Abstract
Self-renewal is the process by which normal stem cells and cancer cells make more of themselves. In cancer, this process is ultimately responsible for the infinite replicative potential of malignant cells and is likely found in residual cell populations that evade conventional therapy. Two intrinsically opposing hypotheses have emerged to explain how self-renewal occurs in cancer. The cancer stem cell hypothesis states that self-renewal is confined to a discrete subpopulation of malignant cells, whereas the stochastic model suggests that all tumor cells have the potential to self-renew. Presently, the gold standard for measuring cancer self-renewal is limiting dilution cell transplantation into immune-matched or immune-deficient animals. From these experiments, tumor-initiating frequency can be calculated based on the number of animals that engraft disease following transplantation of various doses of tumor cells. Here, we describe how self-renewal assays are performed, summarize the current experimental models that support the cancer stem cell and stochastic models of cancer self-renewal, and enumerate how the zebrafish can be used to uncover important pathways in cancer self-renewal.
Self-Renewal in Cancer
Self-renewal is the process by which cells can make more of themselves and has been ascribed to both normal stem cell populations and cancer cells.1 In normal stem cells, self-renewal results in cell division and the production of daughter cells that have the same molecular and functional characteristics as the parental cell type. However, stem cells also have the unique ability to divide and differentiate into specialized cell types. Embryonic stem cells can create more of themselves, but also differentiate into all the cell types contained within an organism. Self-renewal can also be found in tissue-restricted stem cells where potency is limited to the production of a subset of mature cell types. Hematopoietic stem cells (HSCs) are able to make more of themselves and can differentiate into all the blood cell lineages, but HSCs cannot make all types of cells within the body. Thus, tissue-restricted stem cells retain the ability to self-renew and yet can differentiate into lineage-restricted, mature cell types.
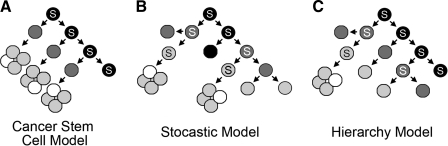
Cancer results from genetic perturbations that cause cells to acquire self-renewal capacity. Some have suggested that tumor heterogeneity may result from sequential step-wise differentiation from a stem cell-like population. The cancer stem cell (CSC) is the only cell type that is capable of self-renewal.1 This concept, known as the CSC hypothesis, has gained much attention over the last decade due, in large part, to the implication that self-renewal is restricted to a subset of the tumor cells (Fig. 1A). Many investigators contend that if self-renewal is confined to one tumor cell type, then new therapies that target the CSC for destruction will cause tumors to stop growing. Although compelling data support this concept in some cancer subtypes, a second less-known hypothesis for self-renewal has also been put forward. In the stochastic model of self-renewal, all tumor cells have the ability to self-renew, but activation of self-renewal is random with only a small population of cells self-renewing at any given time (Fig. 1B). In this model, all tumor cells need to be targeted for destruction because each has the capacity to self-renew.2
FIG. 1.
Models of cancer self-renewal. In these diagrams, black cells give rise to dark gray cells, dark gray cells to light gray, and light gray to white. Self-renewal divisions are denoted by a cell marked with an S. Cancer stem cell model (A), stochastic model (B), and hierarchy model (C).
Although the CSC and stochastic models of self-renewal are the most prominent cancer self-renewal theories, other models can account for how cancer cells self-renew. The hierarchy model is a hybrid between these theories and suggests that self-renewal is restricted to distinct subpopulations of tumor cells, but that the propensity for self-renewal is stratified based on the differentiation status of the cell (Fig. 1C). Less-differentiated cells may have increased capacity for self-renewal, whereas intermediate cell types can also self-renew, but with a substantially reduced capacity. Terminally differentiated cell types would not be capable of self-renewal. A second hybrid model could also account for how cancer cells self-renew. In this model, cancers would follow both the stochastic and CSC models depending on the stage of tumor growth. Early tumors may evolve one dominant cell type that is solely responsible for tumor initiation and self-renewal. This cell—akin to a CSC—would be capable of creating more of itself and producing differentiated progenies. However, as tumors continue to grow, additional genetic/epigenetic events would be acquired which stimulate self-renewal in a larger portion of cancer cell types. In this model, tumor evolution is marked by acquisition of self-renewal programs by all cells.
It is formally possible that each hypothesized model of tumor self-renewal might have physiological relevance depending on the stage of tumor development and type of tumor. In the following sections we summarize how self-renewal is currently assayed and enumerate various experiments that support either the CSC hypothesis or the stochastic model.
Experimentally Assessing Self-Renewal in Cancer
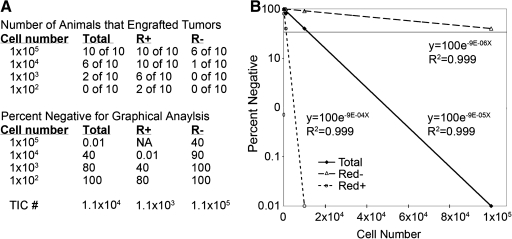
Cancer self-renewal is commonly assessed using limiting dilution cell transplantation into recipient animals. Limiting dilution analysis has been used extensively to quantify self-renewal and to identify stem cell populations in a variety of tissues and cancers.3 Specifically, tumor cells are transplanted into recipient animals at varied doses and then scored for tumor engraftment (a hypothetical example is shown in Fig. 2A, top). The data are plotted on a graph with the Y-axis being a log scale for the percent of animals that failed to engraft tumor (percent negative; Fig. 2A, bottom) and the X-axis indicates the number of cells used for transplantation (Fig. 2B). Tumor-initiating cell number is calculated by the number of cells required to engraft 63% of recipient animals (i.e., 37% of animals are negative for engraftment). By convention, the data are presented as percent negative and linear regression is used to place a best-fit line between the data points. The R2 values show how well the linear regression analysis predicts tumor-initiating cell number and establishes the accuracy of the data. Two additional programs, L-calc from Stem Cell Technologies and Limdil (http://bioinf.wehi.edu.au/software/elda/index.html), have also been used to quantify tumor-initiating cell number. These latter two programs can calculate 95% confidence intervals and make statistical comparisons between data sets.
FIG. 2.
Limiting dilution cell transplantation analysis. Tumor cells were introduced into recipient animals at various doses. In this hypothetical example, the tumor is comprised of two subpopulations of cells (R+ vs. R−). The number of animals that engraft disease from unfractionated tumor cells (total) compared with fractionated cells (R+ or R−) are shown (top, A). Data are converted into percentage of animals that fail to engraft disease (bottom, A) and plotted graphically (B). Specifically, percent negative is plotted as a log scale and cell number is on the X-axis. Linear regression was completed and an equation was fit to the data; R2 values denote the accuracy of the data. A line at 37% negative denotes the number of cells in which one self-renewing cell resides. For the total cell population, this line crosses at 1.1 × 104, indicating that 1 in 1.1 × 104 cells is capable of self-renewal. Tumor-initiating cell number (TIC #) for this illustrative example is also shown (extreme bottom, A).
