption of periostin causes disorganization of the myoseptum during zebrafish embryogenesis (5). In mammals, periostin is expressed in the connective tissues, such as periosteum (a fibrous sheath that covers the bone surface and is connected to muscle), periodontal ligament, aorta, and heart valve (9, 10), which are constantly subjected to mechanical strains from physical exercise, mastication, and blood flow and pressure, respectively. Several groups including ours independently generated periostin−/− mice (11–13). periostin−/− mice are born alive and develop to be indistinguishable from wild-type (WT) littermates except for the disturbed eruption of incisors (11). Recent studies, involving pathological intervention, have shown that periostin−/− mice exhibit several defects in their skin, tendons, and heart valves (13–17). We and others demonstrated that the rate of heart ruptures and death caused by acute myocardial infarction is higher in periostin−/− mice than in WT counterparts (13, 14). Taken together, these previous studies in common suggest that periostin is involved in adaptation of the ECM architecture into the mechanical environment.
To provide a mechanistic insight into the mechanical adaptation mediated by periostin, we investigated an involvement of tenascin-C. The expression profile of tenascin-C coincides with that of periostin (18, 19). tenascin-C−/− mice also develop normally, they are fertile, and no gross defects are found in their principal organ systems (20). Tenascin-C is expressed in the infarcted myocardium (21) and has been suggested to be responsible for the tissue repair after myocardial injury (22), which appears comparable to periostin. Furthermore, a previous study demonstrated that periostin directly binds to tenascin-C coated on a microtiter plate (23). These previous studies potently suggest that tenascin-C is involved in the mechanical adaptation mediated by periostin.
In this study, we showed the extracellular meshwork architecture composed of substantial branched connections between tenascin-C hexabrachions and the ECM fibrils, and demonstrated that periostin functions as a bridge between tenascin-C and the ECM. Furthermore, we found that both periostin−/− mice and tenascin-C−/− mice exhibited confined tibial periostitis, which possibly corresponds to medial tibial stress syndrome in human sports injuries, suggesting a physiological role of the extracellular meshwork architecture.
EXPERIMENTAL PROCEDURES
Antibodies
Rabbit anti-mouse periostin antibodies (anti-RD1 and anti-CT) were described previously (9, 14). Rat monoclonal anti-mouse fibronectin antibody (A15-1) was generated previously in our laboratory.3 Rabbit polyclonal anti-fibronectin antibody Ab-10 (Lab Vision Corp., Fremont, CA), rabbit polyclonal anti-type I collagen antibody (Novotec, Lyon, France), monoclonal mouse anti-HA antibody (Nacalai Tesque, Kyoto, Japan), rabbit polyclonal anti-HA antibody (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-tenascin-C antibody (Chemicon, Millipore, CA), mouse monoclonal anti-tenascin-C antibody 4F10TT (Immuno-Biological Laboratories Co., Ltd., Japan), mouse monoclonal anti-GM130 antibody (BD Transduction Laboratories), and rabbit polyclonal anti-calreticulin antibody (ABR Affinity BioReagents, Golden, CO) were purchased as indicated. Alexa Fluor 488- or 568-labeled goat anti-mouse and rabbit antibodies and Alexa Fluor 488-labeled goat anti-rat antibody, as well as Alexa 488- or 568-labeled phalloidin, were obtained from Molecular Probes (Invitrogen). Alexa Fluor 647-labeled mouse anti-GM130 antibody was purchased from BD Biosciences (Pharmingen).
Plasmid Constructions
The fibronectin expression vector (pAIFNBAC (24)) was provided by Dr. K. Sekiguchi. The tenascin-C expression vector was constructed by subcloning a murine tenascin-C cDNA (25) into pSecTag2 (Invitrogen). Expression vectors for periostin and its deletion constructs were based on the pCAGIPuro backbone, which was provided by Dr. H. Niwa. The bicistronic expression vector pCAGIPuro was constructed by replacing the Zeocin-resistant gene of pPCAGIZ (26) with the puromycin acetyltransferase gene. The substituted mutant Trp-65 → Ala and periostin-HA were also subcloned into the pMXs-puro vector, respectively. For generation of the domain deletion constructs, PCR-based domain replacements were performed. For the generation of the Trp-65 → Ala periostin construct, a pUC119 vector carrying C-terminal HA-tagged periostin cDNA was used as the template, and the substituted mutant form (Trp-65 → Ala) was created by site-directed mutagenesis. Internal complementary PCR primers used were the following: 5′-CCTGTAAGAACGCCTATCAAGGTGC-3′ and 5′-GCACCTTGATAGGCGTTCTTACAGG-3′. For the generation of HA-periostin, the internal PCR primers used were 5′-CAGCGTAGTCTGGGACGTCGTATGGGTAACCTGGACTGTTGGCATTTGCAGG-3′ and 5′-GTGGCTCCGGTGGAAGTAACAGTTACTATGACAAGGTCCTG-3′. KOD plus DNA polymerase (Toyobo Co., Ltd., Osaka, Japan) was used for the PCR reaction. The PCR products were purified by electrophoresis gel extraction, self-ligated, and digested with DpnI before transformation. The mutations were confirmed by sequencing the inserted cDNA. Standard methods were utilized to construct all plasmids. Full details will be provided upon request.
Animals
Care and experiments with animals were in accordance with the guidelines of the animal care and use committees at Tokyo Institute of Technology. Generation of periostin−/− mice was described previously (14). tenascin-C−/− mice were generated as reported previously (20).
Cell Culture and Transfection
Calvarial osteoblast (COB) cells were prepared according to the standard collagenase method. The COB cells were maintained in α-minimal essential medium (Nacalai Tesque) supplemented with 10% fetal bovine serum (JRH Biosciences, Inc., Lenexa, KS), 100 units/ml penicillin, and 100 μg/ml streptomycin. C3H10T1/2 cells, 293T cells, and Plat-E retrovirus-packaging cells (27), were maintained in low glucose Dulbecco's modified Eagle's medium (Nacalai Tesque) supplemented with 10% fetal bovine serum and antibiotics. Cells were transfected with plasmid DNAs by using polyethyleneimine (Sigma-Aldrich (28)).
Immunoprecipitation
Cellular extracts were prepared with extraction buffer (25 mm HEPES-NaOH, pH 7.0, 150 mm NaCl, 0.5% Nonidet P-40) on ice. Clarified lysates were incubated for 1 h with anti-HA antibody-conjugated agarose (Sigma-Aldrich) or protein G-Sepharose (Amersham Biosciences) at 4 °C. The bound proteins were eluted with SDS sample buffer containing 50 mm dithiothreitol.
Formation of the Meshwork Architecture in the in Vitro COB Cell Culture
COB cells were cultured for 3 weeks with α-minimal essential medium supplemented with 5% fetal bovine serum, in which serum fibronectin had been depleted with gelatin-Sepharose (Amersham Biosciences). Depletion of fibronectin was checked by Western blot (data not shown).
Retrovirus Production and Infection
Plat-E retrovirus-packaging cells were transfected with the retroviral vectors, respectively. The virus-containing medium was collected and filtrated. COB cells were infected with a viral supernatant containing 8 μg/ml Polybrene (Sigma-Aldrich) for 6 h. The infected COB cells were cultured in the presence of 4 μg/ml puromycin (Nacalai Tesque) and then fluorescently stained.
Solid-phase Binding Assay
Purification of recombinant periostin proteins was described previously (29). Microtiter plates were coated with 10 μg/ml purified human tenascin-C proteins (Chemicon-Millipore) diluted in PBS overnight at 4 °C. The plates were blocked for 1 h at room temperature with PBS containing 5% BSA. Next, the plates were incubated with 12 μg/ml ΔEMIΔCTR-HA in PBS for 2 h at room temperature, and washed with PBS. Bound ΔEMIΔCTR-HA was detected by using anti-HA antibody followed by incubation with horseradish peroxidase-conjugated anti-mouse antibody. The plates were washed, incubated with peroxidase substrate O-phenylenediamine (0.4 mg/ml) in 50 mm citrate buffer (pH 4.6), 0.003% H2O2 for 30 min, and then read at 490 nm.
