Abstract
Retinoic acid (RA) is a signaling molecule important for photoreceptor development in vertebrates. The purpose of this study was to examine the mechanisms of the effects of RA upon developing rod and cone photoreceptors in the embryonic zebrafish. Exposure to exogenous RA increased the number of photoreceptors expressing rod opsin and red cone opsin, and decreased the number of photoreceptors expressing the blue and UV cone opsins, suggesting targeted effects of RA on photoreceptor development. RA exposure also increased opsin expression in individual rods and red cones, but decreased opsin expression in individual blue and UV cones, as indicated by differences in the strength of opsin hybridization in identified photoreceptors. RA exposure did not, however, significantly alter quantitative measures of photoreceptor pattern in a manner expected for changes in photoreceptor fate. These observations collectively indicate that RA treatment does not affect photoreceptor fate, but rather differentially influences opsin transcription in determined photoreceptors. An enzyme involved in RA synthesis, RALDH2, was immunocytochemically localized to retinal progenitor cells and the retinal pigmented epithelium (RPE), suggesting the presence of RA in the vicinity of developing photoreceptors. However, expression of an RA response element-driven transgene was restricted to the RPE, retinal progenitors, and a small population of neurons in ventral retina, suggesting that the endogenous RA signaling system is spatially limited within the eye.
Keywords: Photoreceptor, Opsin, Cell fate, Retinoic acid, Danio rerio, Retina, Cone, Nuclear receptor signaling, Differentiation
Introduction
Retinoids are vitamin A metabolites that are vital signaling components involved in vertebrate development (reviewed by Means and Gudas, 1995; Durston et al., 1998; Ross et al., 2000). The importance of vitamin A for the development and maintenance of the visual system has been recognized since Dowling’s (1964) observations of degenerative retinal phenotypes in vitamin A-deprived rats. Furthermore, vitamin A has important clinical applications in the treatment of retinal degenerative disorders such as retinitis pigmentosa (Massof and Finkelstein, 1993).
Retinoic acid (RA), a primary metabolite of vitamin A, is known to affect early eye morphogenesis. For example, pharmacological reduction of RA signaling in rats affects the closure of the optic fissure by altering the expression pattern of RA-dependent genes (Stull and Wikler, 2000). Treatment of Xenopus embryos with RA can result in the complete lack of eyes or in severe defects in retinal patterning (Eagleson et al., 2001). In zebrafish, application of exogenous RA at a time when optic primordia begin to develop, causes duplication of the retina (Hyatt et al., 1992), or an expansion of retinal regions expressing ventral retinal markers (Hyatt et al., 1996a). Correspondingly, treatment with an RA synthesis inhibitor selectively disrupts the development of ventral retina (Marsh-Armstrong et al., 1994).
RA is also known to affect later events in ocular development. Specifically, various animal and cell culture models have indicated an important role of RA in regulating the development of retinal photoreceptors. In vitro examinations have revealed that RA increases differentiation and survival of photoreceptors (chick; Stenkamp et al., 1993), and biases progenitor cells toward the photoreceptor cell fate (rat: Kelley et al., 1994, 1995). In rat retinal cultures, RA increases the number of cells that incorporate the thymidine analog BrdU, suggesting that RA influences cells at the level of cell cycle progression (Kelley et al., 1994). An influence of RA on postmitotic cells is also indicated by studies using pellet cultures of embryonic mouse retinal cells (Wallace and Jensen, 1999), in which RA accelerates photoreceptor differentiation. Interestingly, in cultured retinal explants, retinoids stimulate photoreceptor differentiation only in the absence of RPE; when RPE is present, retinoids induce apoptosis (Soderpalm et al., 2000), suggesting a role for RPE in either metabolizing retinoids, or otherwise regulating their effects upon other retinal cell types. Treatment of human retinoblastoma cell lines with RA upregulates the transcription of several photoreceptor-specific genes (Bernard and Klein, 1996; Boatright et al., 2002; Li et al., 2002). Lastly, Hyatt et al. (1996b) demonstrated selective effects of RA upon zebrafish photoreceptor development in vivo: RA treatment stimulates rod differentiation but delays cone maturation, whereas an inhibitor of RA synthesis suppresses rod differentiation. These studies collectively demonstrate that RA can influence disparate cellular aspects of early and late ocular development. But although the presence of RA synthesizing enzymes and RA receptors has been demonstrated in the eye of numerous vertebrates (Marsh-Armstrong et al., 1994; Mey et al., 1997; McCaffery et al., 1999; Hoover et al., 2001; Mori et al., 2001; Azadi et al., 2002), the signaling mechanisms through which RA achieves its specific developmental effects remain unclear.
In the present study, we have addressed these issues by investigating the mechanisms through which RA signaling affects photoreceptor development in the zebrafish embryo. The zebrafish retina contains rods and four classes of cones (with red, green, blue, or UV spectral sensitivity) that can be identified based upon gene expression and morphology (Branchek and BreMiller, 1984; Raymond et al., 1995). Photoreceptor recruitment takes place in a stereotyped pattern; the first photoreceptors of each class differentiate in ventral retina (a site that corresponds to the future site of the optic disk), and the subsequent developmental waves follow a “fan gradient,” from ventral to nasal, to dorsal, to temporal (Raymond et al., 1995; Stenkamp et al., 1996; Schmitt and Dowling, 1996; Hu and Easter, 1999). Effects of extracellular signaling molecules on photoreceptor differentiation can be assessed in zebrafish by how they influence the propagation of each wave of retinal development (Stenkamp et al., 2000; Stenkamp and Frey, 2003). Furthermore, zebrafish cone photoreceptors are arranged in a subtype-specific, geometric mosaic array and in specific phenotypic ratios that are evident upon initial differentiation and that do not show topographical variation (Larison and BreMiller, 1990; Raymond et al., 1993, 1995). Effects of extracellular signaling molecules on photoreceptor (subtype) determination can therefore be assessed by how they influence cone pattern and cone ratios (Stenkamp et al., 2001).
Our results suggest a complex, but targeted, network of RA effects upon photoreceptor development: RA treatment promotes the differentiation of rods and red-sensitive cones, decreases that of blue and ultraviolet-sensitive cones, and has negligible effect on green-sensitive cones. Because these effects suggested a manipulation of photoreceptor fate, we tested the hypothesis that exogenous RA regulates phenotype selection at the level of unspecified photoreceptors by performing pattern analysis on identified cone types following RA treatment. Our data favor the alternative hypothesis that RA treatment differentially regulates photoreceptor gene transcription (i.e., opsin expression) in specified photoreceptors. Additionally, the cell-specific expression patterns of an enzyme involved in RA synthesis, RALDH2, and of an RA response element-driven transgene, indicated loci of direct RA signaling remote from the majority of developing photoreceptors. Our results suggest that RA may regulate opsin expression in specified zebrafish photoreceptors in vivo in a spatially restricted manner.
Methods
Animals and tissue processing
Reproductively mature zebrafish were maintained in monitored aquatic housing units on recirculating system water at 28.5°C. Embryos were collected according to Westerfield (2000), with light onset considered to be zero hours postfertilization (hpf) and embryonic age timed accordingly thereafter. In order to inhibit melanin synthesis and keep embryos transparent for analysis, embryos were treated with 0.003% phenothiourea (PTU) at 11 hpf (Westerfield, 2000).
A transgenic zebrafish line generated to examine the locations of RA signaling was the gift of Elwood Linney (Duke University). These transgenic fish contain RA response elements (RAREs), driving expression of a yellow fluorescent protein reporter (YFP) gene (this line was referred to as RGnY; Perz-Edwards et al., 2001). The transgenic DNA construct consists of three copies of RAREs derived from the mouse RAR-beta gene, a zebrafish basal promoter, an enhanced YFP sequence, an SV40 polyadenylation signal, and a small t intron (Perz-Edwards et al., 2001). The zebrafish RAREs are identical in sequence to mouse RAREs and have been shown to have identical function (Nolte et al., 2003).
Stock solutions of all-trans RA and 9-cis RA (Sigma, St. Louis, MO) were prepared in dimethylsulfoxide (DMSO) and stored under nitrogen in the dark at −20°C. Embryos were manually dechorionated, and stock solution added to embryo water at 1:1000, resulting in a final concentration of 0.3 μM unless otherwise noted. This concentration was selected to be consistent with the previous work of Hyatt et al. (1996b), and based upon preliminary dose–response experiments revealing no additional effects at higher concentrations (data not shown). Control embryos received equivalent volumes of DMSO.