Variations in cell transplantation protocols may limit their ability to correctly calculate the fraction of cells with self-renewal potential. Recent work has shown that cell transplantation of human cells into partially immune-compromised NOD/SCID mice severely underestimated the number of self-renewing cell types in melanoma.4 These investigators utilized NOD/SCID IL2-receptor gamma-deficient recipient animals for transplant assays5 and showed that simply altering the recipient animals used in limiting dilution cell transplantation experiments had a 3- to 100-fold effect on the calculated frequency of melanoma-initiating cells.4 NOD/SCID IL2-receptor gamma-deficient mice that received human CD34+ cord blood engrafted hematopoietic cells better than NOD/SCID.5 NK cell activity was present in NOD/SCID mice but absent in NOD/SCID IL2-receptor gamma-deficient mice, suggesting that loss of NK activity may be responsible for the differences in engraftment rates. However, even when NOD/SCID mice were treated with the anti-asailo-GM1 antibody that suppressed NK activity, human CD34+ cells had approximately eightfold higher engraftment rates in NOD/SCID IL2-receptor gamma-deficient mice than when compared with NOD/SCID recipients.5 Additional immune system factors must be disrupted in NOD/SCID IL2-receptor gamma mice and are likely responsible for superior engraftment into these recipient animals. CD34+CD38+CD19+ and CD34+CD38−CD19+ cells from human B-cell acute lymphoblastic leukemia engrafted disease into NOD/SCID IL2-receptor gamma-deficient mice, whereas only CD34+CD38− cells initiated leukemia in NOD/SCID mice.6,7 These experiments suggest that NOD/SCID animals may fail to identify all tumor cell types that have potential to self-renew.
The site of transplant injection also has a major impact on how well a tumor can engraft. Introduction of tumor cells into the vasculature requires that cells have the ability to move through the blood vessels, extravasate into the correct tissue, and ultimately self-renew. By contrast, introduction of tumor cells into the organ from which the cancer originated may lead to vast differences in the calculated frequency of tumor-initiating cells. Growth factors contained within injection media can also impact limiting dilution cell transplantation assays. Cell transplantation of melanoma cells along with a collagen-containing matrix led to a 10-fold increase in tumor-initiating potential compared with cells introduced without collagen.4 Finally, the last major hurdle for correctly calculating tumor-initiating frequency is determining the time point to end the experiment. Many investigators prematurely stop analyzing animals for engraftment and thus do not capture all the animals that are capable of engrafting disease. Quintana et al. showed that a majority of melanoma xenograft transplant studies stopped assessing animals for engraftment at 8 weeks, but melanomas continued to engraft by 32 weeks.4 These results had a nearly 10-fold effect on correctly determining the self-renewing cell number.
Taken together, cell transplantation and limiting dilution analysis are the gold standard for assessing self-renewal potential. And despite the limitations of this assay, designing carefully controlled experiments that take these four major issues into account has provided unique insights into self-renewal in cancer.
Evidence to Support the CSC Hypothesis
CSCs have been identified in a number of leukemias. A rare leukemia-initiating cell (LIC) was identified in human acute myeloid leukemia (AML) and exhibited similar characteristics to normal CD34+/CD38− stem cells. Importantly, other tumor cell populations were unable to remake tumor.8,9 This rare CD34+/CD38− LIC was selectively targeted by a monoclonal antibody against CD44, resulting in the loss of disease transfer into NOD/SCID recipient mice.10 In a mouse model of AML, MOZ-TIF2-expressing leukemic stem cells expressed the cell surface markers Sca-1−/CD34−/CD4−/CD8−/B220−/CD19−/Mac-1+ and comprised only 1 in 104 of total bone marrow cells.11 Finally, in a CALM/AF10 fusion gene mouse model of AML, LICs were contained in the B220+/Mac−/Gr1− cell population, with 1 in 36 of these cells giving rise to disease in transplant recipient mice, whereas only 1 in 19,717 B220−/Mac+/Gr1+ cells was able to form tumors.12
CSCs have also been identified in chronic myelogenous leukemia (CML). The B-cell receptor (BCR) is often fused to the Abelson tyrosine kinase (ABL) in CML and results in the production of a constitutively active kinase. BCR-ABL when targeted to murine bone marrow cells was necessary and sufficient to cause a transplantable myeloproliferative disorder in mice and was similar to the chronic phase of CML in humans.13 The blast crisis stage of CML was created by simultaneously expressing a second activating mutation, Nup98/Hox9a.14 When both BCR-ABL and Nup98/Hox9a were expressed in primitive HSCs, 1 in 7 Lin−, Kit+/−/Flt3+/Sca+/CD34+/CD150− leukemic blasts were able to transplant disease, whereas Lin+ blasts transplanted very poorly (1 in 1959 cells). CSCs have also been identified in acute promyelocytic leukemia (APL). APL is a malignancy caused by the arrest of leukemic cells in the more primitive promyelocytic stage of myeloid differentiation. A fusion of the retinoic acid receptor-α with promyelocytic leukemia protein gene (PML) is found in a majority of patients. Expression of retinoic acid receptor-α-PML conferred stem cell self-renewal properties to c-Kit+/CD34+/Gr-1+ promyelocytes, which were greatly expanded in disease. One in 100 purified leukemic c-Kit+/CD34+/Gr-1+ promyelocytes induced disease when introduced into syngeneic transplant recipient mice.15 Taken together, these results suggested that CML and APL follow the CSC model of self-renewal.
Rare subpopulations of self-renewing cell types have also been identified in mouse and human T-cell leukemias. A rare LIC population has been identified in Pten-deficient mice that develop T-cell acute lymphoblastic leukemia (T-ALL). The c-KitmidCD3+ LIC engrafted disease into sublethally irradiated SCID mice better than either the CD3− or c-Kit−CD3+ cells. In another study, human T-ALL-initiating cells express either CD34+/CD4− or CD34+/CD7 and engrafted successfully when transplanted into NOD/SCID mice,16 whereas other cell types engrafted far less efficiently. About 1 × 107 unsorted human T-ALL cells were required to transfer disease into NOD/SCID IL2-receptor gamma-deficient mice (n = 2 of 4 or 2 of 5 transplant mice developed leukemia), again suggesting that ALL-initiating cells are rare in this type of leukemia.17 Taken together, the cumulative evidence from many studies in mouse and human leukemias suggests that the CSC hypothesis may be applicable to a large portion of lymphomas and leukemias.
