Abstract
Stomata in the epidermes of terrestrial plants are important for CO2 absorption and transpirational water loss, and are also potential entryways for pathogen infection. Stomatal opening and closure are controlled by distinct mechanisms. Arabidopsis stomata have been shown to close in response to bacteria and pathogen-associated molecular patterns (PAMPs) as part of PAMP-triggered immunity (PTI). Here we show that flg22, a PAMP derived from bacterial flagellin, also inhibits light-induced stomatal opening. Consistent with our observations on stomatal opening, flg22 inhibits the guard cell inward K+ channels (K+in currents) that mediate K+ uptake during stomatal opening. Similar to previously documented K+ current changes triggered by exogenous elevation of H2O2 and nitric oxide (NO), with prolonged duration of flg22 exposure the outward K+ channels (K+out currents) of guard cells are also inhibited. In null mutants of the flg22 receptor, FLS2, flg22 regulation of stomatal opening, K+ currents, and K+out currents is eliminated. flg22 also fails to elicit these responses in null mutants of the sole canonical G protein α subunit, GPA1. The bacterial toxin, coronatine, produced by several pathogenic strains of Pseudomonas syringae, reverses the inhibitory effects of flg22 on both K+in currents and stomatal opening, indicating interplay between plant and pathogen in the regulation of plant ion channels. Thus, PAMP-triggered stomatal response involves K+ channel regulation, and this regulation is dependent on signaling via cognate PAMP receptors and a heterotrimeric G-protein. These new findings provide insights into the largely elusive signaling process underlying PTI-associated guard cell responses.
Keywords: Plant innate immunity, Guard cell, Heterotrimeric G-protein, Stomatal movements, abscisic acid (ABA), K+ channel
Introduction
Plants have co-existed with microbes, including bacterial pathogens, for millions of years, and it is inevitable that the interactions between these organisms have resulted in their coevolution. Higher plants have developed a complicated innate immunity system to resist pathogen attack, including immune responses to conserved pathogen molecules or “pathogen-associate molecular patterns” (PAMPs). This suite of immune responses is called ‘PAMP triggered immunity’ (PTI), and is probably the first reaction when a plant is exposed to a pathogen (Chisholm et al., 2006; Jones and Dangl, 2006).
One well-characterized PAMP is flagellin, a protein subunit of the bacterial flagellum that has highly conserved N- and C- termini and is recognized by the plant during host-microbe interactions (Felix et al., 1999). flg22, a 22-amino-acid peptide derived from flagellin, is commonly used in pathogen studies and is sufficient to trigger PTI in the model species Arabidopsis (Felix et al., 1999; Melotto et al., 2006; Chinchilla et al., 2007). Perception of flg22 in Arabidopsis requires FLS2, a receptor-like kinase (RLK) with an extracellular Leucine-Rich Repeat (LRR) (Gómez-Gómez and Boller, 2000). In opposition to PTI, pathogenic bacteria have evolved virulence factors that overcome host defenses. Among these virulence factors is coronatine (COR), a polyketide toxin that is produced by several pathogenic strains of Pseudomonas syringae (Bender et al., 1999).
Pathogenic microbes need to reach the plant interior to cause disease, and natural openings in the plant surface, particularly stomata, provide portals for pathogen invasion (Melotto et al., 2006; Underwood et al., 2007; Melotto et al., 2008). Recent research has shown that active control of stomatal closure by guard cells serves as the first line of defense against pathogen invasion (Melotto et al., 2006). When an Arabidopsis leaf is exposed to bacteria on the leaf surface, or to the PAMP flg22, guard cells respond so as to decrease stomatal apertures, thereby retarding pathogen invasion. The flg22-elicited response is dependent upon the presence of FLS2, as demonstrated by a lack of stomatal closure in fls2 mutant plants, and can be overcome by the bacterial virulence factor, COR.
Regulation of stomatal closure and opening is best understood in the context of plant responses to environmental signals such as drought and light. Each pair of guard cells regulates the aperture of its circumscribed stomatal pore by turgor-driven volume changes. Stomatal closure in response to drought, in which abscisic acid (ABA) is a major positive regulator, is initiated when the guard cell membrane depolarizes as a result of inhibition of H+ extrusion combined with opening of anion channels that mediate the efflux of Cl-, malate2-, and NO-3 ions. Membrane depolarization activates voltage-regulated outwardly rectifying K+ channels, resulting in K+ efflux. Nitric oxide (NO), an ABA-activated protein kinase, OST1 (Li et al., 2000; Mustilli et al., 2002), and numerous other second messengers, particularly production of reactive oxygen species (ROS), serve as intermediaries, conveying the signal from ABA to these target ion transport molecules (Li et al., 2006). The consequent massive loss of solutes drives water efflux from the guard cells, which deflate against each other, narrowing the stomatal pore. Melotto and coworkers (2006) showed that bacteria/PAMP-triggered stomatal closure requires NO and OST1, two of the intracellular signaling molecules that also operate in guard cells during ABA-induced stomatal closure.
In contrast to stomatal closure, during stomatal opening anion channel activity is reduced and H+-ATPase activity is increased, hyperpolarizing the membrane potential and activating a different set of K+ channels, the inwardly rectifying K+ channels which mediate K+ uptake. Malate2- synthesis from starch breakdown and anion import both combine with this K+ uptake to decrease cellular water potential, resulting in water uptake, guard cell swelling, and stomatal opening. Other solutes such as sucrose also contribute to this process under some conditions (Blatt 2000; Schroeder et al., 2001; Pandey et al., 2007).
As is evident from the above description, stomatal closure and stomatal opening are not simply the reverse of each other, but involve distinct molecular elements. This observation raises the question of whether, in addition to inducing stomatal closure, PAMPs such as flg22 also inhibit stomatal opening. Also, despite genetic and biophysical evidence that ion channel regulation is involved in plant responses to pathogens as a component of specific gene-for-gene resistance mechanisms (Gelli et al., 1997; Yu et al., 1998; Bent and Mackey 2007), to date there has been only one study which has provided evidence that PAMPs regulate ion channel activity in guard cells (Ali et al., 2007).
Heterotrimeric G proteins are composed of Gα, Gβ and Gγ subunits and are important second messengers in cell signaling. Plants have fewer G-protein components than animals. For example, the Arabdopsis genome encodes only one canonical Gα subunit (GPA1), one Gβ subunit (AGB1) and two known Gγ subunits (AGG1 and AGG2). Nevertheless, Arabidopsis heterotrimeric G proteins participate in numerous signal transduction pathways, including gene-for-gene mechanisms of pathogen resistance (Suharsono et al., 2002; Perfus-Barbeoch et al., 2004; Komatsu et al., 2004; Llorente et al, 2005; Trusov et al., 2006, 2007; reviewed in Assmann 2005a, b; Ding et al., in press). However, whether heterotrimeric G-proteins participate in guard cell responses to PAMPs is not known.
To gain further understanding of PTI-associated guard cell responses, we investigated whether, in addition to inducing stomatal closure, PAMPs affect stomatal opening, whether K+ ion channel regulation is targeted by PAMPs and COR, and whether heterotrimeric G proteins participate in guard cell responses to PAMPs.
Results
flg22 inhibits light-induced stomatal opening in an FLS2-dependent manner
Light is one of the most important and well studied factors inducing stomatal opening (reviewed by Shimazaki et al., 2007). By measurement of stomatal apertures in epidermal peels obtained before and after light treatment of leaves, we found that 5 μM flg22 inhibits light-induced stomatal opening in wild-type Col-0 plants (Figure 1). This is a specific effect, since aperture data obtained from fls2 receptor mutant (Salk_line 93905) guard cells show that flg22 has no effect on light-induced stomatal opening (Figure 1). Thus, flg22 not only causes stomatal closure (Melotto et al., 2006), it also inhibits stomata from opening.