Immunofluorescence Microscopy
Cells were fixed with fresh 4% paraformaldehyde (PFA) in PBS for 15 min at room temperature. After a wash with PBS, the cells were incubated with 10 μg/ml glycine in PBS to block free aldehyde groups for 10 min, and then with 0.1% Triton X-100 for 5 min at room temperature. For observation of type I collagen, cells were fixed with cold acetone. After blocking with 1% BSA in TBS (pre-heated at 56 °C for 30 min) for 30 min at room temperature, the cells were incubated with the primary antibodies in 1% BSA in TBS for 2 h at room temperature. Further incubation with an optimal dilution of fluorescence-tagged secondary antibodies was performed for 1 h, followed by washing and post-fixation with 4% PFA. Nuclei were stained with TO-PRO-3 (Molecular Probes, Invitrogen). Fluorescent images were collected on a laser-scanning confocal microscopy (FV1000-BX61, Olympus, Japan) with 40× UPlanApo numerical aperture 0.85 and 100× UPlanApo numerical aperture 1.35 objective lenses using imaging software (Fluoview version 1.6a, Olympus). Images were imported into Photoshop (version CS2, Adobe) for cropping and linear contrast adjustment.
In Situ Proximity Ligation Assay
PLA was performed to visualize protein-protein interactions using fluorescent microscopy. In brief, oligonucleotide conjugated “probe” antibodies are directed against primary antibodies. Annealing of the probes occurs when the target proteins are in close proximity, which initiates the amplification of a reporter signals. C3H10T1/2 cells, which harbor an empty vector or periostin expression vector, were grown on coverslips with α-minimal essential medium/5% fetal bovine serum (fibronectin-depleted) for 1 week in a confluent condition. Cells were fixed with 4% PFA in PBS for 10 min, blocked, and permeabilized with 1% BSA containing 0.2% Triton X-100 and incubated overnight with mouse anti-HA and rabbit anti-tenascin-C antibodies. A PLA was performed according to the manufacturer's protocol using the Duolink Detection Kit with PLA PLUS and MINUS probes for rabbit and mouse antibodies (Olink Bioscience, Nacalai Tesque).
Immunohistochemistry
For tissue preparation, mice were sacrificed after surgery under anesthesia, fixed with 4% PFA in PBS by transcardial perfusion and immediately immersed in 4% PFA in PBS at 4 °C for 12 h. After a wash with PBS, the samples were decalcified in 10% EDTA solution at 4 °C for 3 weeks, and then were dehydrated through a graded series of ethanol prior to embedding in paraffin. Series of 4- to 5-μm-thick paraffin sections were prepared for histological analysis or for immunostaining. For immunostaining of fibronectin, antigen-unmasking techniques with citrate buffer and signal amplification with the TSA (tyramide signal amplification) system (PerkinElmer Life Sciences) were performed. Histological images were collected on a bright-field microscopy (Axioskop, Zeiss) with a charge-coupled device camera (AxioCam HRc, Zeiss), 20× Plan-Neofluar numerical aperture 0.50, and 40× Plan-Neofluar numerical aperture 0.75 objective lenses using imaging software (AxioVision Release 4.4, Zeiss). Images were imported into Photoshop (version CS2) for cropping.
Soft X-ray Analysis
To take radiographs of 22- to 28-week-old WT (n = 15), periostin−/− (n = 22), and tenascin-C−/− (n = 6) mice, we used an x-ray imaging system, the μFX-1000 (Fujifilm, Tokyo, Japan). The skeletons were fixed in 4% PFA and were exposed to x-rays at 0.1 mA and 25 kV for 5 s. The radiographs were scanned with a BAS-2000 IP Reader (Fujifilm). Image analyses were performed with Photoshop (version CS2) for cropping and linear contrast adjustment.
TEM Analysis
Transmission electron microscopy (TEM) analysis of 16-week-old WT and periostin−/− femurs and tibiae was performed as described previously (11).
Quantification of Collagen Cross-linkages and Collagen Contents
Cortical bones of the femur dyaphysis of 14-week-old WT (n = 6) and periostin−/− mice (n = 6) were used. Femur metaphyses, bone marrow, and periosteum were removed. The periosteum was prepared from both tibias and femurs. Pyridinoline, deoxypyridinoline, and total collagen content were determined by using the high-performance liquid chromatography method (30).
Multiple Sequence Alignment of EMI Domains
The alignment was constructed manually on the basis of the GENETYX-MAC (version 13.0.3) results. Protein names and GenBank™ identifiers were as follow: mouse periostin, NM_015784; human periostin, NM_006475; mouse βigh3, NM_009369; human βigh3, NM_000358; mouse Emid1, NM_080595; mouse Emid2, NM_024474; mouse Emilin1, NM_133918; mouse Emilin2, NM_145158; mouse Emilin3, NM_182840; and mouse Multimerin1, AK137556.
Statistics
Data were summarized as the mean ± S.E. Statistical significance was assessed by use of the unpaired t test (p values < 0.05 were considered significant).
RESULTS
Disruption of Collagen Fibrillogenesis in Periosteum of Periostin−/− Mice
Periostin is expressed in periosteum of mice and human (9), which is a collagenous connective tissue surrounding the bone surface, and is connected to muscle. Bone histomorphometric analysis of periostin−/− mice was previously performed, indicating that overall bone contours are unaffected in the absence of periostin (12). We then investigated whether periosteum of the periostin−/− mice was intact, compared with that of the WT mice in detail. Histological sections showed no apparent abnormality of periosteum in the femur from the periostin−/− mice (supplemental Fig. S1). peripheral quantitative computed tomography analysis of cortical bone in the femur showed a decrease in the area and thickness of 84.4% and 86.5%, respectively, in periostin−/− mice (supplemental Table 1). However, the bone mineral density of the periostin−/− cortical bone was indistinguishable from that in the WT one (supplemental Table 1). We next examined the amount of collagen and of collagen cross-linkage in the cortical bone of femurs. The pyridinoline and deoxypyridinoline (cross-linked peptides) content per mole of collagen was decreased in the periostin−/− femur, whereas the collagen content in the femur was not altered (Fig. 1A). The pyridinoline content was also decreased in the periostin−/− periosteum, compared with the WT counterpart (Fig. 1A). The deoxypyridinoline was not detected in periosteum from both the WT and periostin−/− mice (data not shown). These results of collagen deficiency in bone are consistent with those of previous studies on the infarcted myocardium and tendons (14, 17).
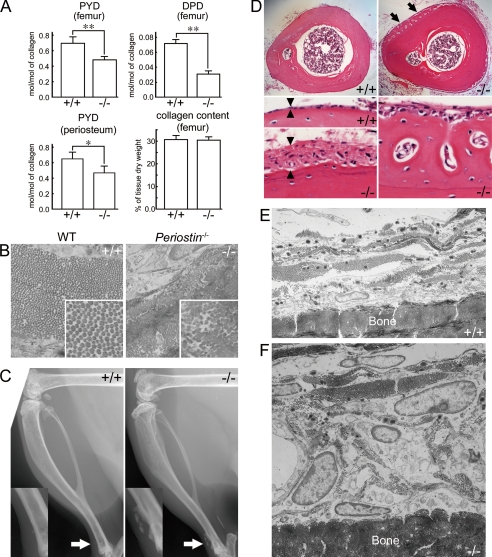
FIGURE 1.
Confined tibial periostitis in periostin−/− mice. A, quantitative analysis of collagen cross-links in femur and periosteum from femur and tibia, and total collagen amount in the femur of 14-week-old WT (+/+, n = 6) and periostin−/− (−/−, n = 6) mice. Bars represent the mean ± S.E. *, p < 0.01; **, p < 0.005. PYD, pyridinoline; DPD, deoxypyridinoline. B, TEM images of cross-sections of collagen fibrils in the osteoblastic layer of the periosteum from 16-week-old mice. Higher magnification images are shown in the white rectangular boxes. C, soft x-ray photomicrographs of hind legs from 7-month-old WT and periostin−/− mice. High magnification images of the areas indicated by the arrows are shown in the rectangular boxes. The arrow indicates ectopically mineralized deposits in the lower region of the tibia from the periostin−/− mouse. D, histological sections of the lower region of tibiae from 16-week-old WT (+/+) and periostin−/− (−/−) mice. Arrows indicate ectopic bone on the cortical surface. Arrowheads indicate the thickness of the periosteum. The right lower column indicates the ectopically mineralized deposit on the tibial surface of the periostin−/− mice. E and F, TEM images of periosteum with cross-sections in the lower region of tibiae from 16-week-old WT (E; +/+) and periostin−/− (F; −/−) mice.