CSCs have also been identified in solid tumors. CD44+CD24low/− CSCs have been isolated from human breast tumors. These cell types successfully engrafted into NOD-SCID mice, whereas other tumor cell populations do not cause tumors.18 CSCs have also been identified in glioblastoma and could be enriched using the CD133 cell surface receptor.19 In this study, the authors found that as few as 100 CD133+ cells were required to engraft glioblastoma into NOD-SCID mice, whereas 1 × 105 CD133− cells could engraft, but failed to form tumors. The exclusivity of CD133 to delineate CSCs in glioblastoma has been recently questioned. It appears that phenotypic heterogeneity can exist within glioblastoma CSC populations and certain CD133− cells can also give rise to tumors.20 Nevertheless, glioblastoma CSCs promoted angiogenesis, resisted radiation treatment, and responded to BMP-differentiation treatments better than other tumor subpopulations.21–24 In total, CSCs have now been reported in many solid tumors including colon cancer,25–27 pancreatic cancer,28,29 and melanoma,30 suggesting that the CSC hypothesis may be broadly applicable to a range of cancer types.
Evidence to Support the Stochastic Model of Cancer Self-Renewal
Recent reports in syngeneic mouse models of lymphoma and leukemia (T- and B-cell lymphoma and AML) have questioned whether all cancer subtypes follow the CSC model of self-renewal. At the core of the debate over which model of self-renewal is correct is the supposition that CSCs are rare and the question of whether xenotransplantation experiments into NOD/SCID mice accurately estimate the number of tumor-initiating cell types. Kelly et al. transplanted well-characterized primary AML and the B- and T-cell lymphomas into syngeneic mice and demonstrated that more than 10% of tumor cells were able to initiate disease.31 Specifically, Eμ-myc-induced B-cell lymphomas engrafted disease into syngeneic recipient animals with as few as 10 cells (n = 10 of 10 across three tumors) and 3 of 8 recipients that received single B-cell lymphoma cells developed disease. These results argue that B-ALLs contain high frequencies of tumor-initiating cells. By contrast, only 2–5% of primary Eμ-myc-induced B-cell lymphoblasts expressed the putative stem cell markers Sca-1 and/or AA4.1. Importantly, both the Sca-1/AA4.1hi and Sca-1/AA4.1lo cells formed lymphomas when transplanted into syngeneic mice.31 These data indicate that a substantial fraction of self-renewing B-ALL cells do not express stem cell markers. In a mouse model of MLL-AF9-induced AML, leukemic stem cells expressed more mature myeloid differentiation markers and comprised 25–30% of total myeloid lineage cells. These Mac+Gr+ leukemic stem cells (LSCs) exhibited superior engraftment potential when compared with MLL-AF9 immortalized bone marrow and progenitor cells.32 In a complementary study of MLL-AF9-induced AML, LICs comprised up to 50% of granulocyte-macrophage progenitors and similarly expressed more differentiated markers of granulocyte-macrophage progenitors, in contrast to the more primitive HSC markers that one would expect.33 These results starkly contrast the human xenograft and mouse transplant studies mentioned in the preceding section, which found that LIC types are rare in AML and lymphoid malignancies.8,9,11,12,14,15,17,34 Although rarity of self-renewing cell types alone is not what defines the CSC hypothesis, the striking differences in number of tumor cells required to engraft human disease into NOD/SCID animals compared with syngeneic mouse tumors suggests that immune barriers may be partially responsible for identifying CSCs in human malignancies. Further experimentation will clearly be required to resolve these discrepancies in the literature.
The existence of rare CSCs in solid tumors has also recently been questioned. In a recent study by Quintana et al., the authors studied the effect of different xenograft assays on tumor formation and discovered that 25% of human melanoma cells had the potential to form tumors in NOD/SCID IL2-receptor gamma-deficient mice.4 These limiting dilution cell transplantation experiments were confirmed by single cell transplants of unsorted melanoma cells into NOD/SCID IL2-receptor gamma-deficient mice and showed that 27% of single cells lead to engraftment of disease. Interestingly, no cell surface markers enriched for melanoma-initiating potential following fluorescence-activated cell sorting (FACS), suggesting that self-renewal potential was not confined to any specific subpopulation of melanoma cells. Taken together, the data from syngeneic mouse models of lymphoma and leukemia31 and xenograft models of melanoma suggest that tumor-initiating cell number may be vastly underestimated using cell transplantation into irradiated NOD or NOD/SCID mice and highlight the need to perform xenotransplants into NOD/SCID IL2-receptor gamma-deficient mice.
Although these findings in lymphoma and melanoma support the stochastic model for self-renewal, it is important to note that most tumors have not been rigorously tested. In the experiments described by Kelly et al., only one Eμ-myc B-cell lymphoma was analyzed for potential stem cell marker expression and was subsequently used in cell transplantation experiments.31 Moreover, the markers analyzed were not extensive, raising the possibility that murine Eμ-myc lymphomas could be fractionated on the basis of additional marker expression. This contrasts starkly with the paper by Quintana et al., where even after extensive marker analysis, no unique antibody markers that could distinguish self-renewing subsets of melanoma cells were identified. A core feature of the CSC hypothesis is that cells can be fractionated based on their ability to self-renew and to remake tumors when transplanted into recipient animals. The frequency of self-renewing tumor cells does not obviate the stem cell hypothesis. However, this subtle distinction may be semantic. If 50% of the tumor cells have self-renewal capacity, then therapeutic intervention would require targeting most tumor cells for destruction. In the end, it will be vitally important to understand how common of an attribute self-renewal is in cancer and the mechanisms governing self-renewal. Importantly, developing experimental models that directly assess self-renewal without making assumptions about which theory is correct will likely usher in a new era for identifying key molecular pathways responsible for cancer self-renewal. It is these molecular pathways that should be targeted by new lines of chemotherapies and small molecule inhibitors.