Dechorionated embryos were fixed in 4% paraformaldehyde in phosphate-buffered, 5% sucrose for 1 h, and were then washed in phosphate-buffered, 5% sucrose. Some embryos were then stored in 100% methanol, for analysis as whole mounts, others were washed in increasing concentrations of sucrose, cryoprotected overnight at 4°C in phosphate-buffered, 20% sucrose, then embedded and frozen in a 1:2 solution of OCT medium (Sakura Finetek, Torrance, CA): 20% sucrose, and sectioned at 3 μm, as described in Barthel and Raymond (1990).
In situ hybridization and immunocytochemistry
The zebrafish opsin cDNAs, in pBK-CMV phagemid, were the gifts of Thomas Vihtelic (University of Notre Dame). Digoxigenin (dig)-labeled cRNA probes were prepared according to the Genius user guide (Roche, Indianapolis, IN). In situ hybridizations were done according to Barthel and Raymond (1993). In brief, tissue was rehydrated and treated with (10 μg/ml) proteinase K, dehydrated, and then hybridized overnight at 56°C with 1 mg/ml probe in a hybridization solution containing 50% formamide. Hybridization was visualized by using an anti-dig antibody coupled to alkaline phosphatase (Roche), and a color reaction using the substrates 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP).
The anti-RALDH2 antibody (used at 1:100) was the gift of Peter McCaffery (Shriver Institute, Massachusetts); zpr-1 (used at 1:200) was purchased from the University of Oregon zebrafish monoclonal stock center; rabbit polyclonal anti-GFP antibody (used at 1:1000) was purchased from Torrey Pines Biolabs (Houston, TX). The green and blue opsin antibodies (each used at 1:250) were the gifts of Thomas Vihtelic (University of Notre Dame). Immunocytochemistry was performed as described (Stenkamp et al., 2000). In brief, tissue was blocked for 30 min in 20% goat serum, then incubated with primary antibody overnight at 4°C, washed in phosphate-buffered saline containing 0.05% Triton X-100 (Sigma), then incubated with Cy3- or fluorescein-conjugated secondary antibody (Jackson Immunoresearch) at 1:200 for 90 min at room temperature (sections) or overnight at 4°C (whole mounts).
Photography and statistics
Tissue was mounted in glycerol, or in glycerol containing phenylenediamine for preservation of fluorescence, and viewed under brightfield (whole mounts), Nomarski (sections), and/or epifluorescence optics on a Leica DMR compound microscope. Images were collected using a Spot digital camera. In some cases, images collected under different optical conditions were superimposed using the ‘merge image’ function in the Spot camera software.
Photoreceptors in the embryonic zebrafish differentiate in a spatiotemporal wave that begins in a ventral patch, spreads nasally, and then across the entire retina (Raymond et al., 1995). Eyes of treated vs. untreated embryos were scored based upon the extent of photoreceptor opsin labeling, according to this stereotyped pattern, using the stages defined by Raymond et al. (1995). The stages are based on the number of opsin-expressing cells such that a higher stage would have greater spatial extent of photoreceptor recruitment with a greater number of opsin-expressing cells. χ2 analysis was used to test if differences in the scored stages were statistically significant; the null hypothesis was that there was no difference in the percentage of eyes at the different stages of photoreceptor recruitment between RA-treated and control embryos.
Appropriate images of a stage micrometer were used to measure diameters of labeled cells in images of the retina. Student’s t test was used to determine if there was any significant difference in diameters of labeled cells between RA-treated and control embryos.
Starting material for pattern analysis consisted of either theoretical patterns based upon the known cone mosaic, or images (representing 40×40 μm2) of whole mounted embryo eyes from either control or RA-treated embryos that had been processed for in situ hybridization or immunocytochemistry. Identified cells were assigned unique, coplanar (x, y) coordinate values by using ScionImage (Frederick, MD) software. The following three methods were then used to quantitatively analyze the resultant patterns, using custom software (Stenkamp et al., 2001; Cameron and Carney, 2004): nearest neighbor distance analysis (Cook, 1996); density recovery profile analysis (Rodieck, 1991); and quadrant analysis (Grieg-Smith, 1964; Stenkamp et al., 2001). Further information on these methods appears in the Supplemental Text.
Results
Effects of RA on rod and cone photoreceptor differentiation
The effects of RA exposure on differentiation of rod photoreceptors and the four spectral classes of cones in the zebrafish were investigated. Embryos were exposed to exogenous all-trans or 9-cis RA continuously from 51–75 hpf, during which time there are both proliferative photoreceptor progenitors and postmitotic, differentiating photoreceptors (Nawrocki et al., 1985; Raymond et al., 1995). Time points beyond 75 hpf were excluded from the analysis because of substantial technical difficulty in the application of the whole mount in situ hybridization/visualization methodology (see Methods). Consistent with the results of Hyatt et al. (1996b), embryos treated with either all-trans or 9-cis RA contained more rod opsin-expressing cells than their control counterparts (Figs. 1A, B). RA-treated embryos also contained a greater number of red cone opsin-expressing cells than controls (Figs. 1C, D) (the red cone opsin cRNA used here corresponds to LWS-2, the first of two red cone opsin genes expressed in zebrafish retina; Takechi and Kawamura, 2005). To determine the effects of RA exposure on green cones, we used indirect immunofluorescence with a green cone antibody; this antibody likely identifies the gene product of RH2-1, the first of four green cone opsin genes expressed in zebrafish retina (Vihtelic et al., 1999; Takechi and Kawamura, 2005). These studies indicated no significant effect of RA exposure upon cones expressing green opsin (data not shown). However, RA treatment also resulted in decreases in the number of cells expressing the blue or UV cone opsins (Figs. 1E–H). These results indicated differential effects of RA treatment upon zebrafish photoreceptor development in vivo.
Fig. 1.
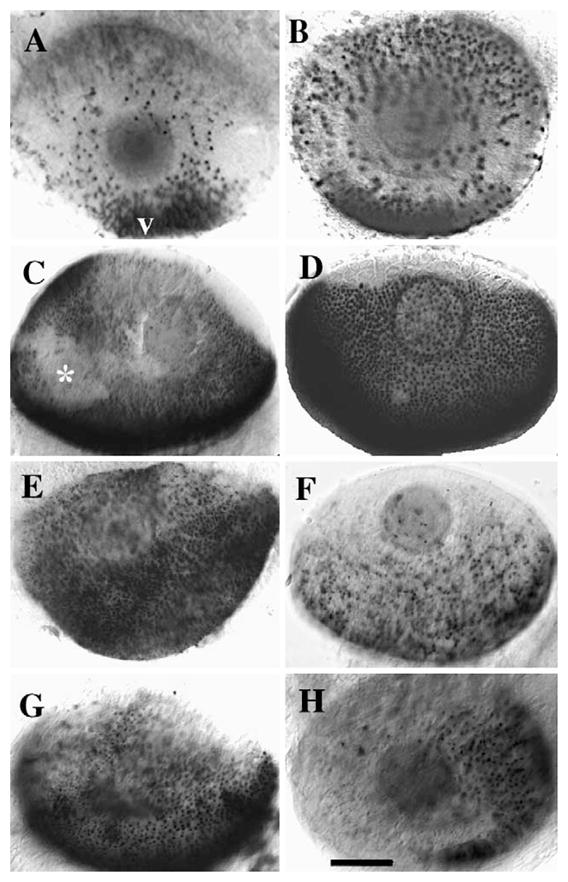
Effects of RA on rod and cone opsin expression. Panels show lateral views of whole-mounted eyes from DMSO-treated zebrafish embryos (A, C, E, G) or embryos treated with RA (B, D, F, H) at 51 hpf and fixed at 75 hpf. Embryos were hybridized with rod opsin riboprobes (A and B), red cone opsin riboprobes (C and D), blue cone opsin riboprobes (E and F), or UV cone opsin riboprobes (G and H). v, ventral (for all panels); scale bar = 40 μm; * indicates slight damage to eye tissue during processing.