To study collagen fibrillogenesis in the periostin−/− mice, we examined the ultrastructure of collagen fibrils in the femoral periosteum by TEM. The periosteum is composed of an osteoblastic layer and a fibrous layer (supplemental Fig. S2), and the cross-sections of collagen fibrils in the osteoblastic layer were examined at a higher magnification (Fig. 1B). Collagen fibrils were coarse and irregular in size and contour in the periostin−/− mice (Fig. 1B, −/−), in contrast to the regular and uniform fibrils in the WT mice (Fig. 1B, +/+). The aberrant nature of these fibrils appeared to be due to multiple and concurrent lateral fusions (Fig. 1B, −/−). These results indicate that collagen fibrillogenesis is disrupted in the periosteal osteoblasts of periostin−/− mice.
Confined Tibial Periostitis in Periostin−/− Mice
The abnormality of the collagen fibrils probably indicates dysfunctions of the periosteum in the periostin−/− mice. We then scrutinized skeletal differences between WT and periostin−/− mice by soft x-ray analysis. We found ectopically mineralized deposits on the surface of the lower region of the tibia from 7-month-old periostin−/− mice (Fig. 1C, arrow). The ectopically mineralized deposit was observed in 20 periostin−/− mice (total of 22 periostin−/− mice); in contrast, this was observed in one WT mouse (total of 15 WT mice). Interestingly, these ectopically mineralized deposits were not found except in the lower region of periostin−/− tibia (data not shown). We then histologically examined the tibia from 20-week-old mice. Cross-sections of the lower one-third region of the tibia revealed a thickened periosteum and extra bone tissue on the cortical surface of the periostin−/− tibia, compared with the thin periosteum of the WT tibia (Fig. 1D). TEM analysis showed an increased number of cells that contained a large nucleus and massive cytoplasm in the periostin−/− periosteum (Fig. 1F), compared with the WT one (Fig. 1E). These characteristic features seen in the periostin−/− tibia appear as periostitis.
Involvement of Tenascin-C in the Confined Tibial Periostitis
To study an involvement of tenascin-C in the confined tibial periostitis of periostin−/− mice, we examined the in vivo deposition of tenascin-C proteins in the periosteum. We then stained paraffin-embedded sections of the femurs with hematoxylin and eosin, anti-fibronectin, anti-type I collagen, and anti-tenascin-C antibodies. Histological sections showed no apparent abnormality in the periostin−/− periosteum, compared with the WT counterpart (Fig. 2A). Immunolocalization of fibronectin and type I collagen also exhibited similar patterns between the WT and periostin−/− periosteum (Fig. 2A and supplemental Fig. S3). A strong immunoreactivity for tenascin-C was detected throughout the osteoblastic layer in the WT periosteum (Fig. 2A). In contrast, a weak and scattered immunolocalization of tenascin-C was discernible in the periostin−/− periosteum (Fig. 2A, arrows). These results indicate that deletion of periostin reduced the deposition of tenascin-C on the ECM.
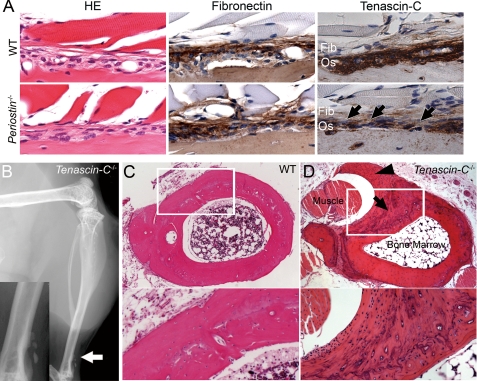
FIGURE 2.
Tenascin-C is involved in the confined tibial periostitis. A, histological sections of periosteum of femur from 16-week-old mice. Sections of the periosteum from WT and periostin−/− mice was stained with hematoxylin and eosin, anti-fibronectin, and anti-tenascin-C antibodies. The immunoreactivity for tenascin-C is reduced in the periostin−/− periosteum (arrows). B, periostitis in the lower region of a tibia from a tenascin-C−/− mouse. Soft x-ray photomicrograph of a hind leg from a 24-week-old tenascin-C−/− mouse. A high magnification image of the area indicated by the arrow is shown in the rectangular box. C and D, histological sections of the lower region of a tibia from a 22-week-old WT mouse (C) and tenascin-C−/− counterpart (D). The arrow indicates ectopic mineralized deposits; and the arrowhead, a thickened periosteum surrounding the tibialis muscle. Highly magnified images of the white rectangular box are shown in the lower columns.
To examine whether the confined tibial periostitis is due to the reduced deposition of tenascin-C in the periostin−/− periosteum, we examined the hind legs of 24-week-old tenascin-C−/− mice. Soft x-ray analysis also revealed ectopically mineralized deposits on the surface of the lower region of the tibia in five tenascin-C−/− mice (total of six tenascin-C−/− mice) (Fig. 2B, arrow). Cross-sections of the lower one-third region of the tibia in tenascin-C−/− mice showed a thickened periosteum surrounding the tibialis muscle and ectopically mineralized bone on the cortical surface of the tibia (Fig. 2D). Thus, the reduced deposition of tenascin-C on the ECM architecture is likely to be involved in the development of the tibial periostitis in periostin−/− mice.
Meshwork Architecture of the ECM Organized by Periostin and Tenascin-C
To investigate effects of the lack of periostin and reduced deposition of tenascin-C, we scrutinized the ECM architecture. Because there is a definite ceiling on histological analysis in vivo, we visualized the ECM architecture by staining an in vitro cell culture with an anti-fibronectin antibody. The ECM architecture is constructed from a framework of fibronectin matrix, which is required for the assembly of multiple ECM proteins (31). Primary COB cells were obtained from WT and periostin−/− neonatal mice and cultured for 3 weeks in the confluent condition. Notably, the immunofluorescent visualization of the ECM in the WT COB cell cultures revealed substantial branched connections between the ECM fibrils that had become integrated in an extensively complex meshwork architecture (Fig. 3A). In contrast, the meshwork architecture was rarely observed in the periostin−/− COB cell cultures (Fig. 3A); branched connections between fibrils were scarce, and the fibrils were thicker than those in the WT cell cultures. These fibrils appeared to be laterally associated with each other, forming a thick and coarse fiber (Fig. 3A, arrowheads). In addition, type I collagen was co-aligned with the fibronectin fibrils both in WT and periostin−/− COB cell cultures (supplemental Fig. S4). Thus, these results indicate that periostin plays a role in construction of the extracellular meshwork architecture.
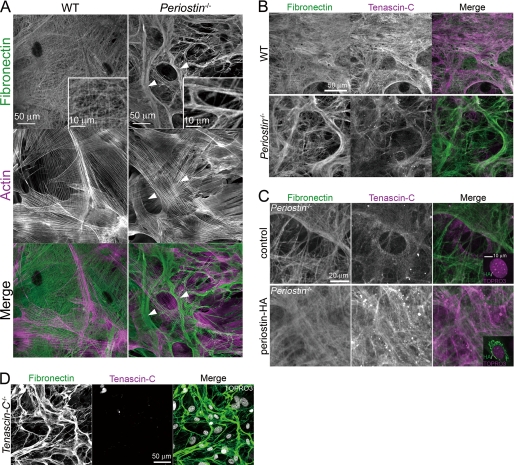
FIGURE 3.
