Abstract
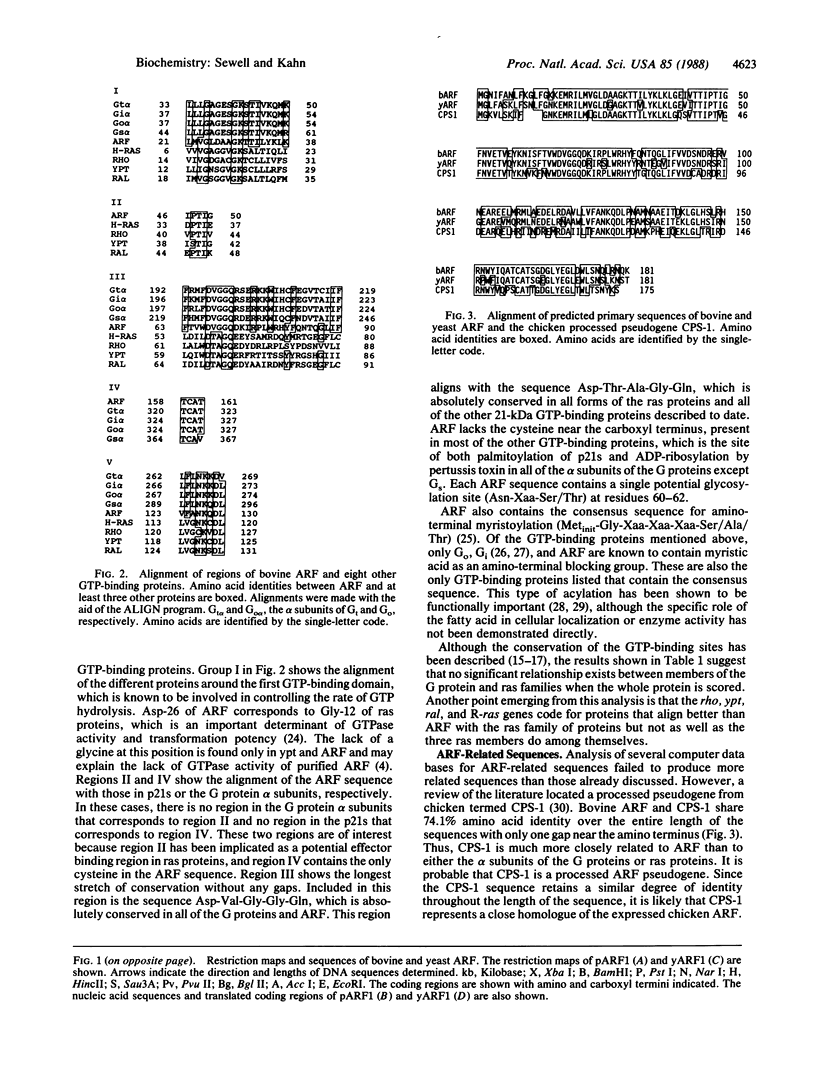
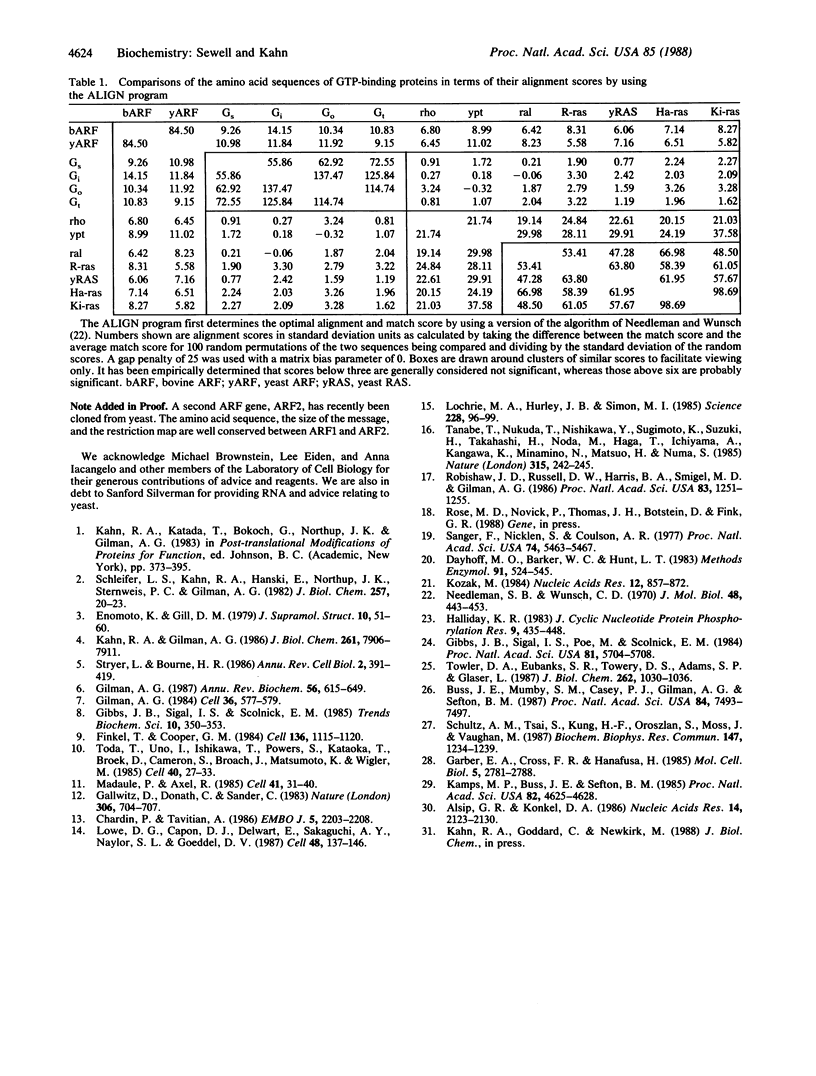
The ADP-ribosylation factor (ARF) is a 21-kDa GTP-binding protein that serves as the cofactor in the cholera toxin-catalyzed activation of the stimulatory guanine nucleotide-binding protein of adenylate cyclase (Gs). An oligonucleotide probe based on the partial amino acid sequence was used to clone ARF from a bovine adrenal chromaffin cDNA library. The yeast (Saccharomyces cerevisiae) ARF gene was then cloned from a YCp50 genomic library by cross-species hybridization by using the coding region of the bovine gene. RNA gel blots of poly(A)+ RNA indicate that only one ARF message size (900 and 2000 base pairs) is present in yeast and cows, respectively. Comparison of the cDNA-derived amino acid sequences of ARF to other GTP-binding proteins reveals a structural relationship between ARF and the ras family of proteins. A slightly better structural relationship is detected when ARF is compared to the alpha subunits of the trimeric GTP-binding proteins, including Gs alpha. All of the biochemical characteristics of the purified ARF, including the lack of GTPase activity and the posttranslational myristoylation, are consistent with the derived sequences. Comparison of the ARF sequences to that of the chicken processed pseudogene (CPS-1), previously reported as a ras homologue, reveals that CPS-1 is actually an ARF-derived gene. These results demonstrate that ARF is a GTP-binding protein with structural features of both the ras and the trimeric GTP-binding protein families.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsip G. R., Konkel D. A. A processed chicken pseudogene (CPS1) related to the ras oncogene superfamily. Nucleic Acids Res. 1986 Mar 11;14(5):2123–2138. doi: 10.1093/nar/14.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P., Tavitian A. The ral gene: a new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986 Sep;5(9):2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Enomoto K., Gill D. M. Requirement for guanosine triphosphate in the activation of adenylate cyclase by cholera toxin. J Supramol Struct. 1979;10(1):51–60. doi: 10.1002/jss.400100106. [DOI] [PubMed] [Google Scholar]