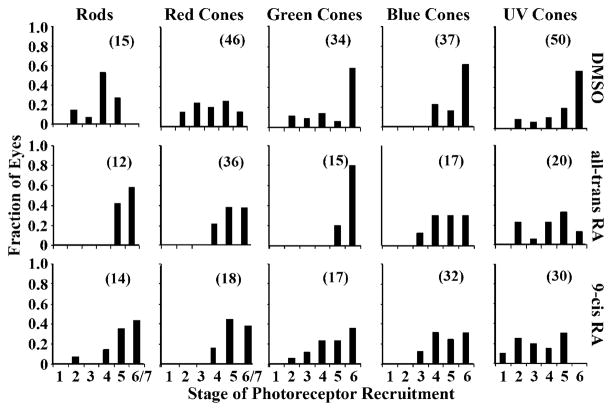
To further quantify the effects of exogenous RA upon photoreceptor development, retinas were scored according to the stages of photoreceptor recruitment, such that higher stages correspond to the presence of more opsin-expressing photoreceptors (Raymond et al., 1995; see Methods). Frequency distributions of the scores derived for the retinas analyzed in this study are provided in Fig. 2. For a given absolute developmental time point, retinas from RA-treated embryos were at higher stages of recruitment for rods and red cones, and at lower stages for blue cones and UV cones, compared to untreated controls. χ2 analysis of the frequency distributions obtained for rods indicated that the null hypothesis (i.e., that RA had no effect on stage of photoreceptors recruitment as indicated by the number of photoreceptors) was rejected at the 5% level for all-trans RA exposure, and at the 10% level for 9-cis RA exposure (Table 1). RA (all-trans and 9-cis) also significantly affects red cone recruitment (P < 0.05, χ2 analysis; Table 1). Green cone recruitment was not significantly affected for 9-cis RA treatment but all-trans RA treatment affected green cone recruitment at the 10% level (Table 1). The null hypothesis could also be rejected for the results of 9-cis and all-trans exposure for blue and UV cones (P < 0.05, χ2 analysis; Table 1). Because 9-cis and all-trans RA treatment showed largely similar effects, and because all-trans RA is a ligand only for RARs (which typically influence gene expression through interaction with RXRs; Glass, 1996), these effects are inferred to be mediated by RAR/RXR heterodimers. These findings also confirm that the observed effects of RA on photoreceptors are statistically significant, and may arise from differential effects upon either the selection of ultimate photoreceptor fate (e.g., red vs. blue cone), or the temporal dynamics of photoreceptor development, such that the development of rods and red cones is accelerated, but the development of blue and UV cones is slowed.
Fig. 2.
Fractions of eyes at different stages of photoreceptor recruitment after RA or a control treatment at 51 hpf and fixation at 75 hpf. Top row: eyes from DMSO-treated control embryos. Middle row: eyes from all-trans RA-treated embryos. Bottom row: eyes from 9-cis RA-treated embryos. Columns of panels, from left to right, show results for rods, red cones, green cones, blue cones, and UV cones. Stages 6 and 7 have been binned together in this figure. The numbers of eyes analyzed for each treatment/riboprobe combination are indicated parenthetically in each panel (the number of embryos analyzed in each case is approximately half the number of eyes).
Table 1.
χ2 analysis of the effects of RA on photoreceptor recruitmenta
| Rods | Red cones | Green conesb | Blue cones | UV cones | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | AT-RAc | 9-cis RA | AT-RA | 9-cis RA | AT-RA | 9-cis RA | AT-RA | 9-cis RA | AT-RA | 9-cis RA |
| P value | 0.0012 | 0.0508 | 0.0005 | 0.018 | 0.089 | 0.2639 | 0.0446 | 0.0253 | 0.0041 | 0.0003 |
Analysis was based upon fraction of eyes in each treatment, assigned to each photoreceptor recruitment stage (see Fig. 2).
Detected by indirect immunofluorescence for green opsin; all others detected by in situ hybridization for a specific opsin.
AT-RA, all-trans retinoic acid.
Effects of RA on rod and cone photoreceptor phenotypic fate
Two-dimensional pattern analyses were performed to test the hypothesis that RA-induced increases in rod opsin- and red opsin-expressing cells, coincident with the decreases in blue opsin- and UVopsin-expressing cells, were the result of changes in photoreceptor phenotypic fate. Cone photoreceptors in the zebrafish retina are arranged in a highly regular, two-dimensional mosaic in which each spectral cone type occupies a stereotypical position (Raymond et al., 1995), and the regularity of this mosaic is apparent upon initial cone differentiation in the embryo (Larison and BreMiller, 1990). Any switches in cone phenotype would therefore be reflected by abnormalities in the cone pattern, and theoretical analysis has confirmed that modest switches in cell fate, even those less than 5%, can be detected by nearest neighbor distance analysis (NND; see Supplemental Figs. 1 and 2). Specifically, the conformity ratios derived from NND analyses of modeled cone patterns (Cook, 1996) predictably decreased as a function of fractional cone fate switches, and were therefore a reliable indicator of cone type repertoires (i.e., cone fate decisions) that deviate from those of normal retina (see Supplemental Figs. 1 and 2).
We wished to confirm that our techniques were also likely to detect pattern abnormalities in small sample sizes (such as those obtained from the spatially limited, optically coplanar area of a zebrafish embryo eye). Therefore, we generated and analyzed theoretical fate change patterns resulting from cone fate changes (UV to red or rod, and blue to red or rod). The theoretical fate change patterns all generated NND data distinct from that of the normal pattern, most evident as reduced conformity ratios that were in most cases correlated with the percentage fate switch (Table 2 and Supplemental Figs. 1 and 2).
Table 2.
Auto-correlative and cross-correlative pattern analysis of theoretical retinal cone mosaics resulting from photoreceptor fate change in small sample sizes
| Cone type | Image type | # of cells | % fate change | NNDa | CRb |
|---|---|---|---|---|---|
| Auto-correlation | |||||
| Red | Control | 69 | n/a | 1.80 | 6.36 |
| Fate change 1 | 81 | 17 | 1.48 | 3.29 | |
| Fate change 2 | 85 | 23 | 1.40 | 3.02 | |
| Fate change 3 | 88 | 28 | 1.36 | 3.09 | |
| Blue | Control | 34 | n/a | 3.00 | 8.37 |
| Fate change 1 | 22 | 35 | 3.08 | 7.72 | |
| Fate change 2 | 15 | 47 | 3.60 | 4.38 | |
| Fate change 3 | 18 | 56 | 3.78 | 4.37 | |
| Cross-correlation | |||||
| R/Gc to blue | Control | 137 (R/G) | n/a | 1.40 | 3.44 |
| Fate change 1 | 149 (R/G) | 9 | 2.00 | 2.12 | |
| Fate change 2 | 153 (R/G) | 12 | 2.20 | 2.22 | |
| Fate change 3 | 156 (R/G) | 14 | 2.60 | 2.00 | |
| Blue to R/G | Control | 34 (blue) | n/a | 1.20 | 3.44 |
| Fate change 1 | 22 (blue) | 35 | 1.80 | 1.95 | |
| Fate change 2 | 18 (blue) | 47 | 2.20 | 2.22 | |
| Fate change 3 | 15 (blue) | 56 | 2.40 | 1.80 | |
NND, nearest neighbor distance, arbitrary units (theoretical patterns).
CR, conformity ratio.
R/G, red- and green-sensitive double cones.
NND analysis was applied to cone photoreceptor patterns derived from control and RA-treated embryos. Although rods can also show a regular pattern (Fadool, 2003) that may be affected by photoreceptor fate change, embryonic rods were present at too low a density for pattern analysis to be feasible. The patterns of each cone type, seen in RA-treated embryos were not different from those of control embryos (Table 3). Most importantly, the conformity ratios of RA-treated patterns predicted fate changes of 0% (see Supplemental Fig. 2B and compare to Table 2), indicating an apparent absence of RA-dependent changes in cone fates.
Table 3.