Periostin and tenascin-C are essential for organization of the extracellular meshwork architecture. A, detection of fibronectin and actin in COB cell cultures from neonatal mice by immunofluorescence. WT COB cells show a fine meshwork architecture of fibronectin matrix. In contrast, periostin−/− COB cells show thick and coarse fibers (arrowheads). A higher magnification image is shown in the white rectangular box. B, detection of fibronectin and tenascin-C in the COB cell cultures by immunofluorescence, showing reduced fluorescent signals for tenascin-C in the periostin−/− COB cell culture, compared with those in the WT COB cell culture. C, stable expression of periostin-HA restored the extracellular meshwork architecture in the periostin−/− COB cell culture. The periostin−/− COB cells were infected with pMXs-puro (control) or pMXs-puro-periostin-HA, cultured in the presence of puromycin, and then stained with anti-fibronectin and anti-tenascin-C antibodies. The infected cells were also stained with anti-HA and TOPRO3 (shown in the right lower rectangular boxes, respectively). D, disorganization of the extracellular meshwork architecture in the tenascin-C−/− COB cell culture. Fibronectin and tenascin-C in the tenascin-C−/− COB cell culture were detected by immunofluorescence. Nuclei are stained with TOPRO3 in the merged image.
To ask whether tenascin-C is involved in construction of the extracellular meshwork architecture, we analyzed the deposition of tenascin-C proteins on the meshwork architecture. WT COB cells deposited tenascin-C predominantly on the meshwork, similar to the deposition of fibronectin (Fig. 3B). periostin−/− COB cells also deposited tenascin-C on their thick and coarse fibers; however, the deposition was drastically decreased, and not uniform, when compared with that in the WT counterparts (Fig. 3B). To confirm disruption of the extracellular meshwork architecture and reduced deposition of tenascin-C in the absence of periostin, we performed a rescue experiment. Stable expression of periostin-HA by retroviral infection restored the meshwork architecture and deposition of tenascin-C in the periostin−/− COB cell culture (Fig. 3C), demonstrating that periostin plays a role in construction of the extracellular meshwork architecture and the deposition of tenascin-C.
To test whether disruption of the extracellular meshwork architecture in the periostin−/− COB cell culture is due to the reduced deposition of tenascin-C on the ECM, we examined the ECM architecture derived from primary tenascin-C−/− COB cell cultures and found that the meshwork architecture was rarely observed (Fig. 3D). Expression of periostin was not altered in the tenascin-C−/− COB cells (data not shown). These results demonstrate that deposition of tenascin-C on the ECM is required for organization of the extracellular meshwork architecture.
Four Tandem Repeats of fas1 Domain of Periostin Interact with Tenascin-C
To provide a mechanistic insight into how periostin incorporates tenascin-C into the ECM and organizes the extracellular meshwork architecture, we examined the interaction between periostin and tenascin-C. Periostin consists of six domains: the N-terminal EMI domain, four tandem repeats of the fas1 domain, and the C-terminal region (CTR) (9). Takayama et al. showed that the CTR deletion form of periostin directly binds to tenascin-C higher than intact periostin in a solid-phase binding assay (23). Furthermore, our previous study demonstrated that the CTR of periostin is cleaved in the infarcted myocardium (14). We first confirmed the CTR cleavage in the periosteum. Tissue lysates were prepared from the periosteum of mouse tibias, subjected to SDS-PAGE, and then blotted with anti-periostin antibodies (anti-RD1 and anti-CT, the recognition sites of these antibodies are shown in Fig. 4A). We found lower molecular mass bands recognized by anti-RD1, but not by anti-CT (Fig. 4B). The sizes of these bands were ∼84 and 74 kDa, indicating the cleavages in the CTR of periostin.
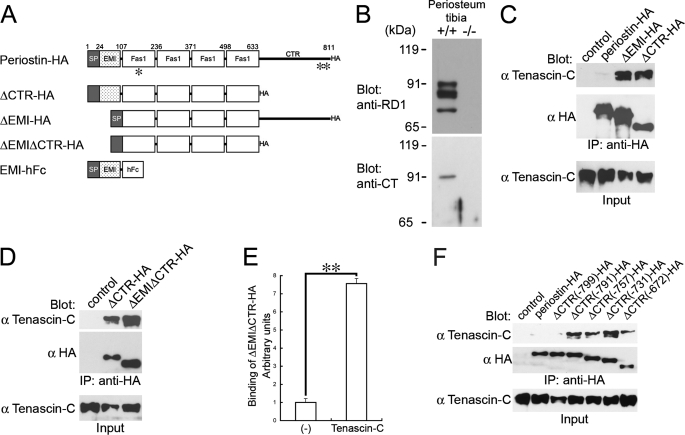
FIGURE 4.
Cleavage of the CTR of periostin is required for the interaction between periostin and tenascin-C. A, domain structures of intact and domain deletion forms of periostin. The EMI domain was expressed as a human Fc (hFc) fusion protein. Recognition sites of the antibodies for periostin are indicated as asterisks (*, anti-RD1; **, anti-CT). SP indicates signal peptide. B, cleavage of periostin CTR in the periosteum. Tissue lysates from the periosteum of tibia were subjected to SDS-PAGE. Proteins were visualized by blotting with anti-RD1 and anti-CT antibodies. Bands corresponding to intact periostin are located at the 90-kDa position; those of cleaved periostin are located at ∼84 and 74 kDa. +/+, wild type; −/−, periostin−/−. C and D, co-immunoprecipitation assays for tenascin-C and periostin-HA or the deletion forms. 293T cells were co-transfected with tenascin-C and periostin-HA or periostin deletion forms. As a control, an empty vector was co-transfected with tenascin-C. The transfected deletion forms are shown above each lane. Blot, antibody used for Western blot analysis; IP, antibody used for immunoprecipitation; Input, crude transfected 293T cell lysates. E, solid-phase binding assay for interaction between tenascin-C and ΔEMIΔCTR-HA. Microtiter plates coated with purified tenascin-C were incubated with ΔEMIΔCTR-HA. Bound ΔEMIΔCTR-HA was detected with anti-HA antibody. Bars represent the mean ± S.E. **, p < 0.005. F, co-immunoprecipitation assay between tenascin-C and serial deletion forms of CTR. The transfected deletion forms are shown above each lane.
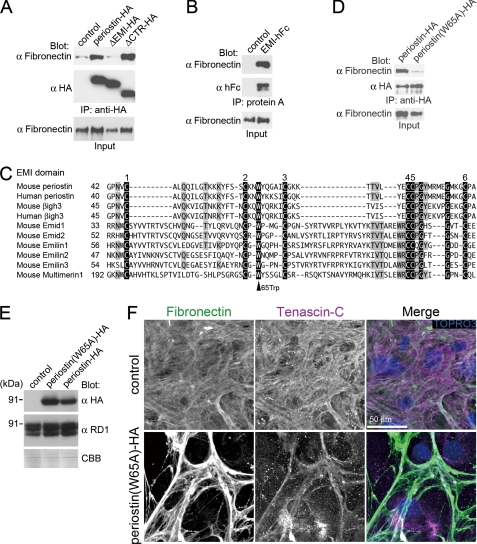
To assess the effect of deletion of the CTR on the interaction with tenascin-C, we performed a co-immunoprecipitation assay. We then generated expression vectors of intact and deletion forms of periostin that had been conjugated with an HA tag at their C-terminal end. The structures of these representative constructs are shown in Fig. 4A. We stably transfected 293T cell lines with the expression vectors. Co-immunoprecipitation was performed with cell lysates from the 293T stable transfectants that had been transiently transfected with expression vectors of tenascin-C. As we predicted, the deletion form of the CTR (ΔCTR-HA) interacted with tenascin-C, although the intact periostin-HA did not (Fig. 4C). The deletion form of the EMI domain (ΔEMI-HA) also interacted with tenascin-C (Fig. 4C). Moreover, the deletion form for both the CTR and the EMI domains (ΔEMIΔCTR-HA) interacted with tenascin-C (Fig. 4D). Furthermore, purified ΔEMIΔCTR-HA proteins bound to tenascin-C coated on a microtiter plate (Fig. 4E), indicating that the four tandem repeats of fas1 domain is the direct binding region for tenascin-C. Because the intact periostin-HA did not interact with tenascin-C, we speculated that there was an inhibitory region present in the CTR. To identify the inhibitory region, we generated five deletion forms of the CTR, as shown in Fig. 4F. ΔCTR(-799)-HA, in which the region C-terminal to amino acid position 799 had been deleted, failed to interact with tenascin-C, whereas ΔCTR(-791)-HA and other shorter deletion forms interacted with tenascin-C (Fig. 4F), indicating that the amino acids at 791–799 are responsible for the inhibition. This region corresponds to the predicted cleavage sites. Taken together, these results suggest that the CTR cleavage enables the fas1 domains of periostin to interact with tenascin-C.