Zebrafish as a Model of Cancer
Zebrafish are a powerful model organism to understand human cancer. Zebrafish tumors are both morphologically and molecularly similar to human malignancies. For example, mutations in the tumor suppressor gene adenomatous polyposis coli cause deregulation of the canonical Wnt-signaling pathway and are implicated in colorectal cancer in humans. Similarly, zebrafish that were heterozygous for a truncating mutation in adenomatous polyposis coli were highly susceptible to neoplasias of the intestine, liver, and pancreas.35 The P53 tumor suppressor gene is frequently mutated or lost in nearly 50% of all human tumors. Zebrafish that have mutations in tp53 which are analogous to those found in human disease were isolated. Homozygous tp53-deficient mutants developed spontaneous malignant peripheral nerve sheath tumors that are similar to human disease.36 RAS proteins are mutationally activated in 25% of human cancers and can also function as oncogenes in zebrafish. In zebrafish, the KRASG12D oncogene can induce rhabdomyosarcomas (RMS) that are highly similar to human embryonal rhabdomyosarcoma.37 Oncogenic KRAS mutations are also associated with 90% of all pancreatic ductal adenocarcinomas. Expression of the KRASG12V mutation under the control of the zebrafish ptf1a promoter resulted in pancreatic cancer with several key features of the human disease.38 Using heat-shock inducible CRE-Lox approaches, Le et al. expressed KRASG12D in various tissues and induced rhabdomyosarcoma, intestinal hyperplasia, and a myeloproliferative disorder in zebrafish. Importantly, these tumors shared many hallmarks with their human diseases, including gene expression patterns and common morphology.39 Transgenic zebrafish models of BRAFV600E-induced melanoma were morphologically similar to human disease and contained pigmented tumor cells.40 Three genetic models of zebrafish T-cell leukemia were recently isolated from a large-scale genetic screen. Most leukemias coexpressed CD4 and CD8, indicating that leukemia cells were arrested at an early stage of thymocyte maturation. However, some leukemias were comprised of mature differentiated T-cells and expressed only CD4 or CD8.41 Taken together, these leukemias mimic a wide array of human T-cell malignancies. By contrast, transgenic zebrafish models of Myc-induced T-ALL expressed scl and lmo2 and mimic the most common and treatment-resistant subtype of pediatric disease.42 Transgenic zebrafish models of TEL-AML1-induced B-cell acute lymphoblastic leukemia were similar to a distinct subtype of human pre-B-ALL that expressed ikaros, rag2, scl, and cd10/NEP.43 Taken together, these studies suggest that zebrafish utilize similar molecular pathways to induce malignancy and accurately mimic various aspects of human disease.
Microarray and cross-species comparisons have identified unique molecular pathways that are conserved between zebrafish and human malignancy. In chemically induced hepatocarcinoma, a zebrafish gene signature revealed that zebrafish and human liver tumors were molecularly similar, but unlike gastric, prostate, or lung tumors. The gene signature associated with zebrafish liver cancer was also associated with disease progression in human patients and identified that the Wnt-β-catenin and RAS-MAPK pathways were deregulated in both human and zebrafish liver tumors.44 Microarray and cross-species comparisons from our group also showed that zebrafish RAS-induced rhabdomyosarcomas were similar to human disease and shared two common molecular signatures with the human embryonal RMS subtype.37 One signature was specific to embryonal RMS (ERMS), whereas the second was a novel RAS-associated signature found in ERMS and pancreatic adenocarcinoma. These experiments established that the RAS pathway is a critical modulator of human ERMS. We have highlighted only a few of the zebrafish models that accurately mimic human disease; however, the cumulative data suggest that zebrafish models recapitulate many important aspects of human malignancy and can be used to identify new pathways involved in disease progression in humans.
Zebrafish as a Model of Cancer Self-Renewal
Zebrafish provide many advantages over mouse models to study cancer self-renewal. For example, large numbers of zebrafish can be housed in a relatively small space, and husbandry costs are 20 times less compared with mice. Additionally, cell transplantation assays can utilize large numbers of animals that are unparalleled in mouse studies. Limiting dilution cell transplantation experiments in mice commonly use three to five animals per dilution and assess only three dilutions per tumor.11,31,32,45 Cell transplantation experiments in zebrafish routinely use 10–12 animals per dilution at four dilutions, greatly facilitating accurate assessment of tumor-initiating potential (Smith et al., unpublished). For example, 300+ adult zebrafish can be transplanted by intraperitoneal injection in 1 day, showing the massive numbers of adult animals that can be used for these experiments. Such large-scale cell transplantation experiments in mice are possible, but not economically feasible for most labs because of both excessive per diem charges and space constraints. Low numbers of tumor cells can be transplanted and can induce tumors in recipient zebrafish. In a transgenic model of ERMS, a subset of tumors engrafted disease into irradiated adult fish with as few as 10 cells.37 The ability to transplant tumors with few engrafting cells makes it possible to design experiments to study clonal evolution and its effects on tumor-initiating potential.
Fluorescent protein expression within cancer cells greatly facilitates the tracking of tumor formation and can be used to quantify transplant engraftment into recipient animals (Fig. 3). Transgenic zebrafish models of leukemia that label tumor cells with fluorescent proteins have been used to quantify the numbers of tumor-initiating cells contained within the bulk of the leukemia mass. In these experiments, limiting dilution cell transplantation analysis of primary zebrafish T-cell leukemias established that 1 in 103 to 1 in 2 × 104 cells are capable of tumor engraftment into irradiated recipient animals.41,46 Remarkably, these numbers are in keeping with published reports from a mouse model of Pten deficiency-induced T-cell leukemia which suggest that tumor-initiating cell number may be low in these leukemias.34 However, we caution that experiments in zebrafish have utilized nonimmune-matched, irradiated recipient animals and likely severely underestimate true leukemia-initiating potential because of incomplete suppression of the immune system following gamma irradiation (Smith et al., unpublished). Further experiments will be required to better address self-renewal in zebrafish (see below).
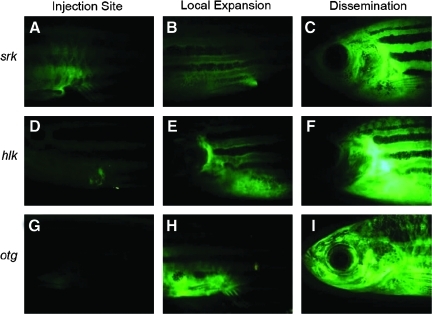
FIG. 3.