- Finkel T., Cooper G. M. Detection of a molecular complex between ras proteins and transferrin receptor. Cell. 1984 Apr;36(4):1115–1121. doi: 10.1016/0092-8674(84)90062-x. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Donath C., Sander C. A yeast gene encoding a protein homologous to the human c-has/bas proto-oncogene product. Nature. 1983 Dec 15;306(5944):704–707. doi: 10.1038/306704a0. [DOI] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Capon D. J., Delwart E., Sakaguchi A. Y., Naylor S. L., Goeddel D. V. Structure of the human and murine R-ras genes, novel genes closely related to ras proto-oncogenes. Cell. 1987 Jan 16;48(1):137–146. doi: 10.1016/0092-8674(87)90364-3. [DOI] [PubMed] [Google Scholar]
- Madaule P., Axel R. A novel ras-related gene family. Cell. 1985 May;41(1):31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Russell D. W., Harris B. A., Smigel M. D., Gilman A. G. Deduced primary structure of the alpha subunit of the GTP-binding stimulatory protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1251–1255. doi: 10.1073/pnas.83.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer L. S., Kahn R. A., Hanski E., Northup J. K., Sternweis P. C., Gilman A. G. Requirements for cholera toxin-dependent ADP-ribosylation of the purified regulatory component of adenylate cyclase. J Biol Chem. 1982 Jan 10;257(1):20–23. [PubMed] [Google Scholar]
- Schultz A. M., Tsai S. C., Kung H. F., Oroszlan S., Moss J., Vaughan M. Hydroxylamine-stable covalent linkage of myristic acid in G0 alpha, a guanine nucleotide-binding protein of bovine brain. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1234–1239. doi: 10.1016/0006-291x(87)90780-7. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Nukada T., Nishikawa Y., Sugimoto K., Suzuki H., Takahashi H., Noda M., Haga T., Ichiyama A., Kangawa K. Primary structure of the alpha-subunit of transducin and its relationship to ras proteins. Nature. 1985 May 16;315(6016):242–245. doi: 10.1038/315242a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]