Spatiotemporal Regulation of the Interaction between Periostin and Tenascin-C
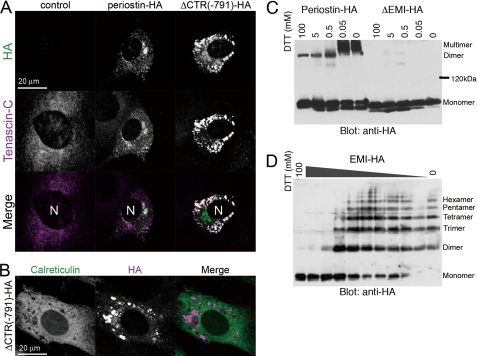
To validate necessity of the CTR cleavage, we set a situation of the interaction between periostin and tenascin-C in the endoplasmic reticulum (ER). We then transiently transfected C3H10T1/2 cells with empty vector (control), periostin-HA, and the CTR deletion form and detected the localization of tenascin-C. Tenascin-C was diffuse inside the 10T1/2-control cells (Fig. 5A, left columns). In the 10T1/2-periostin-HA cells, tenascin-C was partially co-localized with periostin-HA, causing slight aggregation of tenascin-C (Fig. 5A, middle columns). In contrast, ΔCTR(-791)-HA yielded abundant aggregates with tenascin-C in the ER (Fig. 5, A and B). The structure of tenascin-C is a disulfide-bonded hexabrachion (32). Periostin also formed a disulfide-bonded multimer that was mediated by the EMI domain (Fig. 5C). Furthermore, EMI-HA, the EMI domain conjugated with HA tag at its C-terminal end, formed disulfide-bonded multimers (Fig. 5D), suggesting that consecutive interaction between tenascin-C hexabrachions and the multimerized CTR deletion forms in the ER causes huge aggregations of these proteins. These results suggest that the CTR is a safety lock to prevent aggregation between periostin and tenascin-C in the ER.
FIGURE 5.
The CTR deletion form of periostin aggregates with tenascin-C in the ER. A, the CTR deletion form of periostin aggregates with tenascin-C inside the cell. C3H10T1/2 cells were co-transfected with tenascin-C and an empty vector (control), periostin-HA, or ΔCTR(-791)-HA, and then fluorescently stained with anti-tenascin-C and anti-HA antibodies. N indicates the nucleus. B, aggregates of ΔCTR(-791)-HA are localized at the ER. Immunofluorescence detection of calreticulin, an ER chaperone, and ΔCTR(-791)-HA in the C2H10T1/2 cells co-transfected with tenascin-C and ΔCTR(-791)-HA. N indicates the nucleus. C, disulfide bond-mediated multimerization of periostin. Culture supernatants from 293T cells expressing periostin-HA or ΔEMI-HA were mixed with SDS sample buffer containing the indicated concentration of dithiothreitol. The samples were applied to SDS-PAGE and blotted with anti-HA antibody. D, the EMI domain is responsible for the multimerization of periostin. 293T cells expressing the C-terminal HA-tagged EMI domain (EMI-HA) were lysed with SDS sample buffer containing the indicated concentration of dithiothreitol. The EMI domain forms dimers to hexamers.
Close Proximity between Periostin and Tenascin-C
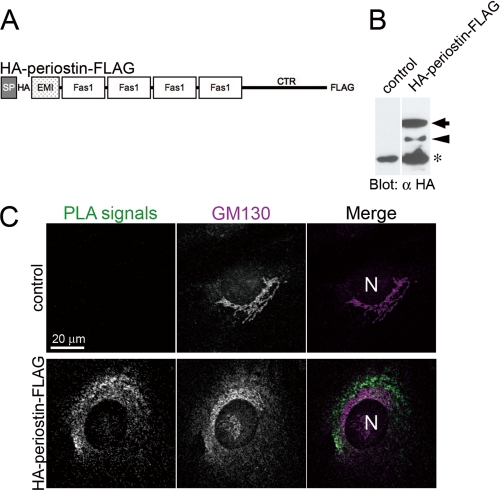
Interestingly, fluorescent signals for periostin were rarely detected in the ECM but in the Golgi of the WT COB cells (supplemental Fig. S5). To investigate where periostin interacts with tenascin-C, we visualized the interaction by performing the in situ PLA, which depicted close proximity (≤40 nm) of cellular molecules as fluorescent signals (PLA signals) (33). Because the cleavage of the CTR is essential for the interaction, we generated a construct of periostin with an HA tag conjugated at its N-terminal end and a FLAG tag at its C-terminal end (HA-periostin-FLAG, Fig. 6A), stably transfected into fibroblastic C3H10T1/2 cells (Fig. 6B), and performed the in situ PLA between HA-periostin-FLAG and tenascin-C. The C3H10T1/2-control and -HA-periostin-FLAG were stained with rabbit anti-tenascin-C and mouse anti-HA antibodies, followed by incubation with anti-rabbit PLUS probe and anti-mouse MINUS probe. If the PLUS and MINUS probes proximate each other, PLA signals emerge as bright fluorescent dots at the proximity site. The in situ PLA is sensitive enough to detect single molecule or interaction. PLA signals between HA-periostin-FLAG and tenascin-C were detected in the periphery of and inside the cells, not in the ECM (Fig. 6C). Although the in situ PLA demonstrates only a close proximity, not a direct binding, these results indicate a possibility of the interaction between periostin and tenascin-C in the matricellular space or inside the cells.
FIGURE 6.
Close proximity between periostin and tenascin-C. A, schematic view of dual tagged periostin (HA tag just after the signal peptide; FLAG tag at the C-terminal end). B, cleavage of HA-periostin-FLAG. C3H10T1/2 cells were stably transfected with an empty vector (control) and HA-periostin-FLAG, and then subjected to SDS-PAGE followed by Western blot analysis by using anti-HA antibody, respectively. The arrow indicates the intact form, and the arrowhead indicates the cleaved form. The asterisk indicates a nonspecific signal. C, in situ PLA between HA-periostin-FLAG and tenascin-C in the C3H10T1/2 cells, which harbor an empty vector (control) and HA-periostin-FLAG expression vector (HA-periostin-FLAG). PLA signals, which indicate close proximity between HA-periostin-FLAG and tenascin-C, are detected in the periphery or inside the cells. The Golgi was stained with anti-GM130 antibody. N, nucleus.
The EMI Domain of Periostin Interacts with Fibronectin
Although periostin interacts with fibronectin, type I collagen, and type V collagen (17, 23), the interaction domain has not been identified. To investigate the mechanism by which periostin incorporates tenascin-C into the ECM, we focused on the N-terminal EMI domain of periostin. EMI domain was identified as a small module rich in cysteines and found in EMILINs and multimerin, which are glycoproteins of the ECM (34). Functional data available for EMILIN proteins suggest that the EMI domain could be a protein-protein interaction module (34). Because the previous study demonstrated that periostin binds directly to fibronectin in a solid-phase binding assay (23) and our unpublished data, we investigated the binding domain of periostin for fibronectin. We then established the stable 293T transfectants with the expression vectors of intact and deletion forms of periostin-HA, respectively. Co-immunoprecipitation was performed with cell lysates from these 293T stable transfectants that had been transiently transfected with expression vectors of fibronectin. Periostin-HA co-immunoprecipitated with fibronectin (Fig. 7A). The deletion form of the N-terminal EMI domain (ΔEMI-HA) did not co-immunoprecipitate with fibronectin (Fig. 7A), indicating that the EMI domain is necessary for the interaction with fibronectin. To test if the EMI domain is sufficient for the interaction, we examined the interaction of fibronectin with the EMI domain conjugated with human Fc fusion protein at its C-terminal end (EMI-hFc, shown in Fig. 4A). The protein A binding to hFc pulled down EMI-hFc and fibronectin proteins (Fig. 7B), indicating that the EMI domain interacts with fibronectin. EMI domains contain a highly conserved tryptophan residue at position 65 in periostin (Fig. 7C). We then substituted the tryptophan residue to alanine (Trp-65 → Ala). The substitution of Trp-65 → Ala inhibited the interaction between periostin and fibronectin (Fig. 7D). These results indicate that the EMI domain, especially the tryptophan residue at position 65, is involved in the interaction with fibronectin. We also found that the EMI-hFc directly binds to type I collagen coated on a microtiter plate (data not shown). These results indicate that the EMI domain and the four tandem repeats of fas1 domain bind to the ECM proteins (e.g. fibronectin and type I collagen), and tenascin-C, respectively.