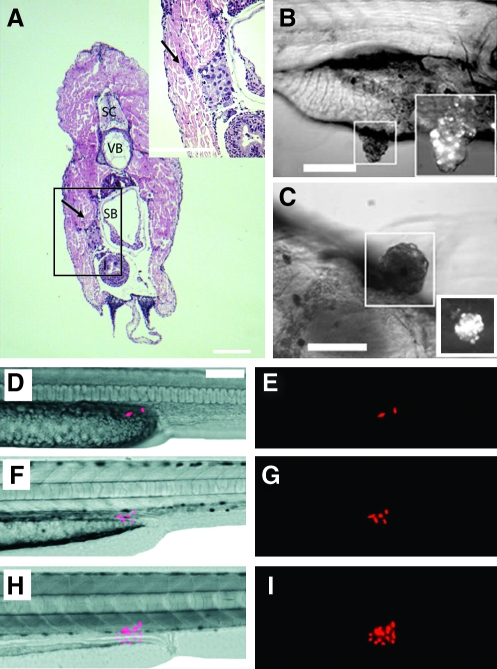
Heritable T-cell malignancies are transplantable and can be easily visualized by fluorescent protein expression in leukemic cells (modified from Frazer et al.41). Green fluorescent protein (GFP+) leukemias from shrek (srk; A–C), hulk (hlk; D–F), and oscar the grouch (otg; G–I) mutants were transplanted into irradiated wild-type hosts. At 1 week, GFP+ cells were seen at the site of transplantation (A, D, G). Subsequently, tumors spread locally (B, E, H) and then disseminated widely (C, F, I).
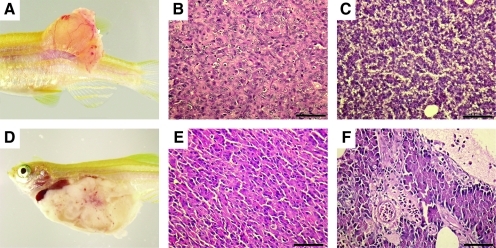
Heterogeneous tumor cell populations can be identified using transgenic lines that express fluorescent proteins in distinct cancer cell subpopulations. Subsequently, FACS and limiting dilution cell transplantation experiments can be used to isolate specific tumor cell types and assess if they are responsible for tumor regrowth and self-renewal. This strategy was used to identify CSCs in embryonal rhabdomyosarcoma.37 The RAS-induced ERMS stem cells expressed rag2-dsRED2 but not the mature muscle marker alpha-actin–green fluorescent protein (GFP) (Fig. 4). Other ERMS cancer cells failed to engraft disease as robustly as the dsRED+/GFP− cell population. Molecular analysis established that the ERMS CSCs were most similar to the normal activated, muscle satellite cells. Together, our results showed that ERMS follows either a CSC model or the hierarchy model of self-renewal. In total, these three published reports were the first to quantify the extent to which self-renewal was found in zebrafish cancer.37,41,46 Importantly, they lay the foundation for new studies to better refine the rules of self-renewal in both leukemias and solid tumors.
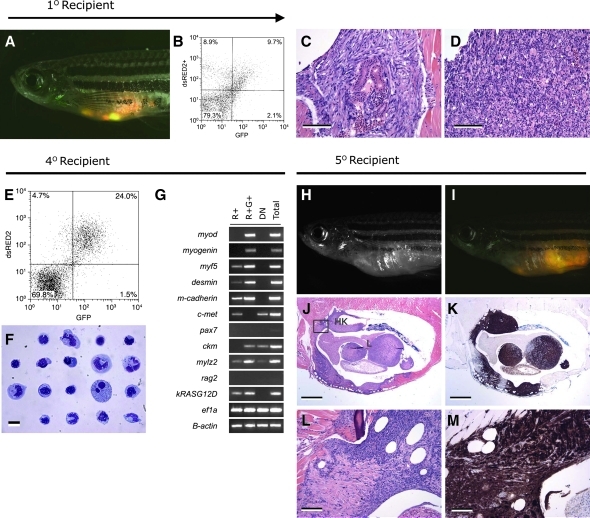
FIG. 4.
The dsRED2+ cell population from double transgenic rag2-dsRED2/alpha-actin-GFP animals contains the serially transplantable cancer stem cell in zebrafish embryonal rhabdomyosarcoma (ERMS). (A–D) Primary transplanted tumors from alpha-actin-GFP+/rag2-dsRED2+ fish (1° Recipient). (A) Merged image of GFP fluorescent, dsRED2 fluorescent, and bright field images. (B) Fluorescence-activated cell sorting (FACS) analysis of primary recipient engrafted with ERMS. (C, D) Histological analysis revealed heterogeneity in transplant animals, with some fish having masses of spindled cells (C) or round cell aggregates (D), or both. Scale bars equal 100 μm in (C, D). (E–G) Cells isolated from serially transplanted animals, in this case a quaternary recipient animal (4° Recipient). (E) FACS plot of tumor cells isolated from a 4° recipient. (F) Wright-Giemsa-stained cytospins of FACS-sorted R+ cells from quaternary tumor. (G) Semiquantitative reverse transcription–polymerase chain reaction analysis of FACS-sorted cell populations. Total refers to total cells isolated from quaternary transplanted ERMS isolated by FACS based on cell viability and serve as an input control. (H–M) Fish transplanted with 50 R+ cells defined in (E)–(G) (5° Recipient). (H) Brightfield image of transplant recipient animal. (I) Merged image of a dsRED2+/GFP+ tumor in same animal. (J) Hematoxylin and eosin-stained and (K) anti-GFP-immunostained section of transplanted fish showing that ERMS cells infiltrate the liver (L), head kidney (HK), and skeletal muscle. (L, M) High-power magnification of boxed region in (J). Scale bar equals 1 mm (J, K) and 100 μm (L, M) (modified from Langenau et al.37).
Fluorescent reporter lines are invaluable for detecting tumor growth and engraftment into recipient zebrafish; however, their use is still limited by directly visualizing tumor cells through translucent zebrafish. In Medaka, see-through fish were generated by creating genetic strains of fish that lack both iridophores and melanocytes.47 In these experiments, Wakamatsu et al. directly visualized organ formation and used a transgenic GFP reporter line to follow gonad growth in vivo. Building on these observations, White et al. have recently created a see-through zebrafish—creatively called “casper”—that also lacks iridophores and melanocytes.48 This breakthrough facilitated the tracking of GFP-labeled blood cells following cell transplantation into irradiated recipient fish and the visualization of melanoma regrowth after injection into casper mutant animals. Remarkably, the authors were able to track melanoma dissemination in these animals over time and suggested that individual cells can be visualized within whole adult fish by laser-scanning confocal microscopy. Such approaches will greatly facilitate the identification of transplant engraftment into recipient animals and will likely aid in identifying tumor niches where self-renewing cells reside. Together, optically clear adult zebrafish provide many advantages over existing vertebrate models of cancer to visualize cancer development and progression.