Auto-correlative and cross-correlative pattern analysis for experimental retinal cone patterns in control and all-trans RA-treated zebrafish embryos
| Cone type | Treatment | Mean NNDa | Mean CRb | Predicted fate changec |
|---|---|---|---|---|
| Auto-correlation | ||||
| Redd | Control | 5.33 ± 0.28 | 5.09 ± 1.3 | 0% |
| RA | 4.79 ± 0.21 | 4.95 ± 1.1 | ||
| Green | Control | 4.57 ± 2.12 | 2.68 ± 1.7 | n/a |
| RA | 5.32 ± 0.89 | 4.57 ± 2.6 | ||
| Blued | Control | 7.10 ± 2.61 | 4.43 ± 0.8 | 0% |
| RA | 5.78 ± 0.59 | 4.40 ± 0.5 | ||
| UVd | Control | 5.13 ± 0.59 | 3.29 ± 1.2 | n/a |
| RA | 5.25 (n = 1) | 3.68 (n = 1) | ||
| Cross-correlation | ||||
| R/Ge to blue | Control | 4.35 ± 1.31 | 2.85 ± 0.6 | 1% |
| RA | 4.49 ± 0.92 | 2.56 ± 0.6 | ||
| Blue to R/G | Control | 3.98 ± 1.27 | 2.93 ± 0.7 | 1% |
| RA | 3.88 ± 0.92 | 2.74 ± 0.6 | ||
NND, nearest neighbor distance, in μm, ±SD.
CR, conformity ratio, ±SD.
Prediction based upon results in Table 2, and in Supplemental Fig. 2.
Cones were detected by in situ hybridization for a specific cone opsin; in other cases, indirect immunofluorescence techniques were used.
R/G, zpr-1-positive red- and green-sensitive double cones.
As an additional test of the phenotypic fate change hypothesis, we performed double immunocytochemical experiments on RA-treated and control embryos, using the following combination of antibodies: zpr-1, which selectively labels red and green-sensitive double cones (Larison and BreMiller, 1990), and a polyclonal antibody against zebrafish blue opsin (Fig. 3). We selected these markers in part because of the experimental simplicity of this immunocytochemical approach, and more importantly because the theoretical studies revealed that cross-correlative NND analysis of double vs. single cones was highly reliable at detecting cone fate changes (see Supplemental Fig. 2B). Eyes of RA-treated embryos displayed evidence of effects of RA on photoreceptors: the spatial extent of zpr-1 immunoreactivity, and thus the extent of red and/or green cone recruitment, was more widespread than in controls; while the spatial extent of immunoreactive blue cones was reduced compared to controls (data not shown). Regions of these retinas that contained both labels were subjected to cross-correlative NND pattern analysis to test for spatial interdependence between the different photoreceptor types (see Supplemental Text). In contrast to the considerable reduction in cross-correlative conformity ratios generated by fate changes in theoretical patterns (Supplemental Fig. 2B and Table 2), RA treatment did not affect the degree of cone spatial interdependence in zebrafish eyes; the decrease in conformity ratios in the RA-treated samples does not correspond to a significant fate change (Table 3, Supplemental Fig. 2B). Furthermore, this decrease in conformity ratios is not statistically significant (R/G to blue, P = 0.53; blue to R/G, P = 0.68). Another potential indicator of RA-dependent cone fate switching –cones coincidently labeled with both antibody markers – was never observed. Collectively, these results do not support the hypothesis that exogenous RA influences in vivo photoreceptor fate in the developing zebrafish.
Fig. 3.
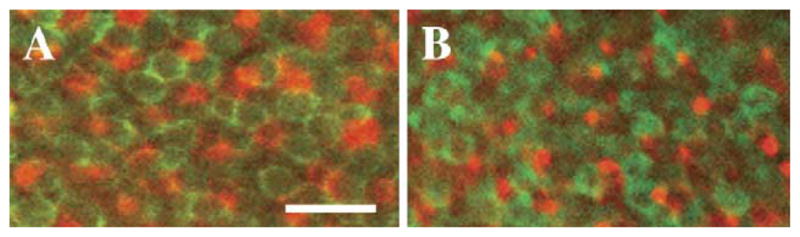
Whole mount double immunocytochemistry using a combination of the zpr-1 (red/green cones) antibody (green fluorescence) and a blue cone opsin antibody (red fluorescence). Regions of whole mounted eye from DMSO-treated control embryos (A) and from RA-treated embryos (B), showing cells stained by each antibody. Embryos were treated at 51 hpf and fixed at 75 hpf. Scale bar = 10 μm.
Effects of RA on rod and cone opsin transcription
The effects of RA exposure on photoreceptor recruitment, as described above, are also consistent with the alternative hypothesis that RA differentially influences gene transcription in photoreceptors already committed to a specific phenotype. A direct test of this hypothesis, in vivo, would require quantitative PCR or a similar method, based on material collected from a single photoreceptor. We instead used an indirect approach, based upon our initial impression that RA treatment resulted in differences in the strength of label present in the photoreceptors, making some photoreceptors appear larger or smaller than others (see Fig. 1). To assess relative strength of labeling, we measured the diameters of labeled photoreceptors in RA-treated vs. untreated embryos. This analysis revealed that RA-treated embryos had significantly larger-diameter, labeled red cones (Fig. 4; 2.63 ± 0.12 μm for RA-treated vs. 2.20 ± 0.10 μm for controls; P = 0.01). However, RA-treated embryos also had significantly smaller-diameter, labeled blue cones (Fig. 4; 2.07 ± 0.23 μm for RA-treated vs. 2.57 ± 0.21 μm for controls; P = 0.05) and labeled UV cones (Fig. 4; 2.10 ± 0.20 μm for RA-treated vs. 2.57 ± 0.15 μm for controls; P = 0.03). Labeled rods were also consistently larger in RA-treated embryos, but this difference was statistically significant only at the 10% level (Fig. 4; 3.20 ± 0.53 μm for RA-treated vs. 2.40 ± 0.10 μm for controls; P = 0.06). These results can be interpreted in at least two ways. Firstly, RA may have influenced actual growth of the photoreceptor cells. Alternatively, RA may have influenced the rate of production of the mRNA present in the cell, resulting in cells that are differentially loaded with hybridization reaction product. We favor the latter interpretation because the diameters of cells labeled with the zpr-1 antibody, which does not label opsin protein but a cell surface epitope on red and green cones (Larison and BreMiller, 1990), were virtually identical in RA-treated vs. control embryos (Fig. 4; 2.20 ± 0.14 μm for RA-treated vs. 2.27 ± 0.22 μm for controls; P = 0.65; see also Fig. 3). We note, however, that the apparently smaller size of individual blue opsin-labeled cones in RA-treated embryos (Fig. 3) may have resulted either from the presence of less blue-sensitive opsin protein, or from decreased growth of blue cones, as low levels of mRNA do not necessarily predict low levels of the corresponding protein. In any case, the effects of exogenous RA are phenotype-specific, and either promote (rods and red cones) or inhibit (blue cones and UV cones) photoreceptor differentiation.
Fig. 4.
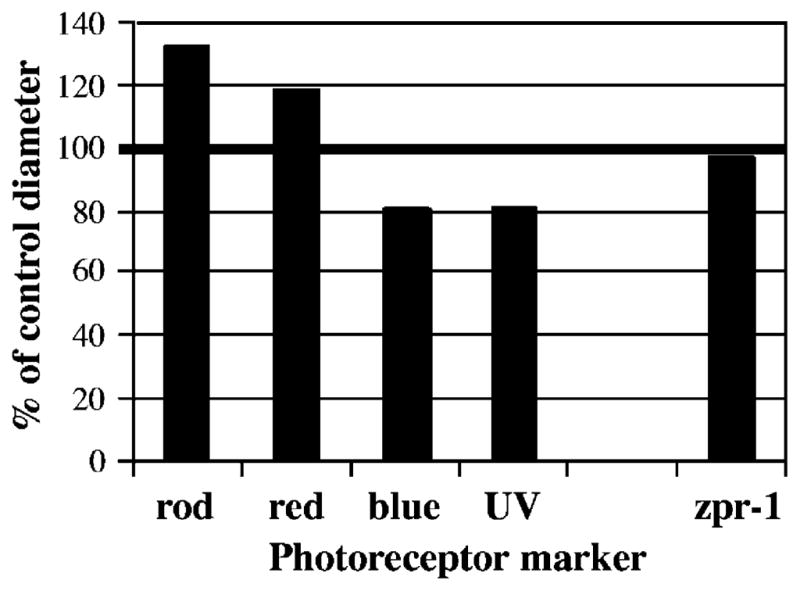
Effects of RA on diameters of labeled photoreceptors, shown as a percent of photoreceptor diameters in control embryos. Embryos were treated at 51 hpf and fixed at 75 hpf. Rods and red cones, hybridized with a specific opsin riboprobe, have larger labeled diameters in RA-treated vs. control embryos, while hybridized blue and UV cones have smaller labeled diameters. The labeled diameters of zpr-1-positive red and green cones do not change as a consequence of RA treatment.