FIGURE 7.
Periostin functions as a bridge for the interaction between tenascin-C and the ECM. A, Western blot of immunoprecipitates from transfected 293T cell lysates. 293T cells were co-transfected with fibronectin and periostin-HA or periostin deletion forms. As a control, an empty vector was co-transfected with fibronectin. The transfected deletion forms are shown above each lane. Blot, antibody used for Western blot analysis; IP, antibody used for immunoprecipitation; Input, crude transfected 293T cell lysates. B, Western blot of immunoprecipitates from transfected 293T cell lysates. 293T cells were co-transfected with fibronectin and an empty vector (control) or EMI-hFc. C, the conserved tryptophan residue in EMI domains. Conserved amino acids are shaded gray. The six conserved cysteines are represented as white on a black background, as well as the strictly conserved glycine and tryptophan. D, the conserved tryptophan residue is required for the interaction with fibronectin. The Western blot represents immunoprecipitates from transfected 293T cell lysates. 293T cells were co-transfected with fibronectin and periostin-HA or the amino acid-substituted form, in which the tryptophan residue was substituted with alanine (W65A). E, expression of the Trp-65 → Ala mutant form in the WT COB cells. The WT COB cells were infected with pMXs-puro (control), pMXs-puro-periostin(W65A)-HA, or pMXs-puro-periostin-HA, cultured in the presence of puromycin. Total cell lysates from the infected COB cells were subjected to SDS-PAGE, followed by Western blot with anti-RD1, and anti-HA antibodies. The gel stained with Coomassie Brilliant Blue is shown as an internal control. F, expression of the Trp-65 → Ala mutant form disturbs organization of the fibronectin meshwork. The infected WT COB cells were cultured in the presence of puromycin and then stained with anti-fibronectin and anti-tenascin-C antibodies. Nuclei were stained with TOPRO3.
Periostin, a Bridge between Tenascin-C and the ECM
The results above raise the possibility that periostin apposes tenascin-C and the ECM in a close proximity and support the deposition of tenascin-C on the ECM. To assess this possibility, we performed a dominant-negative experiment by using the Trp-65 → Ala mutant form of periostin. The Trp-65 → Ala mutant form did not bind to fibronectin (Fig. 7D), whereas the Trp-65 → Ala mutant form possesses the binding domain to tenascin-C (Fig. 4). If periostin functions as a bridge for the interaction between the ECM and tenascin-C, the Trp-65 → Ala mutant form competitively disturbs the function of periostin. We then generated WT COB cells that stably expressed the Trp-65 → Ala mutant form by retroviral infection and confirmed this expression by Western blot analysis (Fig. 7E). Stable expression of the Trp-65 → Ala mutant form in WT COB cells reduced deposition of tenascin-C on the ECM, which resulted in disorganization of the extracellular meshwork architecture (Fig. 7F). On the other hand, stable expression of intact periostin-HA in WT COB cells did not affect the extracellular meshwork architecture (data not shown). These results indicate that the Trp-65 → Ala mutant form inhibits incorporation of tenascin-C into the ECM. Thus, we propose that periostin functions as a bridge between tenascin-C and the ECM, which may result in the incorporation of tenascin-C into the ECM fibrils.
DISCUSSION
Our findings provide a molecular mechanism for construction of the extracellular meshwork architecture composed of substantial branched connections between the ECM fibrils. The present study identifies periostin as a bridge between the ECM fibrils and tenascin-C to support incorporation of tenascin-C into the ECM. The incorporation of tenascin-C hexabrachions may increase bifurcations of the ECM fibrils, which underlie the meshwork architecture of the ECM fibrils. Furthermore, we found confined tibial periostitis both in periostin−/− mice and tenascin-C−/− mice, suggesting a physiological role of the extracellular meshwork architecture in periosteum homeostasis.
Confined Tibial Periostitis Both in Periostin−/− Mice and Tenascin-C−/− Mice
The periosteum serves as a transmitter of muscle contractility to the bones, indicating that a balance between mechanical property of the periosteum and amplitude of muscle contractile force underlies the structural integrity of the periosteum. Loading of muscle contractile force on the periosteum causes stretching of the ECM architecture, and overloading disrupts the ECM architecture and causes periostitis. The permissible force to break down the ECM architecture may be lower in the periosteum of periostin−/− mice and tenascin-C−/− mice than in WT ones. Mechanical strength of the connective tissues is determined by the quality and quantity of collagen fibrils. Collagen fibrils in the periostin−/− periosteum showed an abnormal morphology and reduced covalent cross-linkages. These collagen deficiencies might be involved in the confined tibial periostitis.
In humans, periostitis, a stress reaction in the periosteum (35), is observed in the medial and lower region of the tibia surface, because this region is the attachment site of the tibialis anterior and posterior muscles that stress the periosteum strongly during running and exercise. This periostitis is recognized as the medial tibial stress syndrome in sports injuries, so-called “shin splints” (36), which causes continuous pain along the medial and lower edge of the shin (35). Shin splints is well known to occur not only in athletes but also in growing teenagers (peak at 16 years of age). Although we are not able to explain why the periostitis is confined in the lower region of tibia in mice, the confined tibial periostitis seen both in the periostin−/− mice and tenascin-C−/− mice might correspond to the medial tibial stress syndrome in human sports injuries.
Our studies suggest that the meshwork architecture, organized by periostin, underlies mechanical strength of the connective tissues. The previous study showed that periostin plays a role in the pressure tolerance of the infarcted region of acute myocardial infarction (14), the reason for which may be consistent with that of the development of the confined tibial periostitis in the periostin−/− mice.
The Extracellular Meshwork Architecture Composed of Tenascin-C
A characteristic feature of the extracellular meshwork architecture is the substantial branched connections between the ECM fibrils composed of fibronectin and type I collagen. The extracellular meshwork architecture seems consistent with the structural feature of tenascin-C. Tenascin-C is an extracellular glycoprotein, composed of 14 epidermal growth factor-like repeats and a series of fibronectin type III repeats (32). Notably, tenascin-C forms a typical disulfide-linked hexamer, called the “hexabrachion,” in which six flexible arms emanate from a central globular particle. It is possible that these six flexible arms of tenascin-C catch and stabilize a bifurcation of the ECM fibrils to underlie the extracellular meshwork architecture.
Reduced covalent cross-linkage between collagen fibrils causes a morphological abnormality of collagen fibrils (37–39). Covalent cross-linkage between collagen fibrils depends on lysyl oxidase. Lysyl oxidase has been demonstrated to interact with fibronectin (40). Furthermore, BMP1, which proteolytically activates lysyl oxidase, also interacts with fibronectin (41). Although it remains unclear whether the reduced cross-linkage of collagen fibrils is due to disorganization of the extracellular meshwork architecture, meshwork alignment of fibronectin possibly affects efficiency of the collagen cross-linkage.