Figure 1. flg22 inhibits light-induced stomatal opening in Col-0 but not in fls2 plants.
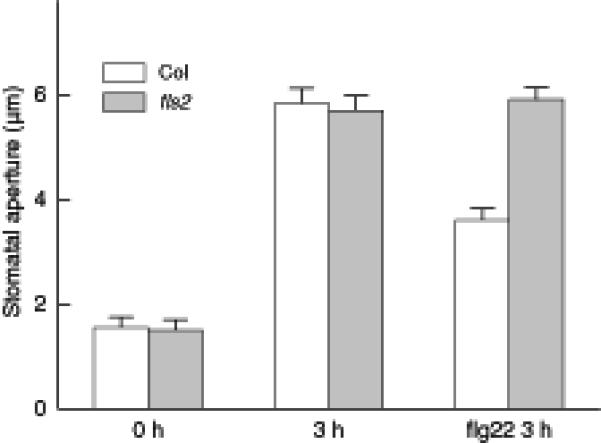
Average stomatal apertures (mean ± SE) in epidermal peels taken from leaves of Col-0 and fls2 before light treatment (0 hr) and after 3 hr in the light with or without 5 μM flg22 treatment. n = 3 experiments, with n>150 apertures measured per experiment.
flg22 inhibits inward K+ current amplitude in Arabidopsis guard cells
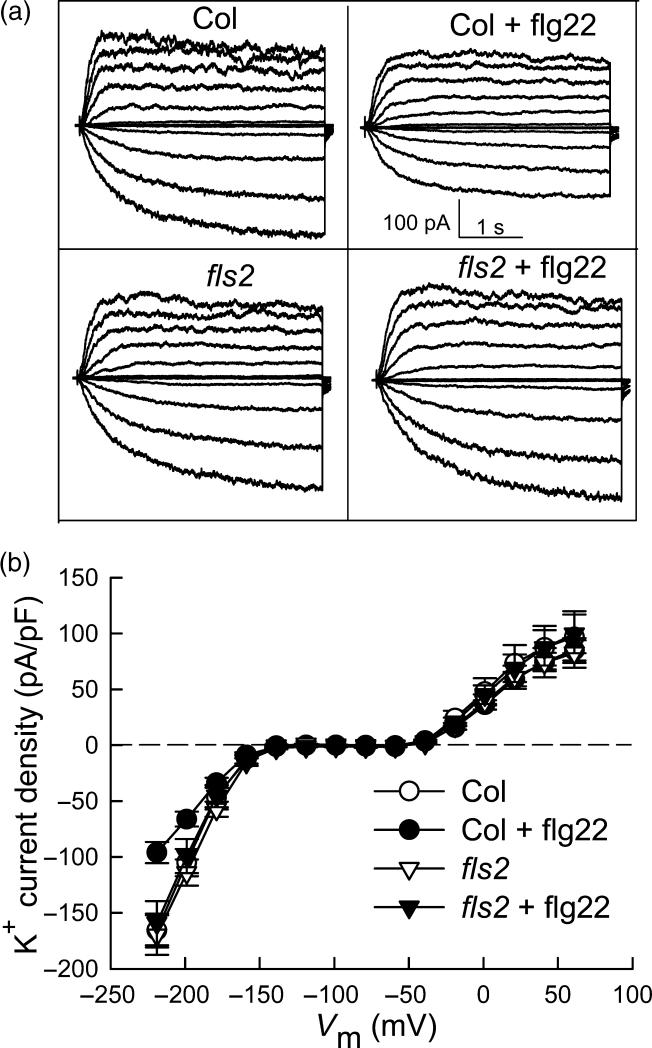
Fluxes of K+ and anions through ion channels into and out of the guard cell are key effectors of stomatal opening and closure, and thus ion channel regulation is an important control point in the determination of stomatal aperture (Schroeder et al., 2001; Garcia-Mata et al., 2003). ABA is well known to inhibit inward K+ channel activity and impair stomatal opening (reviewed by Israelsson et al., 2006; Pandey et al., 2007). Given our observation that flg22, similarly to ABA, inhibits stomatal opening, we hypothesized that flg22 might also inhibit inward K+ channel activity. To test this hypothesis, we used the whole-cell patch-clamp technique to record K+ currents of guard cell protoplasts in the presence and absence of flg22. In wild-type Col-0 guard cells assayed at 10 min. after whole-cell formation, inward K+ currents were significantly inhibited in the presence of 5 μM flg22 in the bath solution as compared to the control. Average inward K+ current density at -219 mV was reduced from -166 ± 15 pA/pF to -96 ± 9 pA/pF (P <0.01) by flg22 treatment. Outward K+ currents were not significantly altered by the flg22 treatment in these recordings (Figure 2).
Figure 2. K+in currents are inhibited by flg22 in Col-0 guard cells but not in fls2 guard cells.
(A) Typical whole-cell K+ current recordings from Col-0 and fls2 with or without 5 μM flg22. Time and current scales are indicated.
(B) Average current-voltage relationship (mean ± SE) of time-activated whole-cell K+ currents from cells recorded as in (A).
n = 22 (Col-0); 11 (Col-0 + flg22); 8 (fls2); and 10 (fls2 + flg22).
flg22 regulates inward K+ currents in an FLS2-dependent manner
To assess whether the inhibitory effect of flg22 on guard-cell inward K+ currents is mediated by a specific interaction between flg22 and the FLS2 receptor, we compared the effects of flg22 on K+ currents of wild-type and fls2 mutant guard cells. The inhibitory effect of flg22 on inward K+ currents was not observed in the fls2 mutant guard cells (Figure 2), indicating that this effect is specific, and requires FLS2.
flg22 inhibits the K+in currents of guard cells in a scalar manner, but does not affect their kinetics
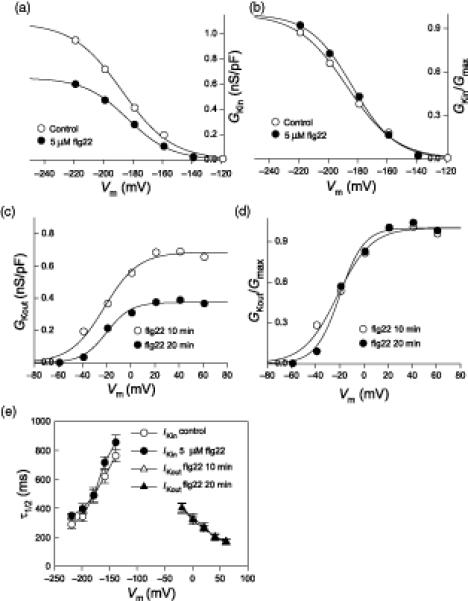
To assess whether flg22 alters K+ channel gating, in addition to its effect on the amplitude of inward K+ currents, we analyzed inward K+ current kinetics. Current values recorded between -219 mV and -119 mV from cells with obvious inward currents were chosen for the analysis. The mean value of conductance at each voltage point was plotted as a function of voltage (Figure 4A) and the relationship was fitted with the Boltzmann equation (see “Material and Methods” for details on data analysis). The average fitted Gmax of the control cells is 1.09 ± 0.04 nS/pF, while the average Gmax of flg22-treated cells is 0.65 ± 0.06 nS/pF (Table 1), a reduction of about 45%. G/Gmax for both control and flg22 treatments were also fitted with the Boltzmann equation (Figure 4B) and the fitted results were used to derive the half-maximal activation voltages (V1/2) and effective gating charge (z). There is no significant difference in V1/2 and z values with or without flg22 treatment (Table 1). Half-activation times (V1/2) of inward K+ currents were also calculated for the two conditions, and were found to be statistically identical (Figure 4E).