Expression pattern of dorsal RA-synthesizing enzyme, RALDH2
In order to identify endogenous cellular sources of RA in the developing zebrafish retina, we performed immunocytochemical analyses of zebrafish embryos using an antibody against retinaldehyde dehydrogenase 2 (RALDH2). Fig. 5 shows that, at 45 hpf, 52 hpf, and 80 hpf, RALDH2 immunoreactivity is present in the neuroepithelial cells and in some developing photoreceptors in the dorsal retina, as well as in the RPE. The level of RALDH2 labeling apparently increased from 45 hpf to 80 hpf. These results indicate that at least one mechanism of RA synthesis is present in the developing zebrafish retina, and is localized to distinct populations of cells that include some photoreceptors. Both the ventral and the dorsal regions of the embryonic zebrafish retina are known to each contain a biochemically distinct enzyme that synthesizes RA (Marsh-Armstrong et al., 1994), but molecular markers for the ventral enzyme are not available.
Fig. 5.
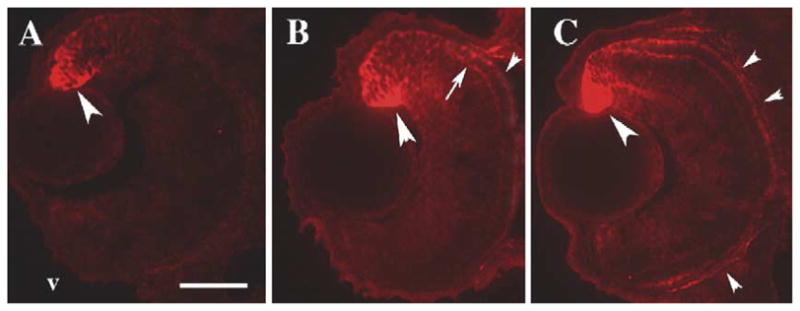
Sectioned zebrafish embryo eyes processed for immunocytochemistry with an anti-RALDH2 (dorsal retinal RA synthesizing enzyme) antibody (red fluorescence). Embryos were fixed at 45 hpf (A), 52 hpf (B), and 80 hpf (C). The antibody labels neuroepithelial cells (large arrowheads) and a few developing neurons, including photoreceptors (arrows), in dorsal retina. At 52 and at 80 hpf, some staining is also evident in ventral retina, throughout the RPE (small arrowheads), and in extraocular locations. v, ventral (in all panels); scale bar = 40 μm.
Expression pattern of RARE-driven YFP in transgenic embryos
In order to identify endogenous mechanisms of RA signaling in the retina, and to assign the mechanisms to particular cellular populations, we examined the expression pattern of a RARE-driven YFP transgene in transgenic embryos (Perz-Edwards et al., 2001). In these transgenic fish, the YFP reporter gene is expressed in cells that contain the necessary components for activation of RAREs, such as RA itself and functional RARs. Transgene expression has previously been demonstrated within the eyes of live embryos (Perz-Edwards et al., 2001).
The cell-specific expression of the RARE-driven YFP transgene was examined with an anti-GFP antibody. At 45 hpf, the transgene is expressed by neuroepithelial cells in ventral retina (Fig. 6A). At 52 hpf, the transgene is expressed by ventral neuroepithelial cells, ventral developing neurons, and by the RPE (Fig. 6B). At 52 hpf, we also detected expression in some dorsal neuroepithelial cells. Unexpectedly, after 52 hpf, the expression of the transgene in the dorsal retina is reduced (Figs. 6C, D), even though RA synthesis continues in the dorsal retina (see Fig. 5; Marsh-Armstrong et al., 1994).
Fig. 6.
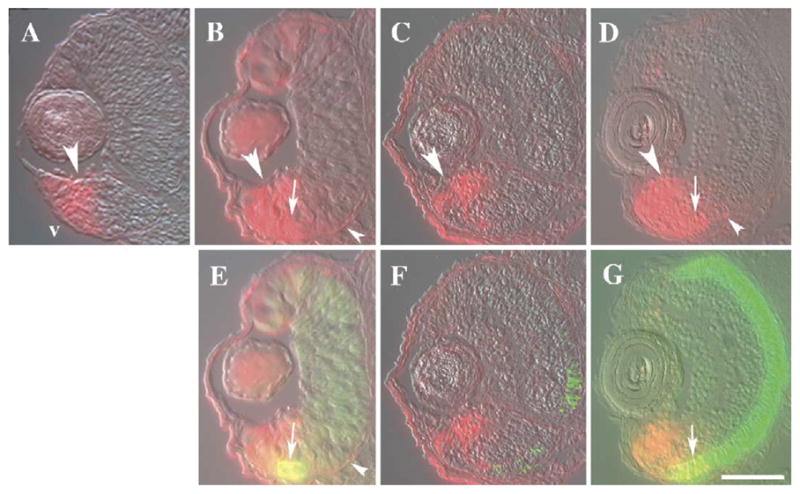
Sectioned eyes derived from embryonic zebrafish, transgenic for a RARE-driven YFP transgene. Embryos were fixed at 45 hpf (A), 52 hpf (B and E), 65 hpf (C and F), and 80 hpf (D and G). (A– D) Immunocytochemistry using an anti-GFP antibody (red fluorescence) shows that neuroepithelial cells (large arrowheads) and a few developing neurons in marginal regions, as well as the RPE (small arrowheads), express the transgene and are therefore likely influenced by RA signaling. Labeling in the dorsal retina decreases after 52 hpf. (E – G) Double immunocytochemistry using the anti-GFP antibody and the zpr-1 (red/green cones) antibody (green fluorescence). Colocalization of the two antibodies (yellow fluorescence) was found in a few ventral photoreceptors at 52 hpf and 80 hpf (arrows). v, ventral (in all panels); scale bar = 40 μm.
To determine if the transgene is expressed in differentiated photoreceptors, we performed double immunocytochemical analyses using the anti-GFP antibody and the zpr-1 antibody. At 52 hpf, we observed a few cones in the ventral retina that expressed both markers (Fig. 6E). In the material derived from 65 hpf embryos, we did not observe any colabeled photoreceptors (Fig. 6F). At 80 hpf, however, differentiated photoreceptors that were colabeled with both antibodies were observed (Fig. 6G).
Our results suggest that endogenous RA signaling mechanisms are located predominantly in the ventral retinal progenitor cells, the RPE, and in a small number of ventral retinal neurons, including only a few photoreceptors. These results further suggest that the dominant mechanism through which RA affects photoreceptor differentiation in vivo involves a spatially restricted cell population, and perhaps cell types other than photoreceptors.
Discussion
Targeted effects of RA on photoreceptor differentiation
Retinoic acid treatment clearly influences photoreceptor development, and in the zebrafish, the nature of the influence depends upon photoreceptor phenotype. Our results largely agree with those of Hyatt et al. (1996b), who demonstrated precocious development of rod photoreceptors and delayed maturation of cone photoreceptors in the continued presence of exogenous RA. We have extended those findings by testing the effects of RA on specific photoreceptor types over the developmental period corresponding to initial photoreceptor determination and differentiation. RA specifically promotes rod- and red cone-specific opsin expression, while apparently downregulating opsins expressed by blue- and UV-sensitive photoreceptors. RA is synthesized in ventral retina at a time when photoreceptors are first generated in that location (Marsh-Armstrong et al., 1994; see also McCaffery et al., 1992). Endogenous, high levels of RA in ventral retina may therefore influence the sequence in which photoreceptor-specific gene expression takes place in that area. Because the apparent effect of RA is to accelerate rod and red cone differentiation, and decelerate blue and UV cone differentiation, it is not surprising that the sequence of opsin expression observed in untreated zebrafish and goldfish embryos is rod, red, green, blue, UV (Raymond et al., 1995; Stenkamp et al., 1996; Schmitt et al., 1999), and in RA-treated embryos, this sequence is exaggerated.