The Adjacent Domains of Periostin Support the Deposition of Tenascin-C on the ECM
A defining feature of periostin is its multidomain structure. The dominant negative experiment using the Trp-65 → Ala mutant form of periostin suggests that periostin apposes the ECM fibrils and tenascin-C in a close proximity and supports the incorporation of tenascin-C into the ECM. Although our study identifies periostin as a bridge between the ECM and tenascin-C, the mechanism by which tenascin-C interacts with the ECM fibrils remains elusive. Ingham et al. suggested that the tenascin-C binding site on fibronectin appears to be cryptic in the whole molecule in solution but is exposed on the fibronectin proteolytic fragments (42). However, the dissociation constant, Kd, is ∼1 μm (42), which seems too weak to stabilize the interaction between fibronectin and tenascin-C. On the other hand, tenascin-C has been demonstrated to possess several heparin-binding domains (43–46), indicating the interaction of tenascin-C with heparan sulfate proteoglycans or glycosaminoglycans. Chung et al. demonstrated that heparan sulfate glycosaminoglycans are essential for matrix deposition of tenascin-C (47). Periostin also possesses the heparin-binding domain at the C-terminal end (48). Thus, heparan sulfate proteoglycans or glycosaminoglycans might play a role in periostin-promoted incorporation of tenascin-C into the ECM.
This study possibly indicates that the interaction between periostin and tenascin-C is transient. Periostin-HA did not co-immunoprecipitate with fibronectin in the cell culture media of the 293T transfectants (data not shown), also suggesting that the interaction between periostin and fibronectin is permissible in the matricellular space or inside the cells. These results imply a mechanism that strictly regulates the interaction between periostin and the ECM.
The CTR Cleavage Promotes the Interaction between Periostin and Tenascin-C
Consecutive interaction between tenascin-C hexabrachions and the multimerized cleaved periostin results in an aggregation of these proteins, which possibly takes an advantage in deposition of tenascin-C on the ECM. However, it remains elusive where periostin is cleaved, and exactly interacts with tenascin-C. A recent study showed that periostin appeared to be anchored in the Golgi (49). We also confirmed the localization of periostin in the Golgi. It is noteworthy that a subcellular localization of periostin has also been indicated (50, 51), while secreted periostin is detected in the cell culture medium (9). Many secretory proteins are synthesized as inactive precursors in the ER and are usually cleaved at sites composed of single or paired basic amino acid residues by members of the subtilisin/kexin-like proprotein convertase family (52). We found several candidates of the cleavage sites in the CTR for the proprotein convertases by using a prediction program (53) (data not shown). Furin proprotein convertase, for example, localizes in the trans-Golgi network (54), indicating that the retention of periostin in the Golgi may increase efficiency of the CTR cleavage and of the subsequent interaction between periostin and tenascin-C. On the other hand, furin is shuttled to the cell surface through the trans-Golgi network and endosomal system (55), also suggesting a possibility of the CTR cleavage in the matricellular space. Further studies are necessary to clear these questions.
The current work provides a novel insight into structural homeostasis of the periosteum and other connective tissues that are subjected to a mechanical environment. Mechanical strains have been proposed to increase expression of periostin mRNA (56). Periostin expression is up-regulated by transforming growth factor-β signaling (9, 14), and the release of the active transforming growth factor-β from the ECM is triggered by mechanical stretching (57, 58). In addition, mechanical strains increase the expression of tenascin-C by the cytoskeletal actin-mediated transcriptional regulation (59). Taken together, mechanical strain-mediated strengthening and conditioning of the connective tissues, which may be due to the periostin-mediated incorporation of tenascin-C into the ECM architecture, maintains the structural homeostasis against the mechanical environment.
Supplementary Material
Acknowledgments
We thank Dr. K. Sekiguchi (Osaka University, Japan), Dr. I. Harada (Tokyo Institute of Technology, Yokohama, Japan), and Dr. H. N. Matsumoto (Tokyo Medical and Dental University, Tokyo, Japan) for reading of this manuscript; Dr. S. Torii (Waseda University, Tokorozawa, Japan) for counsel on medial tibial stress syndrome; Dr. K. Takakuda (Tokyo Medical and Dental University, Tokyo, Japan), Dr. I. Harada, Dr. M. Shimazaki, H. Tanabe, and T. Maruhashi for helpful discussion; Dr. K. Sekiguchi for pAIFNBAC; Dr. H. Niwa (RIKEN, Japan) for pCAGIPuro; Dr. T. Kitamura (University of Tokyo, Tokyo, Japan) for pMXs-puro and Plat-E cells; Dr. Y. Azuma (Teijin Institute for Biomedical Research, Japan) for peripheral quantitative computed tomography analysis; and I. Takayama, K. Nakagawa for technical assistance.
This work was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Culture, and Sports of Japan (to I. K. and A. K.) and by grants from the Ground-based Research Program for Space Utilization promoted by Japan Space Forum (to A. K.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. S1–S5.
I. Kii, T. Nishiyama, M. Li, K.-i. Matsumoto, M. Saito, N. Amizuka, and A. Kudo, unpublished observation.
- ECM
- extracellular matrix
- BSA
- bovine serum albumin
- COB
- calvarial osteoblast
- CTR
- C-terminal region
- ER
- endoplasmic reticulum
- HA
- hemagglutinin
- PFA
- paraformaldehyde
- TEM
- transmission electron microscopy
- WT
- wild type
- PLA
- proximity ligation assay
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Cukierman E., Pankov R., Yamada K. M. (2002) Curr. Opin. Cell Biol. 14, 633–639 [DOI] [PubMed] [Google Scholar]
- 2.Bornstein P. (1995) J. Cell Biol. 130, 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw A. D., Puolakkainen P., Dasgupta J., Davidson J. M., Wight T. N., Helene Sage E. (2003) J. Invest. Dermatol. 120, 949–955 [DOI] [PubMed] [Google Scholar]
- 4.Bornstein P. (2000) Matrix Biol. 19, 555–556 [DOI] [PubMed] [Google Scholar]
- 5.Kudo H., Amizuka N., Araki K., Inohaya K., Kudo A. (2004) Dev. Biol. 267, 473–487 [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer J., Becker T., Lefebvre J., Granato M., Schachner M., Becker C. G. (2005) Dev. Dyn 234, 550–566 [DOI] [PubMed] [Google Scholar]
- 7.Koshida S., Kishimoto Y., Ustumi H., Shimizu T., Furutani-Seiki M., Kondoh H., Takada S. (2005) Dev. Cell 8, 587–598 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H., Amizuka N., Kii I., Kawano Y., Nozawa-Inoue K., Suzuki A., Yoshie H., Kudo A., Maeda T. (2004) Anat. Rec. A Discov. Mol. Cell Evol. Biol. 281, 1264–1275 [DOI] [PubMed] [Google Scholar]
- 9.Horiuchi K., Amizuka N., Takeshita S., Takamatsu H., Katsuura M., Ozawa H., Toyama Y., Bonewald L. F., Kudo A. (1999) J. Bone Miner. Res. 14, 1239–1249 [DOI] [PubMed] [Google Scholar]
- 10.Wang D., Oparil S., Feng J. A., Li P., Perry G., Chen L. B., Dai M., John S. W., Chen Y. F. (2003) Hypertension 42, 88–95 [DOI] [PubMed] [Google Scholar]
