Abstract
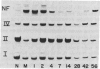
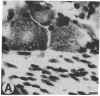
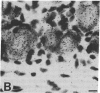
We examined the differential expression of genes encoding three beta-tubulin isotypes (classes I, II, and IV) and the 68-kDa neurofilament protein (NF68) in rat sensory neurons during development, maturation, and axonal regeneration. Expression of the specific beta-tubulin gene encoding the class II isotype was induced to high levels during development and axonal regeneration, whereas the expression of genes encoding the two other isotypes (classes I and IV) remained comparable to mature levels. Conversely, expression of the NF68 gene was relatively low during development and regeneration. Thus, the developmental program for cytoskeletal gene expression is recapitulated during axonal regeneration. The high level of class II beta-tubulin expression found in developing and regenerating neurons occurs during the longitudinal growth of axons. In contrast, induction of NF68 gene expression is associated with the radial growth of axons in maturing neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J Physiol. 1961 Jul;157:315–327. doi: 10.1113/jphysiol.1961.sp006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The multitubulin hypothesis revisited: what have we learned? J Cell Biol. 1987 Mar;104(3):381–383. doi: 10.1083/jcb.104.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G., Kudo N., Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol. 1977 Aug;270(1):165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat Rec. 1970 Aug;167(4):379–387. doi: 10.1002/ar.1091670402. [DOI] [PubMed] [Google Scholar]
- Gillespie M. J., Stein R. B. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983 Jan 17;259(1):41–56. doi: 10.1016/0006-8993(83)91065-x. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Cleveland D. W., Griffin J. W., Landes P. W., Cowan N. J., Price D. L. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci U S A. 1987 May;84(10):3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Griffin J. W., Gold B. G., Price D. L. Slowing of neurofilament transport and the radial growth of developing nerve fibers. J Neurosci. 1985 Nov;5(11):2920–2929. doi: 10.1523/JNEUROSCI.05-11-02920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Griffin J. W., Price D. L. Control of axonal caliber by neurofilament transport. J Cell Biol. 1984 Aug;99(2):705–714. doi: 10.1083/jcb.99.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res. 1980 Dec 8;202(2):317–333. doi: 10.1016/0006-8993(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Thompson G. W., Griffin J. W., Price D. L. Changes in neurofilament transport coincide temporally with alterations in the caliber of axons in regenerating motor fibers. J Cell Biol. 1985 Oct;101(4):1332–1340. doi: 10.1083/jcb.101.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien J. P., Meyer D., Flavell D., Hurst J., Grosveld F. Cloning and developmental expression of the murine neurofilament gene family. Brain Res. 1986 Dec;387(3):243–250. doi: 10.1016/0169-328x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyata Y., Muñoz-Martinez E. J. Properties of fast and slow alpha motoneurones following motor reinnervation. J Physiol. 1974 Oct;242(1):273–288. doi: 10.1113/jphysiol.1974.sp010706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Genetics, evolution, and expression of the 68,000-mol-wt neurofilament protein: isolation of a cloned cDNA probe. J Cell Biol. 1985 Mar;100(3):843–850. doi: 10.1083/jcb.100.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Cowan N. J. Temporal expression of mouse glial fibrillary acidic protein mRNA studied by a rapid in situ hybridization procedure. J Neurochem. 1985 Sep;45(3):913–919. doi: 10.1111/j.1471-4159.1985.tb04080.x. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W. In vivo microtubules are copolymers of available beta-tubulin isotypes: localization of each of six vertebrate beta-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol. 1987 Oct;105(4):1707–1720. doi: 10.1083/jcb.105.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Vaughn J. E. Microtubules and filaments in the axons and astrocytes of early postnatal rat optic nerves. J Cell Biol. 1967 Jan;32(1):113–119. doi: 10.1083/jcb.32.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. L., Porter K. R. The response of ventral horn neurons to axonal transection. J Cell Biol. 1972 Apr;53(1):24–37. doi: 10.1083/jcb.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan J. A., Solomon F. Reformation of the marginal band of avian erythrocytes in vitro using calf-brain tubulin: peripheral determinants of microtubule form. J Cell Biol. 1984 Dec;99(6):2108–2113. doi: 10.1083/jcb.99.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Adachi Y., Hiraoka Y., Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different alpha-tubulin genes in Schizosaccharomyces pombe. Cell. 1984 May;37(1):233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- Weiss P. A., Mayr R. Organelles in neuroplasmic ("axonal") flow: neurofilaments. Proc Natl Acad Sci U S A. 1971 Apr;68(4):846–850. doi: 10.1073/pnas.68.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Spooner B. S., Wessells N. K. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971 Jun;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]