Exogenous RA does not regulate photoreceptor fate
There is considerable debate in the literature regarding the mechanisms by which RA influences photoreceptor development. Clear effects of retinoids on gene transcription have been demonstrated in immortalized cell culture models (Li et al., 2002; Boatright et al., 2002), but a role in retinal cell fate determination has been more controversial. Both primary cell culture (Kelley et al., 1994) and in vivo experiments (Kelley et al., 1999) have shown that RA may favor the photoreceptor phenotype over that of other retinal cells in rat. However, experiments in other animal models have suggested that RA affects other aspects of photoreceptor development. For example, cultured mouse retinal cells respond to RA by accelerating the rate at which they express rod opsin (Wallace and Jensen, 1999), and RA treatment of chick retinal cultures primarily promotes photoreceptor survival (Stenkamp et al., 1993). In our in vivo experiments using the zebrafish, we have not observed overt, RA-dependent effects upon the differentiation of retinal cell types other than photoreceptors (data not shown), consistent with the findings of Hyatt et al. (1996b) and, as above, arguing against a role for RA in influencing cell fate. However, over the time of embryonic photoreceptor development, we did observe striking, differential effects of RA treatment on the different classes of photoreceptor, suggesting that RA may promote specific cone phenotypes.
Genetic manipulation of nuclear receptors that form heterodimers with the RXRs, and of the RXRs themselves, results in changes in the ratios of photoreceptors that express specific opsins (Haider et al., 2000; Ng et al., 2001; Roberts et al., 2005). These receptors may therefore act as ‘phenotypic switches’ that control photoreceptor fate. We therefore tested the hypothesis that RA, which acts via nuclear receptors, may influence photoreceptor phenotype. If RA truly favors the red cone fate over the blue or UV cone fate, this effect would be reflected by irregularities in the cone mosaic. Pattern analysis of singly- and doubly-labeled whole mounted eyes revealed no such irregularities in RA-treated embryos. Furthermore, the spatiotemporal spread of photoreceptor differentiation observed in RA-treated eyes resembled patterns observed at later (in the case of rods and red cones) or earlier (in the case of blue and UV cones) developmental time points (Figs. 1 and 2). These observations suggest that RA induced specified photoreceptors to express phenotype-specific genes earlier (in the case of rods and red cones) or later (in the case of blue and UV cones) in their developmental trajectories, rather than switching their intrinsic phenotype.
In pursuit of this idea, we measured the diameters of labeled, identified rods and cones in RA-treated and -untreated eyes, with the presumption that any differences would reflect either an effect of RA on cell growth, or on the total quantity of hybridized mRNA within that cell. These results supported the hypothesis that RA influences differentiation in specified cones, perhaps by increasing/decreasing transcription of phenotype-specific genes.
Our interpretations are consistent with the predicted functions of nuclear receptors in mouse retina (Haider et al., 2000; Ng et al., 2001; Roberts et al., 2005). In the mouse, the majority of individual cone photoreceptors express both of the mouse cone opsin genes, M-opsin and S-opsin (Applebury et al., 2000; Shupe et al., in press), and nuclear receptor signaling may serve as a ‘toggle’ mechanism that regulates the ratio of the different opsins within each cone (Fig. 7A; Roberts et al., 2005). In a conceptually similar manner, RA signaling in the zebrafish retina appears to regulate the ratios of different opsins, but across the population of cones that are each already specified to express a particular, single opsin (Fig. 7B). Such a mechanism may also be involved in the decrease in UV sensitivity that occurs in salmonids in response to increased nuclear receptor signaling (Browman and Hawryshyn, 1992, 1994).
Fig. 7.
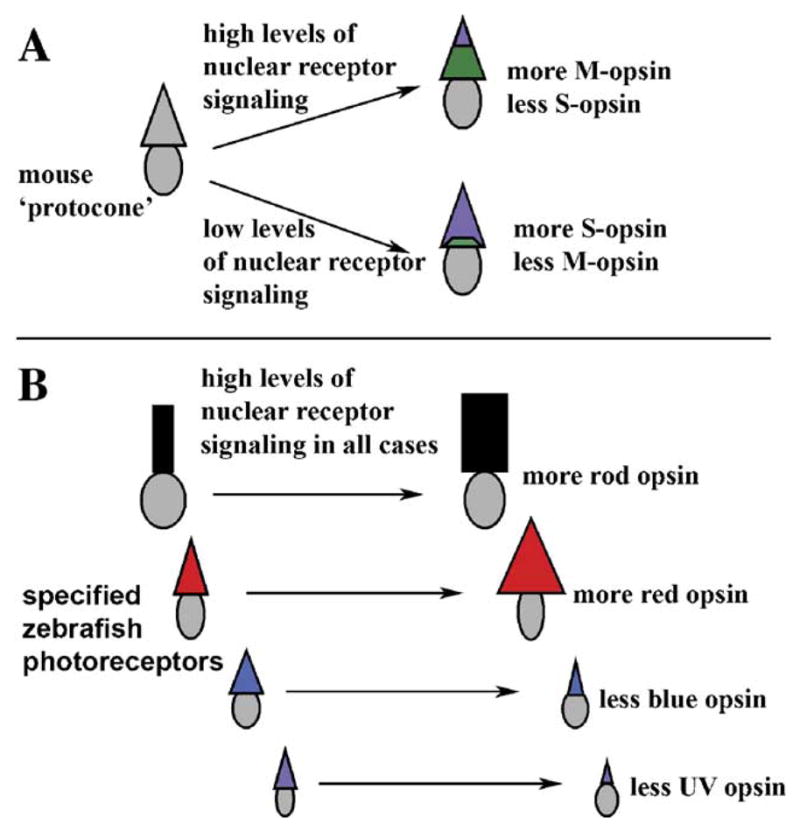
Comparison of the roles of nuclear receptor signaling during photoreceptor development in mouse and zebrafish; high levels of nuclear receptor signaling favor increases in expression of longer wavelength-sensitive opsins in both cases. (A) In mouse, protocones (not yet expressing opsin genes) influenced by high levels of nuclear receptor signaling, express high levels of M-opsin and low levels of S-opsin, while those not influenced by nuclear receptor signaling express high levels of S-opsin and low levels of M-opsin (TRbeta2 or RXRgamma; Ng et al., 2001; Roberts et al., 2005). (B) In zebrafish, cone photoreceptors are structurally as well as molecularly specialized, and a single cone type has not been demonstrated to have the capacity to express more than one opsin (but see Cheng and Novales Flamarique, 2004). In the presence of high levels of nuclear receptor signaling, rods and red cones increase expression of rod opsin and red cone opsin, while blue and UV cones decrease expression of blue and UV cone opsin.
The endogenous RA signaling system in zebrafish retina
Our studies have verified that the critical components of the native RA signaling pathway are present within the zebrafish retina, and with a cell-specific expression pattern consistent with an endogenous role for RA in regulating photoreceptor differentiation. RA-synthesizing enzymes have been biochemically localized to dorsal and ventral embryonic retina (Marsh-Armstrong et al., 1994), indicating that the capacity to generate RA is present in these locations. We have identified the cell types in developing zebrafish retina that express at least one of these enzymes as dorsal retinal neuroepithelial cells, dorsal photoreceptors, and the RPE. It will be important to determine whether similar cell types may be involved in RA synthesis in ventral retina (see also Marsh-Armstrong et al., 1994). These results are interpreted as indicating that relatively high concentrations of RA are likely to be associated with these, and surrounding, cells.
The restricted expression pattern of RARE-driven YFP transgenes in the zebrafish, in contrast, was unexpected. Only ventral progenitor cells, ventral neurons, and the RPE exhibited expression of the transgene, indicating that endogenous RA signaling mechanisms are operational only within these cells. The reduced expression of the transgene in the dorsal retina was especially surprising, because at least one of the components required for RA signaling, RA itself, is likely to be present at this location. The functional significance of this observation is not clear, but suggests complex, spatiotemporal dynamics for RA signaling mechanisms in the developing zebrafish retina.
The restricted expression pattern of the RARE-driven transgene may be explained by any or all of the following factors: the presence of RA binding proteins (Mey et al., 2001) in the dorsal retina; a lack of functional RARs or of positive regulatory elements; or the presence of repressors (Loinder and Soderstrom, 2003). We note that although the predominant nuclear receptors localized to developing vertebrate photoreceptors are RXRs and thyroid hormone receptors (TRs; Ng et al., 2001; Roberts et al., 2005), RXR-TR heterodimers are not generally responsive to all-trans RA or even to 9-cis RA (reviewed by Glass, 1996). Although weak expression of an RAR, RARalpha, is observed throughout the retinas of mice, at all developmental stages (Mori et al., 2001), expression of a RARE-driven transgene in mice is topographically restricted (Stull and Wikler, 2000), suggesting that not all of these RARs are active. These findings collectively indicate the existence of molecular regulatory mechanisms that result in cellular and topographical restriction of RA signaling in vertebrate retinas.