Figure 4. Effect of flg22 on the conductance/voltage relationship of inward- and outward- K+ currents of Col-0 guard cells.
(A) Conductance/voltage curves of inward-rectifying K+ currents fitted with the Boltzmann equation (data were mean values, and n = 9 and 10 for control and flg22 treatment, respectively)
(B) Relative conductance/voltage curves of K+in currents fitted with the Boltzmann equation.
(C) Conductance/voltage curves of K+out at 10 and 20 min after formation of the whole-cell configuration fitted with the Boltzmann equation (data are mean values, n = 10 for each time point)
(D) Relative conductance/voltage curves of K+out fitted with the Boltzmann equation.
(E) Half-activation time (τ1/2) values (mean ± SE) of K+in for control and flg22 treatment (left, voltages are from -219 mV to -139 mV) and K+out at 10 and 20 min with flg22 treatment (right, voltages are from -19 mV to 61 mV).
Table 1.
Fitted parameters for the maximum conductances (Gmax), half-maximal activation voltages (V1/2) and effective gating charge (z), as derived from the conductance/voltage curves. Data are shown as mean ± SE. n = 9 for control and 10 for flg22 treatment.
| IKin | IKout | |||
|---|---|---|---|---|
| Control | 5 μM flg22 10 min | 5 μM flg22 10 min | 5 μM flg22 20 min | |
|
Gmax
(nS/pF) |
1.087±0.043 | 0.650±0.059 | 0.684±0.082 | 0.374±0.046 |
| V1/2 (mV) | -187±2 | -184±3 | -21±3 | -20±2 |
| z | 1.50±0.09 | 1.71±0.10 | 1.79±0.18 | 2.53±0.22 |
flg22 gradually inhibits K+out currents in a scalar manner
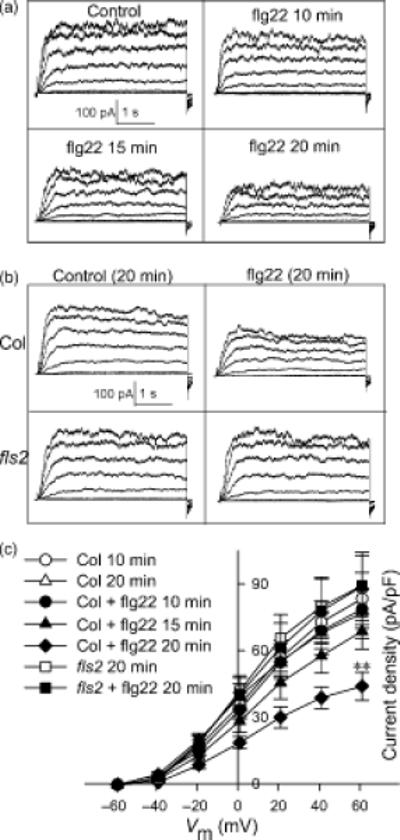
The inhibition of K+in currents of guard cells by 5 μM flg22 was observed within 10 min of whole-cell recording, and no obvious further inhibition was observed at later timepoints (data not shown), indicating that the maximal effect of flg22 had been achieved. At the 10-min time point, no significant inhibitory effect of flg22 on K+out currents was observed when compared with control (Figure 2). To assess whether there was a time-dependent effect of flg22 on the K+out channels of guard cells, recordings taken at a range of time points after formation of the whole-cell configuration were analyzed. In the control recordings (no flg22 in the bath solution), there was no obvious decrease of K+out current amplitude (Figure 3). However, in the presence of flg22, K+out currents decreased gradually over time, and this effect reached statistical significance by 20 min (Figure 3 A, C). In guard cells of fls2 mutants, at 10 min the mean value of K+out currents was about the same as that of Col-0 (Figure 2), and there was no flg22 inhibition of K+out currents when compared to Col-0 at 20 min (Figure 3 B, C).
Figure 3. K+out currents are gradually suppressed by flg22 in Col-0 guard cells but not in fls2 plants.
(A) Typical whole-cell outward K+ current recordings at 10 min without flg22 (control), and recordings at different time points with 5 μM flg22 after formation of the whole cell configuration. Time and current scales are as shown.
(B) Typical whole-cell outward K+ current recordings at 20 min for Col-0 and fls2 without (control) or with 5 μM flg22. Time and current scales are as indicated.
(C) Average current-voltage relationship (mean ± SE) of time-activated whole-cell outward K+ currents indicated in (A) and (B). n = 9 (Col-0; 10 min and 20 min); 10 (Col-0 + flg22; 10 min, 15 min and 20 min); 8 (fls2; 20 min); and 10 (fls2 + flg22; 20 min).
The mean value of the K+out conductance at each voltage step (-59 mV to 61 mV) for Col-0 guard cells was plotted as a function of voltage (Figure 4C) and the relationship was fitted with the Boltzmann equation. The Gmax in 10-min recordings with 5 μM flg22 is 0.68 ± 0.08 nS/pF but it decreased to 0.37 ± 0.05 nS/pF in the 20-min recordings (Table 1). G/Gmax for recordings taken 10 min and 20 min after whole cell formation and in the presence of flg22 were also fitted with the Boltzmann equation (Figure 4D) and the V1/2 and z values were not significantly altered by flg22 treatment (Table 1). The τ1/2 of K+out currents was also calculated for the 10 and 20 min time points, and found to be statistically identical (Figure 4E); τ1/2 values of K+out currents were also identical between control and flg22 treatments at 10 min (data not shown).
COR reverses PAMP-modulation of stomatal opening and K+in but not K+out currents in guard cells
COR is a virulence factor that opposes PTI responses, including flg22-induced stomatal closure (Melotto et al., 2006). Whether COR can also interfere with flg22-mediated inhibition of stomatal opening is not known and was therefore investigated. While COR (0.5 ng.μl-1) alone had no evident effect on this process, it reversed the inhibitory effect of 5 μM flg22 (Figure 5A). We also tested whether COR affects PAMP-regulation of inward K+ currents. While COR (0.5 ng.μl-1) alone had no obvious effects on K+ currents, it reversed the inhibitory effect of flg22 on K+in currents (Figure 5B; C). By contrast, COR could not reverse the slow inhibition of outward K+ currents by flg22 (Figure 6), indicating that distinct mechanisms underlie flg22 regulation of inward vs. outward K+ channels.
Figure 5. flg22 inhibition of stomatal opening and K+in currents is prevented by COR in wild type (Col-0) Arabidopsis guard cells.
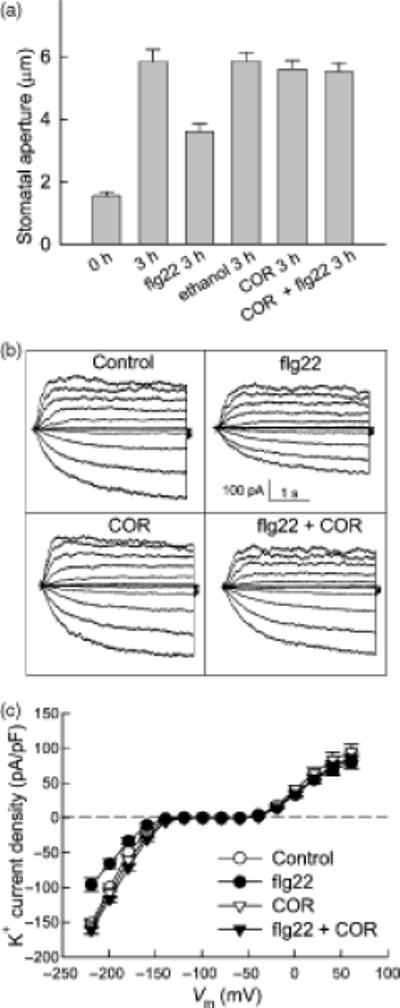
(A) Inhibition of stomatal opening by flg22 is prevented by COR. n = 3 experiments, with n>150 apertures measured per experiment.