Syngeneic zebrafish were recently created and were successfully used to engraft liver and pancreatic tumors into transplant recipients.49 The two clonal lines described by Mizgireuv and Revskoy were created by squeezing eggs from a single female, fertilizing them with ultraviolet-inactivated sperm, and then applying 2 min of heat shock at 41.4°C prior to the first cleavage (∼13 min after fertilization). The exceedingly small fraction of animals that survived this procedure was raised to adulthood. Eggs were obtained from gynogenetic diploid female fish and subjected to a second round of heat shock. The resulting progenies were incrossed to create the CB1 and CW1 clonal fish lines. Importantly, carcinogen-induced liver and pancreatic tumors that developed in these lines could be transplanted into syngeneic recipient animals (Fig. 5). Although a pioneering study, the potential for these and other clonal zebrafish lines has yet to be fully realized. In fact, limiting dilution experiments using these lines should correctly assess and quantify true tumor-initiating cell number in a variety of cancers. Creating clonal fish lines that are see-through will revolutionize the types of experiments that can be completed in zebrafish cell transplantation. Finally, developing truly immune-deficient zebrafish will provide a much needed tool to effectively beat the immune system. Recent advances in targeted gene disruption using zinc finger nucleases should facilitate the development of fully immune-suppressed zebrafish.50–52 Specifically, rag1-deficient53 IL2-gamma receptor-deficient fish would provide a universal recipient line for both zebrafish cancer and xenograft transplantation studies.
FIG. 5.
Tumors from clonal zebrafish can be transplanted into syngeneic recipient animals. Advanced stages of tumor development after intramuscular transplantation of moderately differentiated hepatocellular carcinoma zt34 (A, B) compared with normal liver (C). A spontaneous acinar cell carcinoma of the pancreas was capable of robust engraftment when intraperitoneally injected into clonal fish (D). Histological analysis showed that the tumor contained areas that were morphologically similar to normal pancreas (E, F, respectively). Scale bars (B, C, E, F) equal 50 μm.
Human and mouse cells can be transplanted into larval fish or immune-suppressed adult fish (Fig. 6). Xenograft transplantation experiments in zebrafish have been recently reviewed by Stoletov and Klemke,54 but we will summarize several key findings from this work. Stoletov et al. showed that adenocarcinomas (MDA-435), fibrosarcomas (HT-1080), and melanomas (B16) can engraft into 30-day-old dexamethasone-treated fli1-GFP transgenic zebrafish55 and established that vascular reorganization can be easily visualized in engrafted animals56 (Fig. 6A). In these experiments, RAS family homolog member C (RHOC) played a critical role in cell movement and could partially regulate the early stages of metastasis. Additional experiments by Nicoli et al. demonstrated that tumorigenic FGF2-overexpressing mouse aortic endothelial cells could be transplanted into 2-day-old embryos prior to establishment of the acquired immune system.57 These experiments capitalized on the use of immune-incompetent zebrafish embryos as recipients and showed that FGF2-expressing cells were able to undergo new vascular growth. Cell transplantation of human C8161 melanoma cells into blastula stage embryos showed that the nodal pathway was active in melanomas, despite these fish never developed robust engraftment of tumors.58,59 By contrast, introduction of human metastatic melanoma WM-266-4 cells into the yolk of 2-day-old fish formed observable tumors by 7 days postinjection60 (Fig. 6B, C). Additionally, human U251 glioblastoma cells when transplanted into the yolk sac of blastula-stage embryos proliferated, formed tumors (Fig. 6D–I), and recruited blood vessels. Engrafted glioblastoma cells were more sensitive to radiation treatment when treated in combination with temozolomide.61 Lally et al. also used U251 glioblastoma cells to identify novel small molecule sensitizers and identified a novel drug NS123 (4′-bromo-3′-nitropropiophenone) that enhanced the growth-inhibitory effect of U251 cells to ionizing radiation.62 Although xenograft transplantation into zebrafish is a firmly established method for assessing recruitment of vasculature, response to therapy, and early metastatic potential, their use in determining self-renewal capacity has yet to be described. Xenograft cell transplantation experiments that assess self-renewal potential will likely require fully immune-compromised fish such as those outlined earlier.
FIG. 6.
Human cancers can engraft into zebrafish embryos and larvae. Hematoxylin and eosin-stained cross section of a juvenile zebrafish that had been transplanted with MDA-435 adenocarcinoma cells. Cells were injected intraperitoneally and imaged at 5 days posttransplantation (Stoletov et al.56). Adenocarcinoma cells invaded the body wall (arrow, A). Anatomical structures include spinal cord (SC), vertebrae (VB), and swim bladder (SB). Transplanted metastatic WM-266-4 melanoma cells formed pigmented masses in the intestinal wall by 7 days postinjection (Haldi et al.60). Lateral view (B) and ventral view (C) of the same fish. Boxed area: pigmented tumor masses seen with brightfield illumination are also fluorescent-labelled with em-Di dye (bottom right). Red fluorescent protein–labeled human U251 glioblastoma cells can engraft into larval fish (D–I). Two U251 glioblastoma cells transplanted into a 2-day-old zebrafish embryo proliferate over time (Geiger et al.61): 2 days (D, E), 4 days (F, G), and 9 days posttransplantation (H, I).
Remaining Challenges
Zebrafish transplantation models hold much promise for assessing cancer self-renewal; however, many challenges remain. For example, cell transplantation protocols still require further optimization. Irradiation has been commonly used to ablate immune responses and facilitated engraftment of tumors into adult fish. In these protocols, 23–25 Gy whole body irradiation was applied to recipient animals at 2 days prior to cell transplantation. Although irradiation protocols can dampen the immune response in recipient fish, animals regained immune competency by 21 days postirradiation63–65 and mounted immune responses to kill tumor cells (Smith et al., unpublished). Other groups have used dexamethasone treatment to ablate the lymphocyte populations and were subsequently able to engraft tumors into 30-day-old recipient animals.56 However, in these experiments, dexamethasone must be present throughout the experiment to block immune responses and was lethal when at a large dose to engrafted fish. Dexamethasone and other glucocorticoids are immunosuppressive drugs that are also commonly used in the treatment of leukemia. Thus, dexamethasone would likely kill transplanted leukemia cells and alter overall tumor growth, which would affect accurate quantitation of tumor-initiating cells following limiting dilution cell transplantation. Finally, xenograft transplantation can be completed in larvae that have yet to develop a functional acquired immune system.57–60,62,66 T and B cells develop by 3 days of life, but are not functional until much later in development. Thus, blastulas and 2–5-day-old zebrafish can be used as transplant recipients. However, a severe disadvantage of this system is that transplant engraftment can be assessed only during the short time period prior to maturation of the acquired immune system. In fact, most experiments did not follow tumor engraftment past 9 days of life.57,58,60–62,66
Detecting subpopulations of tumor cells through use of antibodies and/or transgenic approaches remains a severe limitation for the field. Zebrafish-specific antibodies are limited, and cell surface antibodies commonly used in FACS are currently not in use. Creating new cell surface antibodies to identify subpopulations of tumor cells will be critically important for realizing the full potential of zebrafish as a model of self-renewal. As an alternative, investigators have put great efforts into creating transgenic zebrafish lines that label distinct subpopulations of cells with fluorescent proteins. However, there remains a major need to create stable transgenic zebrafish that express fluorescent proteins other than GFP. For example, numerous transgenic lines that label discrete muscle cell populations including myf5-GFP,67 myogenin-GFP, mylz2-GFP,68 creatine kinase-GFP,69 and alpha-actin-GFP have been described,70 but only few transgenic lines that utilize additional fluorescent proteins have been generated. If there are new transgenic lines that labeled cell types based on expression of Amcyan, GFP, zsYellow, and mCherry, it would greatly aid in the prospective identification of muscle cell subpopulations in various models of disease and malignancy. Moreover, multicolored transgenic zebrafish would also facilitate the tracking and imaging of tumor cell populations in vivo through use of confocal microscopy. In fact, use of multifluorescent animals and time-lapse microscopy would allow investigators to directly assess self-renewal in vivo without the need for complex cell transplantation protocols. Such approaches would revolutionize how we assess self-renewal in cancer and are currently unavailable to any model of disease.