It is intriguing that the only photoreceptors displaying molecular components of endogenous RA signaling are those likely to be the first photoreceptors to differentiate (Raymond et al., 1995; Schmitt and Dowling, 1996; Stenkamp et al., 1996). It is possible that only these early photoreceptors are influenced by native RA signaling mechanisms. Alternatively, these early photoreceptors may be the source of an RA-regulated secondary signal that is, in turn, propagated across the developing sheet of photoreceptors. Another alternative is that the RPE may provide the source of an RA-regulated secondary signal that influences photoreceptor differentiation. The RPE is known to be the source of differentiation and cell survival cues for photoreceptors, such as the signaling protein sonic hedgehog (Levine et al., 1997; Stenkamp et al., 2000); it will be interesting to test whether any of these may be regulated by RA signaling in vivo.
Functional significance of RA effects upon photoreceptors
What is the adaptive significance of these effects of RA? Teleost rod photoreceptors are established from a progenitor lineage that includes additional rounds of cell division compared to the cone lineage (Otteson et al., 2001), resulting in rod cell birth occurring after cone cell birth (Johns and Fernald, 1981). However, in zebrafish and goldfish, rods are the first photoreceptors to express opsin genes (Raymond et al., 1995; Stenkamp et al., 1996). Perhaps a mechanism that favors rapid development of rods has been selected for, in order to generate sufficiently sensitive visual capacity early in embryogenesis. Zebrafish have recordable ERGs and behavioral responses to visual stimuli at 3 dpf (Branchek, 1984; Easter and Nicola, 1996), the same developmental point at which we evaluated the effects of RA. Interestingly, however, the rod contribution to visual responses is negligible in zebrafish larvae, even in scotopic conditions (Bilotta et al., 2001), suggesting little or no visual advantage for the rapid differentiation of rods. One alternative explanation considers the speculative role proposed for red cones in organizing the zebrafish cone mosaic (Stenkamp and Cameron, 2002; Raymond and Barthel, 2004). The orderly mosaic of the teleost fish retina bears a resemblance to the crystalline photoreceptor mosaic of the fruit fly; the fly retinal mosaic is established by an early-differentiating ‘founder photoreceptor’ that sets up spacing between mosaic units and initiates signaling interactions that promote the differentiation of other photoreceptor types at specific positions relative to the founder (Banerjee and Zipursky, 1990). Because the red cones are the first cones to differentiate in some teleosts (Raymond et al., 1995; Stenkamp et al., 1996), they have been considered as candidates for the role of founder photoreceptor (Wan and Stenkamp, 2000; Raymond and Barthel, 2004). Our results suggest that RA signaling may be important for promoting the more rapid differentiation of the red cone photoreceptor as compared to other cone types. This mechanism may therefore ensure that that putative founder cell of the cone mosaic – the red cone – is available to orchestrate subsequent patterning interactions. A test of this hypothesis is underway using RA signaling knockdown methods.
Supplementary Material
Acknowledgments
The authors thank Dr. Elwood Linney for providing the RGnY (RARE-YPF) transgenics, Dr. Thomas Vihtelic for zebrafish opsin cDNAs and antibodies, Dr. Peter McCaffery for the RALDH2 antibody, and Dr. Nicholas Marsh-Armstrong for providing comments on the manuscript. This work was supported by NIH EY012146 (DLS), NIH P20 RR016454 (BRIN/INBRE program of NCRR), and a Fight for Sight summer graduate fellowship (SNP).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2005.08.045.
References
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27 (3):513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Azadi S, Zhang Y, Caffe AR, Holmqvist B, van Veen T. Thyroid-beta2 and the retinoid RAR-alpha, RXR-gamma and ROR-beta2 receptor mRNAs; expression profiles in mouse retina, retinal explants and neocortex. NeuroReport. 2002;13 (6):745–750. doi: 10.1097/00001756-200205070-00003. [DOI] [PubMed] [Google Scholar]
- Banerjee U, Zipursky SL. The role of cell–cell interaction in the development of the Drosophila visual system. Neuron. 1990;4 (2):177–187. doi: 10.1016/0896-6273(90)90093-u. [DOI] [PubMed] [Google Scholar]
- Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38 (9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- Barthel LK, Raymond PA. Subcellular localization of alpha-tubulin and opsin mRNA in the goldfish retina using digoxigenin-labeled cRNA probes detected by alkaline phosphatase and HRP histochemistry. J Neurosci Methods. 1993;50:145–152. doi: 10.1016/0165-0270(93)90002-9. [DOI] [PubMed] [Google Scholar]
- Bernard M, Klein DC. Retinoic acid increases hydroxyindole-O-methyltransferase activity and mRNA in human Y-79 retinoblastoma cells. J Neurochem. 1996;67 (3):1032–1038. doi: 10.1046/j.1471-4159.1996.67031032.x. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Saszik S, Sutherland SE. Rod contributions to the electroretinogram of the dark-adapted developing zebrafish. Dev Dyn. 2001;222 (4):564–570. doi: 10.1002/dvdy.1188. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Stodulkova E, Do VT, Padove SA, Nguyen HT, Borst DE, Nickerson JM. The effect of retinoids and butyrate on the expression of CRX and IRBP in retinoblastoma cells. Vision Res. 2002;42 (8):933–938. doi: 10.1016/s0042-6989(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Branchek T. The development of photoreceptors in the zebrafish, Brachydanio rerio: II. Function J Comp Neurol. 1984;224 (1):116–122. doi: 10.1002/cne.902240110. [DOI] [PubMed] [Google Scholar]
- Branchek T, BreMiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio: I. Structure. J Comp Neurol. 1984;224 (1):107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Browman HI, Hawryshyn CW. Thyroxine induces a precocial loss of ultraviolet photosensitivity in rainbow trout (Oncorhynchus mykiss Teleostei) Vision Res. 1992;32 (12):2303–2312. doi: 10.1016/0042-6989(92)90094-y. [DOI] [PubMed] [Google Scholar]
- Browman H, Hawryshyn C. Retinoic acid modulates retinal development in the juveniles of a teleost fish. J Exp Biol. 1994;193 (1):191–207. doi: 10.1242/jeb.193.1.191. [DOI] [PubMed] [Google Scholar]
- Cameron DA, Carney LH. Cellular patterns in the inner retina of adult zebrafish: quantitative analyses and a computational model of their formation. J Comp Neurol. 2004;471 (1):11–25. doi: 10.1002/cne.11040. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Novales Flamarique I. Opsin expression: new mechanism for modulating colour vision. Nature. 2004;428 (6980):279. doi: 10.1038/428279a. [DOI] [PubMed] [Google Scholar]
- Cook JE. Spatial properties of retinal mosaics: an empirical evaluation of some existing measures. Vis Neurosci. 1996;13 (1):15–30. doi: 10.1017/s0952523800007094. [DOI] [PubMed] [Google Scholar]
- Dowling JE. Nutritional and inherited blindness in the rat. Exp Eye Res. 1964;15:348–356. doi: 10.1016/s0014-4835(64)80042-7. [DOI] [PubMed] [Google Scholar]
- Durston AJ, van der Wees J, Pijnappel WW, Godsave SF. Retinoids and related signals in early development of the vertebrate central nervous system. Curr Top Dev Biol. 1998;40:111–175. doi: 10.1016/s0070-2153(08)60366-x. [DOI] [PubMed] [Google Scholar]
- Eagleson GW, Johnson-Meeter LJ, Frideres J. Effects of retinoic acid upon eye field morphogenesis and differentiation. Dev Dyn. 2001;221 (3):350–364. doi: 10.1002/dvdy.1149. [DOI] [PubMed] [Google Scholar]
- Easter SS, Jr, Nicola GN. The development of vision in the zebrafish (Danio rerio) Dev Biol. 1996;180 (2):646–663. doi: 10.1006/dbio.1996.0335. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev Biol. 2003;258 (2):277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Glass CK. Some new twists in the regulation of geneexpressionby thyroid hormone and retinoic acid receptors. J Endocrinol. 1996;150 (3):349–357. doi: 10.1677/joe.0.1500349. [DOI] [PubMed] [Google Scholar]
- Grieg-Smith P. Quantitative Plant Ecology. 2. Butterworths; London: 1964. [Google Scholar]
- Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24 (2):127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- Hoover F, Gundersen TE, Ulven SM, Michaille JJ, Blanchet S, Blomhoff R, Glover JC. Quantitative assessment of retinoid signaling pathways in the developing eye and retina of the chicken embryo. J Comp Neurol. 2001;436 (3):324–335. [PubMed] [Google Scholar]