(B) Typical whole-cell recordings of guard cell K+ currents of Col-0 for control, 5 μM flg22, 0.5 ng.μl-1 COR or 5 μM flg22 + 0.5 ng.μl-1 COR treatments. Time and current scales are indicated.
(C) Average current-voltage relationship (mean ± SE) of time-activated whole-cell K+ currents from cells recorded as in (A).
n = 9 (Control); 10 (flg22); 7 (COR); and 6 (flg22 + COR)
Figure 6. flg22 inhibition of outward K+ currents of Arabidopsis Col-0 guard cells could not be reversed by COR.
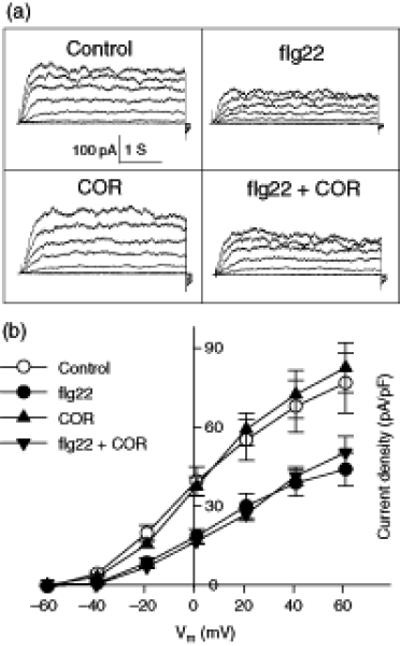
(A) Typical whole-cell outward K+ currents at 20 min for Col-0 guard cells subjected to control, 5 μM flg22, 0.5 ng.μl-1 COR or 5 μM flg22 + 0.5 ng.μl-1 COR treatments. Time and current scales are as indicated.
(B) Average current-voltage relationship (mean ± SE) of whole-cell outward K+ currents from the cells recorded as in A.
n = 9 (control); 10 (flg22); 7 (COR); and 6 (flg22 + COR)
flg22 regulation of stomatal opening and K+ currents in guard cells requires the G-protein α subunit GPA1
Given the demonstrated role of the Gα subunit, GPA1, in mediating ABA-inhibition of inward K+ currents and stomatal opening (Wang et al., 2001), we hypothesized that GPA1 might also mediate the inhibitory effects of flg22 on these processes. To assess whether GPA1 plays a role in guard cell response to PAMPs, Gα subunit null mutants (gpa1-3 and gpa1-4) (Jones et al., 2003) were also used in assays of light-induced stomatal opening. We found that flg22 inhibition of stomatal opening is impaired in the gpa1-3 and gpa1-4 mutants (Figure 7A).
Figure 7. Involvement of Gα subunit (GPA1) in flg22 inhibition of guard cell K+ currents.
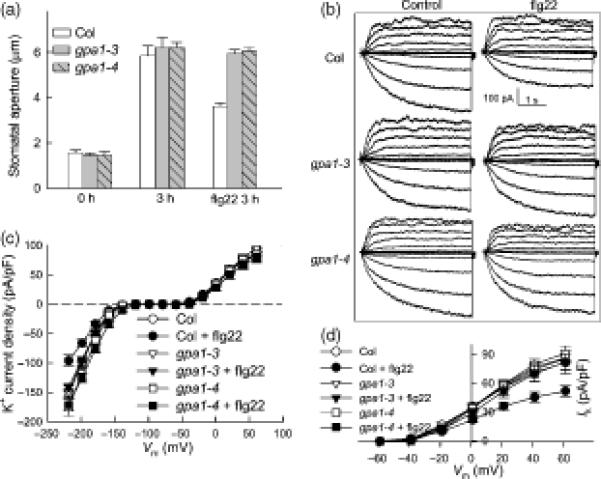
(A) Stomatal apertures of Col-0, gpa1-3 and gpa1-4 plants before light treatment (0 hr) and after 3 hr in the light with or without 5 μM flg22 treatment. n = 3 experiments, with n>110 apertures measured per experiment.
(B) Typical whole-cell recordings at 10 min of Col-0, gpa1-3 and gpa1-4 guard cell K+ currents in the absence or presence of 5 μM flg22. Time and current scales are indicated.
(C) Average current-voltage relationship (mean ± SE) of time-activated whole-cell K+ currents from cells recorded as in (B). n = 22 (Col-0); 17 (Col-0 + flg22); 31 (gpa1-3); 47 (gpa1-3 + flg22); 16 (gpa1-4) and 26 (gpa1-4 + flg22).
(D) Average current-voltage relationship (mean ± SE) of whole-cell outward K+ currents at 20 min from the cells recorded as in B. n = 22 (Col-0); 17 (Col-0 + flg22); 31 (gpa1-3); 47 (gpa1-3 + flg22); 16 (gpa1-4) and 26 (gpa1-4 + flg22).
To investigate whether alterations in K+ channel response are associated with the alterations in stomatal movement responses to flg22 that were observed in the gpa1 mutants, we performed electrophysiological experiments analogous to those of Figure 2 using gpa1-3 and gpa1-4 mutant guard cells. In the absence of flg22 application, the K+ currents of both gpa1-3 and gpa1-4 guard cells are the same as those of Col-0, a result that is in accordance with previous reports (Wang et al., 2001; Coursol et al., 2003; Fan et al., 2008). When 5 μM flg22 was applied, no flg22 inhibition of K+in currents was observed in either gpa1 mutant as compared with Col-0 (Figure 7B; C). Furthermore, the slow inhibition of outward K+ currents by flg22 also did not occur in guard cells of gpa1-3 and gpa1-4 null mutants (Figure 7D).
Discussion
The survival of plants following pathogen exposure requires the plant immune system, which consists of two interconnected branches (Jones and Dangl, 2006; Chisholm et al., 2006). One branch almost always occurs inside of the host cell and relies on the expression of R genes, most of which encode NB (nucleotide binding)-LRR proteins. These NB-LRR proteins allow the recognition of specific pathogen virulence effectors to cause effector-triggered immunity (ETI), also known as gene-for-gene immunity. The other branch, PTI, is based on transmembrane receptors associated with recognition of PAMPs and it triggers rapid responses as the first line of defense against pathogen attack. While there has been significant progress in the understanding of PTI at the level of leaf tissue, which mainly consists of mesophyll cells, little is understood regarding PAMP regulation of stomatal movement and PAMP signaling specifically in stomatal guard cells. We show here that (i) the PAMP flg22 inhibits opening of stomata induced by light, and the bacterial virulence factor COR counteracts this process; (ii) flg22 inhibits K+in currents in guard cells and COR prevents this inhibition, and; (iii) flg22 PAMP-inhibition of stomatal opening and K+ currents requires the G-protein α subunit GPA1. These new findings advance our understanding of the largely elusive process of guard cell-based PTI.