Although we have focused this review on highlighting our belief that zebrafish will be a valuable model of cancer self-renewal, the ultimate goal for all this work is to provide new mechanistic insights into self-renewal processes. As elegantly reviewed in this issue of Zebrafish by Taylor et al., powerful transplantation techniques can be used in conjunction with mutant analysis, heat-shock transgenic approaches, morpholino knockdown, and loss-of-function mutants produced by zinc finger nucleases. Such approaches will provide unprecedented access into the molecular underpinnings of self-renewal. In summary, the zebrafish is now widely used as an experimental model system to uncover important pathways in malignancy. Zebrafish cancer models have many unique attributes that are unavailable in mouse models of disease and thus provide new and complementary approaches to assess important questions related to cancer biology and self-renewal. Although in its infancy, using zebrafish to assess cancer self-renewal will likely provide new and exciting insights into human disease.
Disclosure Statement
No competing financial interests exist.
References
- 1.Reya T. Morrison SJ. Clarke MF. Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM. Strasser A. Is tumor growth sustained by rare cancer stem cells or dominant clones? Cancer Res. 2008;68:4018–4021. doi: 10.1158/0008-5472.CAN-07-6334. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefoix T. Bonnefoix P. Verdiel P. Sotto JJ. Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. J Immunol Methods. 1996;194:113–119. doi: 10.1016/0022-1759(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 4.Quintana E. Shackleton M. Sabel MS. Fullen DR. Johnson TM. Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M. Hiramatsu H. Kobayashi K. Suzue K. Kawahata M. Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 6.Cobaleda C. Gutierrez-Cianca N. Perez-Losada J. Flores T. Garcia-Sanz R. Gonzalez M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human Philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–1013. [PubMed] [Google Scholar]
- 7.Kong Y. Yoshida S. Saito Y. Doi T. Nagatoshi Y. Fukata M, et al. CD34+CD38+CD19+ as well as CD34+CD38−CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia. 2008;22:1207–1213. doi: 10.1038/leu.2008.83. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D. Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Lapidot T. Sirard C. Vormoor J. Murdoch B. Hoang T. Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 10.Jin L. Hope KJ. Zhai Q. Smadja-Joffe F. Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 11.Huntly BJ. Shigematsu H. Deguchi K. Lee BH. Mizuno S. Duclos N, et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell. 2004;6:587–596. doi: 10.1016/j.ccr.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande AJ. Cusan M. Rawat VP. Reuter H. Krause A. Pott C, et al. Acute myeloid leukemia is propagated by a leukemic stem cell with lymphoid characteristics in a mouse model of CALM/AF10-positive leukemia. Cancer Cell. 2006;10:363–374. doi: 10.1016/j.ccr.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Daley GQ. Van Etten RA. Baltimore D. Blast crisis in a murine model of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 1991;88:11335–11338. doi: 10.1073/pnas.88.24.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neering SJ. Bushnell T. Sozer S. Ashton J. Rossi RM. Wang PY, et al. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 2007;110:2578–2585. doi: 10.1182/blood-2007-02-073031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wojiski S. Guibal FC. Kindler T. Lee BH. Jesneck JL. Fabian A, et al. PML-RARalpha initiates leukemia by conferring properties of self-renewal to committed promyelocytic progenitors. Leukemia. 2009;8:1462–1471. doi: 10.1038/leu.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox CV. Martin HM. Kearns PR. Virgo P. Evely RS. Blair A. Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukemia. Blood. 2007;109:674–682. doi: 10.1182/blood-2006-06-030445. [DOI] [PubMed] [Google Scholar]
- 17.Agliano A. Martin-Padura I. Mancuso P. Marighetti P. Rabascio C. Pruneri G, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer. 2008;123:2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hajj M. Wicha MS. Benito-Hernandez A. Morrison SJ. Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh SK. Hawkins C. Clarke ID. Squire JA. Bayani J. Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 20.Beier D. Hau P. Proescholdt M. Lohmeier A. Wischhusen J. Oefner PJ, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 21.Bao S. Wu Q. McLendon RE. Hao Y. Shi Q. Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 22.Bao S. Wu Q. Sathornsumetee S. Hao Y. Li Z. Hjelmeland AB, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 23.Piccirillo SG. Reynolds BA. Zanetti N. Lamorte G. Binda E. Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 24.Taylor MD. Poppleton H. Fuller C. Su X. Liu Y. Jensen P, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien CA. Pollett A. Gallinger S. Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 26.Dalerba P. Dylla SJ. Park IK. Liu R. Wang X. Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci-Vitiani L. Lombardi DG. Pilozzi E. Biffoni M. Todaro M. Peschle C. De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 28.Li C. Heidt DG. Dalerba P. Burant CF. Zhang L. Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 29.Hermann PC. Huber SL. Herrler T. Aicher A. Ellwart JW. Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Schatton T. Murphy GF. Frank NY. Yamaura K. Waaga-Gasser AM. Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly PN. Dakic A. Adams JM. Nutt SL. Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 32.Somervaille TC. Cleary ML. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 2006;10:257–268. doi: 10.1016/j.ccr.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Krivtsov AV. Twomey D. Feng Z. Stubbs MC. Wang Y. Faber J, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 34.Guo W. Lasky JL. Chang CJ. Mosessian S. Lewis X. Xiao Y, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haramis AP. Hurlstone A. van der Velden Y. Begthel H. van den Born M. Offerhaus GJ. Clevers HC. Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 2006;7:444–449. doi: 10.1038/sj.embor.7400638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berghmans S. Murphey RD. Wienholds E. Neuberg D. Kutok JL. Fletcher CD, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langenau DM. Keefe MD. Storer NY. Guyon JR. Kutok JL. Le X, et al. Effects of RAS on the genesis of embryonal rhabdomyosarcoma. Genes Dev. 2007;21:1382–1395. doi: 10.1101/gad.1545007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SW. Davison JM. Rhee J. Hruban RH. Maitra A. Leach SD. Oncogenic KRAS induces progenitor cell expansion and malignant transformation in zebrafish exocrine pancreas. Gastroenterology. 2008;134:2080–2090. doi: 10.1053/j.gastro.2008.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le X. Langenau DM. Keefe MD. Kutok JL. Neuberg DS. Zon LI. Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA. 2007;104:9410–9415. doi: 10.1073/pnas.0611302104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patton EE. Widlund HR. Kutok JL. Kopani KR. Amatruda JF. Murphey RD, et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Frazer JK. Meeker ND. Rudner L. Bradley DF. Smith AC. Demarest B, et al. Heritable T-cell malignancy models established in a zebrafish phenotypic screen. Leukemia. 2009;10:1825–1835. doi: 10.1038/leu.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langenau DM. Feng H. Berghmans S. Kanki JP. Kutok JL. Look AT. Cre/lox-regulated transgenic zebrafish model with conditional myc-induced T cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2005;102:6068–6073. doi: 10.1073/pnas.0408708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabaawy HE. Azuma M. Embree LJ. Tsai HJ. Starost MF. Hickstein DD. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2006;103:15166–15171. doi: 10.1073/pnas.0603349103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam SH. Wu YL. Vega VB. Miller LD. Spitsbergen J. Tong Y, et al. Conservation of gene expression signatures between zebrafish and human liver tumors and tumor progression. Nat Biotechnol. 2006;24:73–75. doi: 10.1038/nbt1169. [DOI] [PubMed] [Google Scholar]
- 45.Kennedy JA. Barabe F. Poeppl AG. Wang JC. Dick JE. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science. 2007;318:1722. doi: 10.1126/science.1149590. author reply 1722. [DOI] [PubMed] [Google Scholar]
- 46.Langenau DM. Keefe MD. Storer NY. Jette CA. Smith AC. Ceol CJ, et al. Co-injection strategies to modify radiation sensitivity and tumor initiation in transgenic Zebrafish. Oncogene. 2008;27:4242–4248. doi: 10.1038/onc.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakamatsu Y. Pristyazhnyuk S. Kinoshita M. Tanaka M. Ozato K. The see-through medaka: a fish model that is transparent throughout life. Proc Natl Acad Sci USA. 2001;98:10046–10050. doi: 10.1073/pnas.181204298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White RM. Sessa A. Burke C. Bowman T. LeBlanc J. Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizgireuv IV. Revskoy SY. Transplantable tumor lines generated in clonal zebrafish. Cancer Res. 2006;66:3120–3125. doi: 10.1158/0008-5472.CAN-05-3800. [DOI] [PubMed] [Google Scholar]
- 50.Doyon Y. McCammon JM. Miller JC. Faraji F. Ngo C. Katibah GE, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meng X. Noyes MB. Zhu LJ. Lawson ND. Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foley JE. Yeh JR. Maeder ML. Reyon D. Sander JD. Peterson RT, et al. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN) PLoS One. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wienholds E. Schulte-Merker S. Walderich B. Plasterk RH. Target-selected inactivation of the zebrafish rag1 gene. Science. 2002;297:99–102. doi: 10.1126/science.1071762. [DOI] [PubMed] [Google Scholar]
- 54.Stoletov K. Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- 55.Lawson ND. Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 56.Stoletov K. Montel V. Lester RD. Gonias SL. Klemke R. High-resolution imaging of the dynamic tumor cell vascular interface in transparent zebrafish. Proc Natl Acad Sci USA. 2007;104:17406–17411. doi: 10.1073/pnas.0703446104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nicoli S. Ribatti D. Cotelli F. Presta M. Mammalian tumor xenografts induce neovascularization in zebrafish embryos. Cancer Res. 2007;67:2927–2931. doi: 10.1158/0008-5472.CAN-06-4268. [DOI] [PubMed] [Google Scholar]
- 58.Lee LM. Seftor EA. Bonde G. Cornell RA. Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 59.Topczewska JM. Postovit LM. Margaryan NV. Sam A. Hess AR. Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 60.Haldi M. Ton C. Seng WL. McGrath P. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis. 2006;9:139–151. doi: 10.1007/s10456-006-9040-2. [DOI] [PubMed] [Google Scholar]
- 61.Geiger GA. Fu W. Kao GD. Temozolomide-mediated radiosensitization of human glioma cells in a zebrafish embryonic system. Cancer Res. 2008;68:3396–3404. doi: 10.1158/0008-5472.CAN-07-6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lally BE. Geiger GA. Kridel S. Arcury-Quandt AE. Robbins ME. Kock ND, et al. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007;67:8791–8799. doi: 10.1158/0008-5472.CAN-07-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traver D. Paw BH. Poss KD. Penberthy WT. Lin S. Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 64.Traver D. Winzeler A. Stern HM. Mayhall EA. Langenau DM. Kutok JL, et al. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 2004;104:1298–1305. doi: 10.1182/blood-2004-01-0100. [DOI] [PubMed] [Google Scholar]
- 65.Langenau DM. Ferrando AA. Traver D. Kutok JL. Hezel JP. Kanki JP, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci USA. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marques IJ. Weiss FU. Vlecken DH. Nitsche C. Bakkers J. Lagendijk AK, et al. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen YH. Wang YH. Chang MY. Lin CY. Weng CW. Westerfield M, et al. Multiple upstream modules regulate zebrafish myf5 expression. BMC Dev Biol. 2007;7:1. doi: 10.1186/1471-213X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ju B. Chong SW. He J. Wang X. Xu Y. Wan H, et al. Recapitulation of fast skeletal muscle development in zebrafish by transgenic expression of GFP under the mylz2 promoter. Dev Dyn. 2003;227:14–26. doi: 10.1002/dvdy.10273. [DOI] [PubMed] [Google Scholar]
- 69.Ju B. Xu Y. He J. Liao J. Yan T. Hew CL, et al. Faithful expression of green fluorescent protein (GFP) in transgenic zebrafish embryos under control of zebrafish gene promoters. Dev Genet. 1999;25:158–167. doi: 10.1002/(SICI)1520-6408(1999)25:2<158::AID-DVG10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 70.Higashijima S. Okamoto H. Ueno N. Hotta Y. Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev Biol. 1997;192:289–299. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]