- Hu M, Easter SS., Jr Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong NR, Dowling JE. Retinoic acid induced duplication of the zebrafish retina. Proc Natl Acad Sci U S A. 1992;89 (17):8293–8297. doi: 10.1073/pnas.89.17.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong N, McCaffery P, Drager UC. Retinoic acid establishes ventral retinal characteristics. Development. 1996a;122:195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Fadool JM, Dowling JE. Retinoic acid alters photoreceptor development in vivo. Proc Natl Acad Sci U S A. 1996b;93 (23):13298–13303. doi: 10.1073/pnas.93.23.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Fernald RD. Genesis of rods in teleost fish retina. Nature. 1981;293 (5828):141–142. doi: 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Retinoic acid promotes differentiation of photoreceptors in vitro. Development. 1994;120 (8):2091–2102. doi: 10.1242/dev.120.8.2091. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Turner JK, Reh TA. Ligands of steroid/thyroid receptors induce cone photoreceptors in vertebrate retina. Development. 1995;121 (11):3777–3785. doi: 10.1242/dev.121.11.3777. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Williams RC, Turner JK, Creech-Kraft JM, Reh TA. Retinoic acid promotes rod photoreceptor differentiation in rat retina in vivo. NeuroReport. 1999;10 (11):2389–2394. doi: 10.1097/00001756-199908020-00031. [DOI] [PubMed] [Google Scholar]
- Larison KD, BreMiller R. Early onset of phenoytpe and cell patterning in the embryonic zebrafish retina. Development. 1990;109:567–576. doi: 10.1242/dev.109.3.567. [DOI] [PubMed] [Google Scholar]
- Levine EM, Roelink H, Turner J, Reh TA. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J Neurosci. 1997;17 (16):6277–6288. doi: 10.1523/JNEUROSCI.17-16-06277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhu X, Craft CM. Retinoic acid upregulates cone arrestin expression in retinoblastoma cells through a cis element in the distal promoter region. Invest Ophthamol Visual Sci. 2002;43 (5):1375–1383. [PubMed] [Google Scholar]
- Loinder K, Soderstrom M. The nuclear receptor corepressor (N-CoR) modulates basal and activated transcription of genes controlled by retinoic acid. J Steroid Biochem Mol Biol. 2003;84 (1):15–21. doi: 10.1016/s0960-0760(03)00007-4. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, McCaffery P, Gilbert W, Dowling JE, Drager UC. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc Natl Acad Sci U S A. 1994;91 (15):7286–7290. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massof RW, Finkelstein D. Supplemental vitamin A retards loss of ERG amplitude in retinitis pigmentosa. Arch Opthamol. 1993;111 (6):751–754. doi: 10.1001/archopht.1993.01090060039019. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Lee MO, Wagner MA, Sladek NE, Drager UC. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992;115:371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Wagner E, O’Neil J, Petkovich M, Drager UC. Dorsal and ventral retinoic territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mech Dev. 1999;85 (1–2):203–214. doi: 10.1016/s0925-4773(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Means AL, Gudas LJ. The roles of retinoids in vertebrate development. Annu Rev Biochem. 1995;64:201–233. doi: 10.1146/annurev.bi.64.070195.001221. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P, Drager UC. Retinoic acid synthesis in the developing chick retina. J Neurosci. 1997;17 (19):7441–7449. doi: 10.1523/JNEUROSCI.17-19-07441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey J, McCaffery P, Klemeit M. Sources and sink of retinoic acid in the embryonic chick retina: distribution of aldehyde dehydrogenase activities, CRABP-I, and sites of retinoic acid inactivation. Brain Res Dev Brain Res. 2001;127 (2):135–148. doi: 10.1016/s0165-3806(01)00127-4. [DOI] [PubMed] [Google Scholar]
- Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Visual Sci. 2001;42 (6):1312–1318. [PubMed] [Google Scholar]
- Nawrocki L, BreMiller R, Streisinger G, Kaplan M. Larval and adult visual pigments of the zebrafish, Brachydanio rerio. Vision Res. 1985;25 (11):1569–1576. doi: 10.1016/0042-6989(85)90127-0. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27 (1):94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Nolte C, Amores A, Nagy Kovacs E, Postlethwait J, Featherstone M. The role of a retinoic acid response element in establishing the anterior neural expression border of Hoxd4 transgenes. Mech Dev. 2003;120:325–335. doi: 10.1016/s0925-4773(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232 (1):62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic-acid mediated gene expression in transgenic reporter zebrafish. Dev Biol. 2001;229 (1):89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK. A moving wave patterns the cone photoreceptor mosaic array in the zebrafish retina. Int J Dev Biol. 2004;48 (8–9):935–945. doi: 10.1387/ijdb.041873pr. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones. Neuron. 1993;10:1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Curran GA. Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. J Comp Neurol. 1995;359:537–550. doi: 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor gamma is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Visual Sci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. The density recovery profile: a method for the analysis of points in the plane applicable to retinal studies. Vis Neurosci. 1991;6 (2):95–111. doi: 10.1017/s095252380001049x. [DOI] [PubMed] [Google Scholar]
- Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80 (3):1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Dowling JE. Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. J Comp Neurol. 1996;371 (2):222–234. doi: 10.1002/(SICI)1096-9861(19960722)371:2<222::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Schmitt EA, Hyatt GA, Dowling JE. Erratum: temporal and spatial patterns of opsin gene expression in the zebrafish (Danio rerio): corrections with additions. Vis Neurosci. 1999;16:601–605. doi: 10.1017/s0952523899163181. [DOI] [PubMed] [Google Scholar]
- Shupe JM, Kristan DM, Austad SN, Stenkamp DL. The eye of the laboratory mouse remains anatomically adapted for natural conditions. Brain Behav Evol. doi: 10.1159/000088857. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm AK, Fox DA, Karlsson JO, van Veen T. Retinoic acid produces rod photoreceptor selective apoptosis in developing mammalian retina. Invest Ophthamol Visual Sci. 2000;41 (3):937–947. [PubMed] [Google Scholar]
- Stenkamp DL, Cameron DA. Cellular pattern formation in the retina: retinal regeneration as a model system. Mol Vision. 2002;8:280–293. [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol. 2003;258:349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Gregory JK, Adler R. Retinoid effects in purified cultures of chick embryo retina neurons and photoreceptors. Invest Ophthalmol Visual Sci. 1993;34 (8):2425–2436. [PubMed] [Google Scholar]
- Stenkamp DL, Hisatomi O, Barthel LK, Tokunaga F, Raymond PA. Temporal expression of rod and cone opsins in embryonic goldfish retina predicts the spatial organization of the cone mosaic. Invest Ophthalmol Visual Sci. 1996;37 (2):363–376. [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for Hedgehog genes in zebrafish retinal development. Dev Biol. 2000;220 (2):238–252. doi: 10.1006/dbio.2000.9629. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431 (4):363–381. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Stull DL, Wikler KC. Retinoid dependent gene expression regulates early morphological events in the development of the murine retina. J Comp Neurol. 2000;417 (3):289–298. doi: 10.1002/(sici)1096-9861(20000214)417:3<289::aid-cne3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Takechi M, Kawamura S. Temporal and spatial changes in the expression pattern of multiple red and green subtype opsin genes during zebrafish development. J Exp Biol. 2005;208:1337–1345. doi: 10.1242/jeb.01532. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Wallace VA, Jensen AM. IBMX, taurine and 9-cis retinoic acid all act to accelerate rhodopsin expression in postmitotic cells. Exp Eye Res. 1999;69 (6):617–627. doi: 10.1006/exer.1999.0741. [DOI] [PubMed] [Google Scholar]
- Wan J, Stenkamp DL. Cone mosaic development in the goldfish retina is independent of rod neurogenesis and differentiation. J Comp Neurol. 2000;423 (2):227–242. [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish Danio rerio. 4. University of Oregon Press: Eugene; 2000. The Zebrafish Book. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.