flg22 inhibits stomatal opening and K+in currents
During stomatal opening, activation of plasma membrane H+-ATPases, and the resultant extrusion of H+ causes hyperpolarization of the membrane potential. This hyperpolarization provides a driving force for K+ uptake and activates K+in channels while deactivating K+out channels, and thus facilitates net K+ accumulation within the guard cell. Concurrently, Cl- and NO-3 enter the cell via presumed anion transporters in the plasma membrane, and catabolic processes also result in intracellular accumulation of malate and sugars (Schroeder et al., 2001; Shimazaki et al., 2007). A central role of inward K+ channels in effecting stomatal opening in response to environmental stimuli is well established (e.g. Kwak et al., 2001; Lebaudy et al., 2008). Here we show that flg22 inhibits light-induced stomatal opening and that this effect is dependent on flg22 perception by the FLS2 receptor (Figure 1). Consistent with flg22 inhibition of stomatal opening, flg22 inhibited K+in currents in an FLS2-dependent manner. Other mechanisms may also contribute to flg22 inhibition of stomatal opening. For example, K+ uptake is dependent on membrane hyperpolarization mediated in part via active H+ extrusion, and flagellin may oppose this process: net H+ influx was measured after flg22 application to tomato leaf tissue (Lanfermeijer et al., 2008), and other elicitors inhibit H+ ATPase activity (Amborabe et al., 2008).
flg22 inhibition of K+in currents occurs in a scalar fashion. Even though the Gmax of K+in currents was dramatically inhibited by 5 μM flg22, the relative value of Gk/Gmax was about the same with and without flg22, and the half-activation voltage V1/2 and the half-activation times (τ1/2) were the same with and without flg22 application (Figure 4E). These data indicate that flg22 does not change the kinetics of the guard cell K+in channels. The whole-cell inward current is the sum of the currents through all the open K+in channels in the plasma membrane. A decrease in current can occur by a decrease in the open probability of the channel, a decrease in the single channel conductance, or a decrease in the number of activatable channels in the membrane. Because the time constants and voltage-dependence of the K+in currents did not change upon flg22 treatment (indicating that the open probability of the channels is not affected by flg22), yet overall current magnitude was decreased, we can conclude that flg22 inhibits the K+in currents either by decreasing the single channel conductance or by decreasing the number of K+in channels available for voltage-activation. The latter phenomenon could occur if flg22 signaling resulted in the conversion of a proportion of the K+in channels to a nonactivatable (silent) state, as occurs, e.g. for low pH suppression of guard cell K+out currents (Miedema and Assmann, 1996), or if flg22 resulted in endocytosis of K+in channels. Regarding the latter possibility, it is interesting to note that flg22 is known to induce endocytosis of its own receptor, FLS2, (Robatzek et al., 2006); one could speculate that these endocytotic vesicles also contain K+ channels.
PAMP regulation of guard cell K+ channels had not been previously evaluated. Only one previous study reported pathogen regulation of these channels, in the context of gene-for-gene interaction, in which it was shown that the Avr9 elicitor inhibits K+in channels and activates K+out channels of transgenic tobacco guard cells expressing the Cf9 resistance transgene. In response to Avr9 elicitor, both magnitude and kinetics of the K+ currents were changed, indicating that the Avr9 elicitor alters K+in and K+out channel gating (Blatt et al., 1999), in contrast to the apparent gating-independent effects of the PAMP flg22.
flg22 inhibits K+out currents
We found that flg22 suppressed K+out currents (Figures 3, 4, 6). This effect developed slowly after whole-cell formation, even though the cells had been pre-incubated in flg22. These results suggest that some change in cytosolic composition as a result of intracellular perfusion by the pipette solution is a necessary condition for the subsequent slow activation of a signaling pathway that is responsible for the inhibition of K+out. Slow inhibition was also observed when flg22 was added to the bath solution immediately after whole cell formation (Supplemental Figure S1). By contrast, inhibition of the K+in currents was complete and stable at the first recording timepoint, 10 min. after whole cell formation, regardless of whether the guard cell protoplasts had been pre-exposed to flg22 (Figures 2, 7) or exposed to flg22 immediately after whole cell formation (Supplemental Figure S1). The flg22-inhibition of K+out currents does not appear to be a non-specific deleterious effect: it is absent from fls2 guard cells, indicating that it is mediated by specific PAMP-receptor interaction. Analogous to the regulation of K+in currents, the kinetics of K+out currents were not altered by flg22 treatment, indicating that channel open probability was unchanged and thus the decrease in current presumably resulted from a decrease in activatable K+out channels or single channel conductance.
The flg22-inhibition of K+out channels in guard cells that we observed is in contrast to P. syringae pv. pisi -induced K+ efflux from suspension-cultured tobacco cells, as determined by atomic absorption spectroscopy of extracellular K+ concentration (Atkinson et al., 1985). This result is also unexpected given the observation that flg22 can induce stomatal closure (Melotto et al., 2006). Suppression of K+ efflux channels would be expected to oppose stomatal closure because stomatal closure is dependent on loss of K+. However, these results could be reconciled if flg22 enhances the driving force for K+ efflux, i.e. the magnitude of membrane depolarization, sufficiently to overcome the flg22-induced decrease in available K+out channels. This could occur if flg22 also activated anion efflux channels or H+ influx, or decreased H+ ATPase activity (Amborabe et al., 2008; Lanfermeijer et al., 2008). Another phenomenon that occurs after bacteria or flg22 application is medium alkalinization (Atkinson et al., 1985; Felix et al., 1999). pH is a well-known regulator of guard cell function, and external alkalinization activates the K+out channels and inactivates the K+in channels (Blatt 1992; Ilan et al., 1994; 1996). While our patch clamp study of guard cell protoplasts used a bath solution that included a pH buffer, which would have counteracted any activation of K+out by external alkalinization, in situ the K+out channels of guard cells may be activated by flg22 or P. syringae via external alkalinization.
A proposed mechanism of flg22 action on guard cell K+ channels
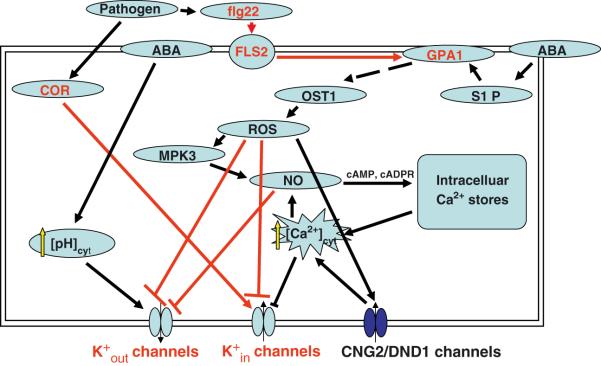
In ABA-regulation of stomatal movements, the movement of K+ into and out of guard cell is one of the most downstream responses of the guard cell. Based on published reports, the following signaling cascade can be assembled for ABA inhibition of K+in channels (Figure 8). ABA activates sphingosine kinase which produces the lipid metabolite, S1P (Coursol et al., 2003). S1P, a known ligand of GPCRs in mammalian systems, inhibits K+inchannels in a GPA1-dependent manner (Coursol et al., 2003), and genetic elimination of either GPA1 or OST1 prevents ROS production in guard cells (Joo et al., 2005; Mustilli et al., 2002). Therefore, a logical order of these components is S1P activation of GPA1 which directly or indirectly activates OST1, resulting in ROS production, which activates Ca2+-permeable channels at the plasma membrane (Pei et al., 2000; Kwak et al., 2003). Production of ROS (e.g., H2O2) appears to act at least in part through a MAPK, MPK3 (Gudesblat et al., 2007), to elicit NO production in guard cells (Garcia-Mata et al., 2003; Sokolovski et al., 2005; Bright et al., 2006; Yan et al., 2007). NO, acting via cGMP and cADP ribose (cADPR), elicits Ca2+ release from intracellular stores (Garcia-Mata et al., 2003; Sokolovski et al., 2005), and elevation of cytosolic Ca2+ is known to inhibit K+in channels (Schroeder and Hagiwara, 1989).
