Abstract
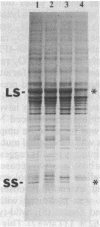
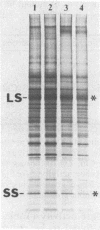
The Chlamydomonas reinhardtii chloroplast mutant 68-4PP is phenotypically indistinguishable from wild type at 25 degrees C but fails to grow photosynthetically at 35 degrees C. It had about 30% of the wild-type level of ribulose-1,5-bisphosphate carboxylase/oxygenase (EC 4.1.1.39) holoenzyme and carboxylase activity when grown at 25 degrees C, but less than 15% when grown at 35 degrees C. Pulse-labeling with 35S showed that the decrease in enzyme level at the restrictive temperature was not a result of reduced synthesis of enzyme subunits. The CO2/O2 specificity factor (VCKO/VOKC, where VC and VO are Vmax values for carboxylation and oxygenation and KC and KO are Km values for CO2 and O2) of the mutant enzyme was found to be significantly less than that of the wild-type enzyme (54 +/- 2 and 62 +/- 1, respectively), and this alteration was accompanied by increases in KO and KC and a decrease in VC/VO. DNA sequencing revealed a single missense mutation in the 68-4PP chloroplast large-subunit gene. This mutation causes leucine to be replaced by phenylalanine at position 290 in the large-subunit polypeptide sequence. These results (i) support previous studies that implicated this region of the large subunit as an important structural component of the enzyme's function and (ii) demonstrate that chloroplast genetic modification of the CO2/O2 specificity factor of a plant-type carboxylase/oxygenase is feasible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Suh S. W., Cascio D., Smith W. W., Eisenberg D. Sliding-layer conformational change limited by the quaternary structure of plant RuBisCO. Nature. 1987 Sep 24;329(6137):354–356. doi: 10.1038/329354a0. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D., Mets L. First DNA sequence of a chloroplast mutation: a missense alteration in the ribulosebisphosphate carboxylase large subunit gene. Plasmid. 1983 May;9(3):321–324. doi: 10.1016/0147-619x(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast DNA region of Chlamydomonas reinhardii containing the gene of the large subunit of ribulose bisphosphate carboxylase and parts of its flanking genes. J Mol Biol. 1982 Dec 25;162(4):775–793. doi: 10.1016/0022-2836(82)90547-2. [DOI] [PubMed] [Google Scholar]
- Estelle M., Hanks J., McIntosh L., Somerville C. Site-specific mutagenesis of ribulose-1,5-bisphosphate carboxylase/oxygenase. Evidence that carbamate formation at Lys 191 is required for catalytic activity. J Biol Chem. 1985 Aug 15;260(17):9523–9526. [PubMed] [Google Scholar]
- Gutteridge S., Sigal I., Thomas B., Arentzen R., Cordova A., Lorimer G. A site-specific mutation within the active site of ribulose-1,5-bisphosphate carboxylase of Rhodospirillum rubrum. EMBO J. 1984 Dec 1;3(12):2737–2743. doi: 10.1002/j.1460-2075.1984.tb02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman F. C., Soper T. S., Niyogi S. K., Mural R. J., Foote R. S., Mitra S., Lee E. H., Machanoff R., Larimer F. W. Function of Lys-166 of Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase as examined by site-directed mutagenesis. J Biol Chem. 1987 Mar 15;262(8):3496–3501. [PubMed] [Google Scholar]
- Hartman F. C., Stringer C. D., Lee E. H. Complete primary structure of ribulosebisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1984 Jul;232(1):280–295. doi: 10.1016/0003-9861(84)90544-7. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., McFadden B. A., el-Gul T. Active site histidine in spinach ribulosebisphosphate carboxylase/oxygenase modified by diethyl pyrocarbonate. Biochemistry. 1985 Jul 16;24(15):3957–3962. doi: 10.1021/bi00336a024. [DOI] [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981 Feb;67(2):237–245. doi: 10.1104/pp.67.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D. B., Ogren W. L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys. 1983 Dec;227(2):425–433. doi: 10.1016/0003-9861(83)90472-1. [DOI] [PubMed] [Google Scholar]
- Laing W. A., Ogren W. L., Hageman R. H. Bicarbonate stabilization of ribulose 1,5-diphosphate carboxylase. Biochemistry. 1975 May 20;14(10):2269–2275. doi: 10.1021/bi00681a035. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miziorko H. M., Lorimer G. H. Ribulose-1,5-bisphosphate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- Niyogi S. K., Foote R. S., Mural R. J., Larimer F. W., Mitra S., Soper T. S., Machanoff R., Hartman F. C. Nonessentiality of histidine 291 of Rhodospirillum rubrum ribulose-bisphosphate carboxylase/oxygenase as determined by site-directed mutagenesis. J Biol Chem. 1986 Aug 5;261(22):10087–10092. [PubMed] [Google Scholar]
- Norton I. L., Welch M. H., Hartman F. C. Evidence for essential lysyl residues in ribulosebisphosphate carboxylase by use of the affinity label 3-bromo-1,4-dihydroxy-2-butanone 1,4-bisphosphate. J Biol Chem. 1975 Oct 25;250(20):8062–8068. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G., Lindqvist Y., Brändén C. I., Lorimer G. Three-dimensional structure of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum at 2.9 A resolution. EMBO J. 1986 Dec 20;5(13):3409–3415. doi: 10.1002/j.1460-2075.1986.tb04662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Al-Abed S. R., Huether M. J. Temperature-Sensitive, Photosynthesis-Deficient Mutants of Chlamydomonas reinhardtii. Plant Physiol. 1988 Mar;86(3):773–777. doi: 10.1104/pp.86.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Brown T., Chen Z., Zhang D., Al-Abed S. R. Missense Mutation in the Chlamydomonas Chloroplast Gene that Encodes the Rubisco Large Subunit. Plant Physiol. 1988 Apr;86(4):987–989. doi: 10.1104/pp.86.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Goldschmidt-Clermont M., Rahire M., Rochaix J. D. Nonsense mutations in the Chlamydomonas chloroplast gene that codes for the large subunit of ribulosebisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5460–5464. doi: 10.1073/pnas.82.16.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Mets L. Photosynthesis-deficient Mutants of Chlamydomonas reinhardii with Associated Light-sensitive Phenotypes. Plant Physiol. 1981 Mar;67(3):565–569. doi: 10.1104/pp.67.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Laing W. A., Christeller J. T., Petersen G. B., Hill D. F. Ribulose 1,5-bisphosphate carboxylase. Effect on the catalytic properties of changing methionine-330 to leucine in the Rhodospirillum rubrum enzyme. Biochem J. 1986 May 1;235(3):839–846. doi: 10.1042/bj2350839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]