- 11.Kii I., Amizuka N., Minqi L., Kitajima S., Saga Y., Kudo A. (2006) Biochem. Biophys. Res. Commun. 342, 766–772 [DOI] [PubMed] [Google Scholar]
- 12.Rios H., Koushik S. V., Wang H., Wang J., Zhou H. M., Lindsley A., Rogers R., Chen Z., Maeda M., Kruzynska-Frejtag A., Feng J. Q., Conway S. J. (2005) Mol. Cell. Biol. 25, 11131–11144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oka T., Xu J., Kaiser R. A., Melendez J., Hambleton M., Sargent M. A., Lorts A., Brunskill E. W., Dorn G. W., 2nd, Conway S. J., Aronow B. J., Robbins J., Molkentin J. D. (2007) Circ. Res. 101, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimazaki M., Nakamura K., Kii I., Kashima T., Amizuka N., Li M., Saito M., Fukuda K., Nishiyama T., Kitajima S., Saga Y., Fukayama M., Sata M., Kudo A. (2008) J. Exp. Med. 205, 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snider P., Hinton R. B., Moreno-Rodriguez R. A., Wang J., Rogers R., Lindsley A., Li F., Ingram D. A., Menick D., Field L., Firulli A. B., Molkentin J. D., Markwald R., Conway S. J. (2008) Circ. Res. 102, 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris R. A., Moreno-Rodriguez R. A., Sugi Y., Hoffman S., Amos J., Hart M. M., Potts J. D., Goodwin R. L., Markwald R. R. (2008) Dev. Biol. 316, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norris R. A., Damon B., Mironov V., Kasyanov V., Ramamurthi A., Moreno-Rodriguez R., Trusk T., Potts J. D., Goodwin R. L., Davis J., Hoffman S., Wen X., Sugi Y., Kern C. B., Mjaatvedt C. H., Turner D. K., Oka T., Conway S. J., Molkentin J. D., Forgacs G., Markwald R. R. (2007) J. Cell. Biochem. 101, 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton D. W. (2008) J. Cell Commun. Signal. 2, 9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyama E., Shimazu A., Leatherman J. L., Golden E. B., Nah H. D., Pacifici M. (1996) J. Orthop. Res. 14, 403–412 [DOI] [PubMed] [Google Scholar]
- 20.Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. (1992) Genes Dev. 6, 1821–1831 [DOI] [PubMed] [Google Scholar]
- 21.Imanaka-Yoshida K., Hiroe M., Yoshida T. (2004) Histol. Histopathol. 19, 517–525 [DOI] [PubMed] [Google Scholar]
- 22.Tamaoki M., Imanaka-Yoshida K., Yokoyama K., Nishioka T., Inada H., Hiroe M., Sakakura T., Yoshida T. (2005) Am. J. Pathol. 167, 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takayama G., Arima K., Kanaji T., Toda S., Tanaka H., Shoji S., McKenzie A. N., Nagai H., Hotokebuchi T., Izuhara K. (2006) J. Allergy Clin. Immunol. 118, 98–104 [DOI] [PubMed] [Google Scholar]
- 24.Manabe R., Ohe N., Maeda T., Fukuda T., Sekiguchi K. (1997) J. Cell Biol. 139, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saga Y., Tsukamoto T., Jing N., Kusakabe M., Sakakura T. (1991) Gene 104, 177–185 [DOI] [PubMed] [Google Scholar]
- 26.Niwa H., Burdon T., Chambers I., Smith A. (1998) Genes Dev. 12, 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita S., Kojima T., Kitamura T. (2000) Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 28.Boussif O., Lezoualc'h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7297–7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama I., Kii I., Kudo A. (2009) J. Biochem. 146, 713–723 [DOI] [PubMed] [Google Scholar]
- 30.Saito M., Marumo K., Fujii K., Ishioka N. (1997) Anal. Biochem. 253, 26–32 [DOI] [PubMed] [Google Scholar]
- 31.Pankov R., Yamada K. M. (2002) J. Cell Sci. 115, 3861–3863 [DOI] [PubMed] [Google Scholar]
- 32.Hsia H. C., Schwarzbauer J. E. (2005) J. Biol. Chem. 280, 26641–26644 [DOI] [PubMed] [Google Scholar]
- 33.Söderberg O., Leuchowius K. J., Gullberg M., Jarvius M., Weibrecht I., Larsson L. G., Landegren U. (2008) Methods 45, 227–232 [DOI] [PubMed] [Google Scholar]
- 34.Callebaut I., Mignotte V., Souchet M., Mornon J. P. (2003) Biochem. Biophys. Res. Commun. 300, 619–623 [DOI] [PubMed] [Google Scholar]
- 35.Tweed J. L., Avil S. J., Campbell J. A., Barnes M. R. (2008) J. Am. Podiatr. Med. Assoc. 98, 107–111 [PubMed] [Google Scholar]
- 36.Gaeta M., Minutoli F., Scribano E., Ascenti G., Vinci S., Bruschetta D., Magaudda L., Blandino A. (2005) Radiology 235, 553–561 [DOI] [PubMed] [Google Scholar]
- 37.Hong H. H., Pischon N., Santana R. B., Palamakumbura A. H., Chase H. B., Gantz D., Guo Y., Uzel M. I., Ma D., Trackman P. C. (2004) J. Cell. Physiol. 200, 53–62 [DOI] [PubMed] [Google Scholar]
- 38.Pischon N., Babakhanlou-Chase H., Darbois L., Ho W. B., Brenner M. C., Kessler E., Palamakumbura A. H., Trackman P. C. (2005) J. Cell. Physiol. 203, 111–117 [DOI] [PubMed] [Google Scholar]
- 39.Takaluoma K., Hyry M., Lantto J., Sormunen R., Bank R. A., Kivirikko K. I., Myllyharju J., Soininen R. (2007) J. Biol. Chem. 282, 6588–6596 [DOI] [PubMed] [Google Scholar]
- 40.Fogelgren B., Polgár N., Szauter K. M., Ujfaludi Z., Laczkó R., Fong K. S., Csiszar K. (2005) J. Biol. Chem. 280, 24690–24697 [DOI] [PubMed] [Google Scholar]
- 41.Huang G., Zhang Y., Kim B., Ge G., Annis D. S., Mosher D. F., Greenspan D. S. (2009) J. Biol. Chem. 284, 25879–25888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingham K. C., Brew S. A., Erickson H. P. (2004) J. Biol. Chem. 279, 28132–28135 [DOI] [PubMed] [Google Scholar]
- 43.Aukhil I., Joshi P., Yan Y., Erickson H. P. (1993) J. Biol. Chem. 268, 2542–2553 [PubMed] [Google Scholar]
- 44.Fischer D., Chiquet-Ehrismann R., Bernasconi C., Chiquet M. (1995) J. Biol. Chem. 270, 3378–3384 [DOI] [PubMed] [Google Scholar]
- 45.Weber P., Zimmermann D. R., Winterhalter K. H., Vaughan L. (1995) J. Biol. Chem. 270, 4619–4623 [DOI] [PubMed] [Google Scholar]
- 46.Jang J. H., Hwang J. H., Chung C. P., Choung P. H. (2004) J. Biol. Chem. 279, 25562–25566 [DOI] [PubMed] [Google Scholar]
- 47.Chung C. Y., Erickson H. P. (1997) J. Cell Sci. 110, 1413–1419 [DOI] [PubMed] [Google Scholar]
- 48.Sugiura T., Takamatsu H., Kudo A., Amann E. (1995) Protein Expr. Purif. 6, 305–311 [DOI] [PubMed] [Google Scholar]
- 49.Kim B. Y., Olzmann J. A., Choi S. I., Ahn S. Y., Kim T. I., Cho H. S., Suh H., Kim E. K. (2009) J. Biol. Chem. 284, 19580–19591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Litvin J., Chen X., Keleman S., Zhu S., Autieri M. (2007) Am. J. Physiol. Cell Physiol. 292, C1672–C1680 [DOI] [PubMed] [Google Scholar]
- 51.Yoshioka N., Fuji S., Shimakage M., Kodama K., Hakura A., Yutsudo M., Inoue H., Nojima H. (2002) Exp. Cell Res. 279, 91–99 [DOI] [PubMed] [Google Scholar]
- 52.Zhou A., Webb G., Zhu X., Steiner D. F. (1999) J. Biol. Chem. 274, 20745–20748 [DOI] [PubMed] [Google Scholar]
- 53.Duckert P., Brunak S., Blom N. (2004) Protein Eng. Des. Sel. 17, 107–112 [DOI] [PubMed] [Google Scholar]
- 54.Thomas G. (2002) Nat. Rev. Mol. Cell Biol. 3, 753–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molloy S. S., Anderson E. D., Jean F., Thomas G. (1999) Trends Cell Biol. 9, 28–35 [DOI] [PubMed] [Google Scholar]
- 56.Wilde J., Yokozeki M., Terai K., Kudo A., Moriyama K. (2003) Cell Tissue Res. 312, 345–351 [DOI] [PubMed] [Google Scholar]
- 57.Wipff P. J., Rifkin D. B., Meister J. J., Hinz B. (2007) J. Cell Biol. 179, 1311–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Annes J. P., Chen Y., Munger J. S., Rifkin D. B. (2004) J. Cell Biol. 165, 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiquet M., Gelman L., Lutz R., Maier S. (2009) Biochim. Biophys. Acta 1793, 911–920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.