Figure 8. Model of PAMP regulation of Arabidopsis guard cell K+ channels.
A model of how proposed common signaling components in ABA and PAMP responses act to regulate guard cell K+ channels. The model is drawn based on the new information provided by this study (shown in red) and references cited in the Discussion. Note that a parsimonious model is shown; thus, if component A is known to activate both component B and component C, and component B is also known to activate component C, then the pathway is drawn as A activates B and B activates C.
Many of the above ABA signaling elements are also known to be related to pathogen signaling and therefore can be implicated in the regulation of K+ channels observed here. To organize this information, and provide a framework for future research, we assembled this information into a speculative model of pathogen signaling in guard cells (Figure 8). Melotto et al. (2006) previously demonstrated in Arabidopsis guard cells that NO production and the presence of the ABA-activated Ca2+-independent protein kinase, OST1, were required for flg22-promotion of stomatal closure. Both ABA and bacteria or flg22 induce the production of NO and reactive oxygen species (ROS) (Keppler et al., 1989; Felix et al., 1999; Durner et al., 1998; Delledonne et al., 1998; Ali et al., 2007). The MAPK kinase, MPK3, is well-known for its involvement in pathogen signaling in leaves (Asai et al., 2002). Ali et al. (2007) provided evidence that lipopolysaccharides, another bacterial PAMP, could activate Ca2+ influx through the cyclic-nucleotide-gated channel, CNG2/DND1, in guard cells and that this process functions upstream of NO production (Garcia-Mata and Lamattina, 2007), and non-specific elicitors have also been implicated in activation of Ca2+-permeable guard cell channels (Klüsener et al., 2002).
Since experimental elevation of H2O2 (Zhang et al., 2001; Kohler et al., 2003), NO (Garcia-Mata et al., 2003; Sokolovski et al., 2005), and cytosolic Ca2+ (Schroeder and Hagiwara, 1989; Kelly et al., 1995) all have been shown by electrophysiological methods to inhibit K+in currents of guard cells, it is reasonable to implicate these elements in flg22-inhibition of K+in currents. However, one important distinction is that, at least in Vicia faba guard cells, NO (Garcia-Mata et al., 2003) and cytosolic Ca2+ elevation (Schroeder and Hagiwara, 1989; Grabov and Blatt, 1997) inhibit K+in currents by altering K+in channel gating, i.e. in a non-scalar manner, whereas our data show scalar inhibition of K+in currents by flg22. This scalar inhibition is most similar to that produced by exogenous H2O2 treatment, which, like flg22, has been reported to inhibit K+in currents without a change in half-activation time (Kohler et al., 2003).
With regard to flg22-inhibition of K+out currents, the scalar effects of flg22 are similar to the scalar effects of NO on these K+ channels observed in Vicia faba. Unlike the K+in channels, K+out channels are Ca2+-insensitive, and are proposed to instead be regulated by NO via NO-based protein nitrosylation (Sokolovski and Blatt, 2004). H2O2 effects on these channels are also likely to be scalar (Kohler et al., 2003). These similarities suggest that PAMP regulation of K+out channels in guard cells utilize H2O2 and NO as signaling elements. NO inhibition of K+out channels of guard cells occurs at higher concentration than NO inhibition of K+in channels (Garcia-Mata et al., 2003; Sokolovski and Blatt, 2004), which may account for our observation showing that flg22 inhibited K+in currents quickly (Figure 3) while suppression of K+out currents developed slowly (Figure 8). K+out currents are also activated in a scalar manner by cytosolic alkalinzation (Blatt, 1992; Miedema and Assmann, 1996). However, because cytosolic pH was buffered in our study, pH seems less likely as a mediator of the flg22 effect under our experimental conditions.
Other pathways also may be involved in guard cell response to PAMPs, in addition to elements shared with abiotic (drought, ABA) signaling cascades. Melotto and coworkers (2006) showed that in addition to the requirement for ABA-related signaling elements, bacterium/PAMP-triggered stomatal closure is dependent on salicylic acid (SA). Whether and how SA-controlled pathways are connected to the ABA pathway in the guard cell is currently not known. However, SA has been shown to trigger stomatal closure via generation of ROS (Manthe et al., 1992; Lee, 1998; Mori et al., 2001). Perhaps microbial and abiotic signals generate both shared and distinct ROS and NO signatures that trigger both common and unique downstream responses.
Coronatine action on guard cell K+ channels
One interesting contrast in PAMP regulation of K+in vs. K+out channels is evident in their responsiveness to COR. While the plant immune system has evolved to resist microbial invasion and colonization, virulent pathogens can still infect host plants because successful pathogens have presumably developed strategies to conquer or avoid plant defense responses. COR is necessary for successful infection of P. syringae pv. tomato DC3000 in Arabidopsis leaves and plays multiple roles during pathogenesis (Bender et al., 1999; Melotto et al., 2006; Underwood et al., 2007; Melotto et al., 2008). Melotto et al. (2006) showed that COR could reverse flg22- and ABA-induced stomatal closure even though flg22-induction of NO elevation still occurred. Accordingly, we can infer that COR interferes with flg22 signaling downstream of OST1, H2O2, and NO, but upstream of the ion channels controlling solute fluxes across the guard cell plasma membrane (Figure 8). Interestingly, while COR is able to reverse flg22-inhibition of K+in currents (Figure 5), it has no obvious effect on flg22-inhibition of K+out currents (Figure 6). One possibility is that the NO-based protein nitrosylation postulated to mediate NO effects on K+out but not K+in channels (Sokolovski and Blatt, 2004) is not reversible by COR. Failure of the flg22-mediated K+out current inhibition to be reversed by COR could be advantageous to the pathogen, as such failure could potentially contribute to retention of open stomata.
The G-protein α subunit GPA1 is required for flg22 regulation of stomatal movements and K+ channels
In Arabidopsis, there is only one gene, GPA1, encoding a classical Gα subunit of heterotrimeric G proteins. By using Arabidopsis gpa1 T-DNA null mutants, GPA1 has been shown to be required for ABA- and sphingosine-1-phosphate (S1P)-inhibition of K+in channels and stomatal opening, and for pH independent S-anion channel activation by ABA (Wang et al., 2001; Coursol et al., 2003). Our data showing absence of flg22-inhibition of K+ currents and stomatal opening in gpa1 guard cells (Figure 7) identifies GPA1 as another common element in PAMP and ABA signaling. Since GPA1 is required for ROS production in guard cell responses to ozone (Joo et al., 2005), it is reasonable to propose that GPA1 also functions upstream of ROS in guard cell PAMP-signaling (Figure 8).
Conclusion
The regulation of stomatal movements is a venerable system for the study of the effects of pathogen products as it has been known since 1969 that the toxin, fusicoccin, produced by the pathogenic fungus Fusicoccum amygdali Del., stimulates stomatal opening (Turner and Graniti, 1969), with subsequent biochemical (reviewed in Marre 1979) and electrophysiological (Assmann and Schwartz, 1992; Lohse and Hedrich, 1992) studies demonstrating that fusicoccin activates the guard cell H+-ATPase (Kinoshita and Shimazaki, 2001; Merlot et al., 2007). More recent research has identified Ca2+-permeable channels (Gelli et al., 1997; Zimmermann et al., 1997; Klüsener et al., 2002; Ali et al., 2007) as targets of pathogen products. Our study provides evidence that guard cell K+ channels are regulated by PAMPS, and that this regulation occurs in a manner distinct from that evoked by Avr elicitors during effector-triggered immunity (Blatt et al., 1999).
The multiplicity of shared and distinct downstream responders to microbial and abiotic signals suggests a fruitful area for further understanding of signal integration and prioritization in the guard cell. Guard cell signaling has traditionally been studied in the context of abiotic stresses, yet stomata are a major portal through which many foliar pathogens enter and exit plants, representing two key steps in disease cycles and epidemiology (Boureau et al., 2002; Guimaraes and Stotz, 2004; Melotto et al., 2008). Outbreak of many foliar plant diseases requires high humidity, which could promote stomatal opening. Future studies integrating abiotic and biotic signals may shed light on the important question of whether abiotic conditions that promote stomatal opening diminish the effectiveness of stomata-based restriction of pathogen entry into and exit from the plant, thereby influencing disease epidemics.
Experimental procedures
Stomatal aperture assay
Wild type Col-0, knockout mutant fls2 (Salk_line 93905) and the gpa1 (gpa1-3 and gpa1-4)) lines, were grown in controlled conditions in growth chambers with a 8h/16h light/dark photoperiod with a light intensity of 120 μmol m-2 s-1, relative humidity of 80%, and day/night temperatures of 22°C/20°C. For all assays of stomatal aperture, fully expanded young leaves from 4-5 week-old plants were used. Whole leaves were excised in the morning before the lights came on, and were immediately placed in opening solution (10 mM KCl, 7.5 mM iminodiacetic acid, 10 mM Mes-KOH, pH 6.15) with the adaxial surface upward. These leaves were kept in darkness for 3 hr to ensure stomatal closure and then the abaxial epidermes of two leaves of each genotype used were peeled and photographed at 400x total magnification for subsequent measurement to obtain a 0 hr baseline stomatal aperture. Next, 5 μM flg22 peptide (Alpha Diagnostics, Inc.) (same volume of water was added for control) or 0.5 ng μl-1COR (Sigma-Aldrich, Inc.) (same volume of ethanol was added as solvent control) was added to the opening solution and the leaves were subjected to white light (450 μmol m-2 s-1) illumination for another 3 hr to induce stomatal opening. Then the abaxial leaf epidermes were peeled and photographed as for 0 hr leaves. Stomatal apertures were subsequently measured from these images using ImageJ (open access software, version 1.37) and at least 110 stomatal apertures were measured in each replicate. All the data reported here were obtained from 3 independent replicate experiments. Student’s t-test was used to compare the flg22 effect, and P value < 0.01 was considered as a significant difference. Aperture measurements were performed blind.
Electrophysiology
Plants used for patch clamp experiments were grown under the same conditions as for stomatal aperture assays. Arabidopsis guard cell protoplast isolation and solutions used for K+ current recordings were as previously described (Wang et al., 2001; Coursol et al., 2003). Except for the instantaneous flg22 treatments (Supplemental Figure 1) all data were obtained 0.5 hr to 2 hr after protoplasts were introduced into the bath solutions (control solution or bath solution with 5 μM flg22 and/or 0.5 ng.μl-1 COR added), and recordings were obtained 10 min after formation of the whole-cell configuration except as otherwise noted. For the instantaneous flg22 treatments, bath solution with flg22 added was directly perfused into the chamber immediately after formation of the whole-cell configuration. For all recordings, the holding potential was set at -79 mV and the voltage family applied was from -219 to +61 mV with +20-mV increments of 3.9-s duration with 6-s interpulse intervals.
Data analysis
Time-activated K+ current (Ik for K+ or K+ in out) was calculated for each recording as the average steady state current between 3.60 to 3.88 s minus the instantaneous current at 20 ms. Cell capacitances were read directly from the Axon 200B amplifier after full compensation and immediately before whole-cell recordings. Data were compared with the Student’s t-test, and results with P ≤ 0.01 were considered as significantly different. The software for data recording and analysis was pCLAMP 6.0.3 and figures were drawn with SigmaPlot 8.0.
The calculation of K+ channel conductance (Gk) is based on the relationship Gk = Ik/(Em-Erev), where Em is the applied potential, Erev is the reversal potential, and Ik is the time-activated K+ current (Ilan et al., 1995; Wang et al., 1998). We obtained the Erev from tail-current recordings from control and flg22-treated cells, and the value was -59 mV in both conditions (data not shown). Current values from -219 mV to -119 mV from cells with obvious inward currents were chosen for the analysis, and conductance was calculated at each voltage. To derive the maximum conductance, Gmax, the cell conductance/voltage data were fitted with the Boltzmann equation: Gk=Gmax/{1+exp[-(V-V1/2)zF/RT]} (where V is the applied potential, V1/2 is the half-activation voltage (i.e., the voltage where G is 50% of Gmax), z is the effective gating charge,, and R, T and F are the ideal gas constant, absolute temperature, and Faraday’s constant respectively). Gmax, V1/2and are obtained directly from the fitted curve. The relationship of Gk/Gmax to voltage was also fitted with the Boltzmann equation. The calculation of K+out kinetics followed the identical method as for analysis of K+in currents, except that the current densities at voltages from -59 mV to 61 mV were selected. Data were fitted with SigmaPlot 8.0.
Half-activation time (τ1/2) was defined as the time point at which half-maximal current amplitude was reached, and was calculated directly from the current traces obtained at the voltages specified in Figure 5E. Current amplitude was defined as the difference in current magnitude at 20 ms and the mean current value from 3.60 to 3.88 s, measured using pCLAMP 6.03.
Supplementary Material
Acknowledgements
Funded by USDA grant 2006-35100-17254 (SMA) and grants from the Department of Energy Bioscience Program and National Institutes of Health (SYH).
References
- Ali R, Ma W, Lemtiri-Chlieh F, Tsaltas D, Leng Q, von Bodman S, Berkowitz GA. Death don’t have no mercy and neither does calcium, Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell. 2007;19:1081–1095. doi: 10.1105/tpc.106.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amborabe BE, Bonmort J, Fleurat-Lessard P, Roblin G. Early events induced by chitosan on plant cells. J Exp Bot. 2008;59:2317–2324. doi: 10.1093/jxb/ern096. [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gómez-Gómez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Assmann SM. G protein regulation of disease resistance during infection of rice with rice blast fungus. Sci.STKE 2005. 2005a doi: 10.1126/stke.3102005cm13. cm13. [DOI] [PubMed] [Google Scholar]
- Assmann SM. G proteins go green: A plant G protein signaling FAQ sheet. Science. 2005b;310:71–73. doi: 10.1126/science.1118580. [DOI] [PubMed] [Google Scholar]
- Assmann SM, Schwartz A. Synergistic effect of light and fusicoccin on stomatal opening: Epidermal peel and patch clamp experiments. Plant Physiol. 1992;98:1349–1355. doi: 10.1104/pp.98.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Huang JS, Knopp JA. The hypersensitive reaction of tobacco to Pseudomonas syringae pv. pisi: Activation of a plasmalemma K+/H+ exchange mechanism. Plant Physiol. 1985;79:843–847. doi: 10.1104/pp.79.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CL, Alarcon-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999;63:266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent AF, Mackey D. Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 2007;45:399–436. doi: 10.1146/annurev.phyto.45.062806.094427. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Grabov A, Brearley J, Hammond-Kosack K, Jones JDG. K+ channels of Cf-9 transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9 elicitor-dependent signal transduction. Plant J. 1999;19:453–462. doi: 10.1046/j.1365-313x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Blatt MR. K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J.Gen.Physiol. 1992;99:615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR. Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- Boureau T, Routtu J, Roine E, Taira S, Romantschuk M. Localization of hrpA-induced Pseudomonas syringae pv. tomato DC3000 in infected tomato leaves. Mol. Plant Pathol. 2002;3:451–460. doi: 10.1046/j.1364-3703.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JDG, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Coursol S, Fan LM, Stunff HL, Spiegel S, Gilroy S, Assmann SM. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature. 2003;423:651–654. doi: 10.1038/nature01643. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Ding L, Chen JG, Jones AM, Assmann SM. Heterotrimeric G-protein-coupled signaling in higher plants. In: Yang Z, editor. Plant Cell Signaling. Blackwell Scientific; Oxford: in press. [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. PNAS. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. Nitric oxide regulates K+ and Cl- channels in guard cells through a subset of abscisic acid-evoked signaling pathways. PNAS. 2003;100:11116–11121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Lamattina L. Abscisic acid (ABA) inhibits light-induced stomatal opening through calcium- and nitric oxide-mediated signaling pathways. Nitric Oxide. 2007;17:143–151. doi: 10.1016/j.niox.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta. 1997;201:84–95. [Google Scholar]
- Gudesblat GE, Iusem ND, Morris PC. Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytologist. 2007;173:713–721. doi: 10.1111/j.1469-8137.2006.01953.x. [DOI] [PubMed] [Google Scholar]
- Guimaraes RL, Stotz H,U. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004;136:3703–3711. doi: 10.1104/pp.104.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Moran N, Schwartz A. The role of potassium channels in the temperature control of stomatal aperture. Plant Physiol. 1995;108:1161–1170. doi: 10.1104/pp.108.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Schwartz A, Moran N. External pH effects on the depolarization-activated K channels in guard cell protoplasts of Vicia faba. J.Gen.Physiol. 1994;103:807–831. doi: 10.1085/jgp.103.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Schwartz A, Moran N. External protons enhance the activity of the hyperpolarization-activated K channels in guard cell protoplasts of Vicia faba. J. Membr. Biol. 1996;154:169–181. doi: 10.1007/s002329900142. [DOI] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 2006;9:654–663. doi: 10.1016/j.pbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Jones AM, Ecker JR, Chen JG. A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 2003;131:1623–1627. doi: 10.1104/pp.102.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17:957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WB, Esser JE, Schroeder JI. Effects of cytosolic calcium and limited, possible dual, effects of G protein modulators on guard cell inward potassium channels. The Plant J. 1995;8:479–489. [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM. Active oxygen production during a bacteria induced hypersensitive reaction in tobacco suspension cells. Phytopathology. 1989;79:974–978. [Google Scholar]
- Kinoshita T, Shimazaki K-I. Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol. 2001;42:424–432. doi: 10.1093/pcp/pce055. [DOI] [PubMed] [Google Scholar]
- Klüsener B, Young JJ, Murata Y, Allen GJ, Mori IC, Hugouvieux V, Schroeder JI. Convergence of calcium signaling pathways of pathogenic elicitors and abscisic acid in Arabidopsis guard cells. Plant Physiol. 2002;130:2152–2163. doi: 10.1104/pp.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler B, Hills A, Blatt MR. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol. 2003;131:385–388. doi: 10.1104/pp.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Yang G, Hayashi N, Kaku H, Umemura K, Iwasaki Y. Alterations by a defect in a rice G protein alpha subunit in probenazole and pathogen-induced responses. Plant, Cell & Environment. 2004;27:947–957. [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, Schroeder JI. Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in arabidopsis. Plant Physiol. 2001;127:473–485. [PMC free article] [PubMed] [Google Scholar]
- Lanfermeijer FC, Staal M, Malinowski R, Stratmann JW, Elzenga JT. Micro-electrode flux estimation confirms that the Solanum pimpinellifolium cu3 mutant still responds to systemin. Plant Physiol. 2008;146:129–139. doi: 10.1104/pp.107.110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, Véry AA, Simonneau T, Sentenac H. Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. PNAS. 2008;105:5271–5276. doi: 10.1073/pnas.0709732105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-S. The mechanism of stomatal closing by salicylic acid in Commelina communis L. J. Plant Biol. 1998;41:97–102. [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006;4:1732–1748. doi: 10.1371/journal.pbio.0040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A. ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. The Plant J. 2005;43:165–180. doi: 10.1111/j.1365-313X.2005.02440.x. [DOI] [PubMed] [Google Scholar]
- Lohse G, Hedrich R. Characterization of the plasma-membrane H+-ATPase from Vicia faba guard cells. Planta. 1992;188:206–214. doi: 10.1007/BF00216815. [DOI] [PubMed] [Google Scholar]
- Marre E. Fusicoccin: A tool in plant physiology. Annu. Rev. Plant Biol. 1979;30:273–288. [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, He SY. Role of plant stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008 doi: 10.1146/annurev.phyto.121107.104959. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthe B, Schulz M, Schnable H. Effects of salicylic acid on growth and stomatal movements on Vicia faba L. evidence for salicylic acid metabolism. J. Chem. Ecol. 1992;18:1525–1539. doi: 10.1007/BF00993226. [DOI] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, Giraudat J, Leung J. Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J. 2007;26:3216–3226. doi: 10.1038/sj.emboj.7601750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema H, Assmann SM. A membrane-delimited effect of internal pH on the K+ outward rectifier of Vicia faba guard cells. J. Membr. Biol. 1996;154:227–237. doi: 10.1007/s002329900147. [DOI] [PubMed] [Google Scholar]
- Mori IC, Pinontoan R, Kawano T, Muto S. Involvement of superoxide generation in salicylic acid-induced stomatal closure in Vicia faba. Plant Cell Physiol. 2001;42:1383–1388. doi: 10.1093/pcp/pce176. [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. Febs Letters. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Jones AM, Assmann SM. Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr. Opin. Plant Biol. 2004;7:719–731. doi: 10.1016/j.pbi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007;58:219–247. doi: 10.1146/annurev.arplant.57.032905.105434. [DOI] [PubMed] [Google Scholar]
- Sokolovski S, Blatt MR. Nitric oxide block of outward-rectifying K+ channels indicates direct control by protein nitrosylation in guard cells. Plant Physiol. 2004;136:4275–4284. doi: 10.1104/pp.104.050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolovski S, Hills A, Gay R, Garcia-Mata C, Lamattina L, Blatt MR. Protein phosphorylation is a prerequisite for intracellular Ca2+ release and ion channel control by nitric oxide and abscisic acid in guard cells. Plant J. 2005;43:520–529. doi: 10.1111/j.1365-313X.2005.02471.x. [DOI] [PubMed] [Google Scholar]
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K. The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. PNAS. 2002;99:13307–13312. doi: 10.1073/pnas.192244099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol. 2006;140:210–220. doi: 10.1104/pp.105.069625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NC, Graniti A. Fusicoccin: a fungal toxin that opens stomata. Nature. 1969;223:1070–1071. [Google Scholar]
- Underwood W, Melotto M, He SY. Role of plant stomata in bacterial invasion. Cell. Microbiol. 2007;9:1621–1629. doi: 10.1111/j.1462-5822.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Ullah H, Jones AM, Assmann SM. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Wu WH, Assmann SM. Differential responses of abaxial and adaxial guard cells of broad bean to abscisic acid and calcium. Plant Physiol. 1998;118:1421–1429. doi: 10.1104/pp.118.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Tsuichihara N, Etoh T, Iwai S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007;30:1320–1325. doi: 10.1111/j.1365-3040.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- Yu I.c., Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. PNAS. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Miao YC, Aa GY, Zhou Y, Shangguan ZP, Gao JF, Song CP. K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res. 2001;11:195–202. doi: 10.1038/sj.cr.7290086. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Nurnberger T, Frachisse JM, Wirtz W, Guern J, Hedrich R, Scheel D. Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. PNAS. 1997;94:2751–2755. doi: 10.1073/pnas.94.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.