Summary
Matrix production during biofilm formation by Bacillus subtilis is governed by a gene control circuit at the heart of which are three dedicated regulatory proteins, the antirepressor SinI, the repressor SinR, and the downstream regulator SlrR. Matrix production is triggered by the synthesis of SinI, which binds to and inactivates SinR, thereby derepressing genes for matrix production as well as the gene for SlrR. Recently, two additional regulators of matrix genes were identified: SlrA, which was reported to be an activator of SlrR, and YwcC, a repressor of SlrA synthesis. We present evidence indicating that SlrA, which is a paralog of SinI, is like SinI, an antirepressor that binds to, and inactivates, SinR. We also show that SlrA does not activate SlrR for expression of matrix genes. Instead, SlrR binds to, and inhibits the activity of, SlrA. Thus, the YwcC-SlrA-SinR-SlrR pathway is a negative feedback loop in which SlrA indirectly stimulates the synthesis of SlrR, and SlrR, in turn, inhibits the activity of SlrA. Finally, we report that under standard laboratory conditions SlrA makes only a small contribution to the expression of genes for matrix production. We propose that in response to an unknown signal recognized by the YwcC repressor, SlrA transiently boosts matrix production.
Introduction
Most bacteria can form complex, multicellular communities known as biofilms (Branda et al., 2005, O’Toole & Kaplan, 2000, Stoodley et al., 2002). One bacterium that forms particularly robust biofilms is the endospore-forming soil organism Bacillus subtilis (Aguilar et al., 2007, Branda et al., 2001, Lemon et al., 2008). Wild (undomesticated) strains of B. subtilis form morphologically complex communities on solid medium and at the surface of standing cultures (floating biofilms or pellicles) (Branda et al., 2001). The cells in these communities grow in long, parallel chains bound together by an extracellular matrix consisting of polysaccharide and a specific protein (TasA) (Branda et al., 2006, Branda et al., 2001, Chu et al., 2006, Kearns et al., 2005, Kobayashi, 2007). The exopolysaccharide is produced by enzymes encoded by the fifteen-gene epsA-O operon (hereafter simply the eps operon) and the protein (TasA) is encoded and deployed by the three-gene yqxM-sipW-tasA operon (hereafter the yqxM operon) (Branda et al., 2006, Chu et al., 2006, Kearns et al., 2005). B. subtilis biofilms consist of multiple, and spatially separated, types of cells, including matrix-producing cells, spore-forming cells, and cells that produce the cyclic lipopeptide surfactin (López et al., 2009b, López et al., 2009c, Vlamakis et al., 2008). In addition to its role as a surfactant, surfactin is a signaling molecule that stimulates the activity of the kinase KinC, which, in turn, leads to the activation by phosphorylation of the response regulator Spo0A (López et al., 2009a). Finally, Spo0A~P triggers a regulatory cascade that leads to matrix production and cell filamentation (Branda et al., 2001, Chu et al., 2008, Kobayashi, 2007). The circuitry for this cascade consists of two kinds of regulatory proteins: those that also play a global role in gene expression and those that are dedicated to biofilm formation. Examples of the former are Spo0A itself and the transition-state regulator AbrB (Banse et al., 2008, Burbulys et al., 1991, Fujita et al., 2005, Gonzalez-Pastor et al., 2003, Hamon & Lazazzera, 2001, Molle et al., 2003). Examples of the latter are sinI, sinR, slrR (Chu et al., 2008, Kearns et al., 2005, Kobayashi, 2008), and two newly described genes, ywcC and slrA (Kobayashi, 2008), which are the subject of this investigation.
The core circuitry for biofilm formation is as follows. Spo0A~P stimulates the transcription of sinI (Chai et al., 2008, Fujita et al., 2005, Shafikhani et al., 2002), whose product binds to, and inactivates, the repressor SinR (Bai et al., 1993, Kearns et al., 2005, Lewis et al., 1996). SinR is the master regulator for biofilm formation that directly represses the eps and yqxM operons (Chu et al., 2006, Kearns et al., 2005). Thus, SinI is an antirepressor that derepresses genes under SinR control by antagonizing (reversing the action of) the master regulator. The eps and yqxM operons are also under the negative control of AbrB (Chu et al., 2008, Hamon et al., 2004, Stover & Driks, 1999), which is eliminated in post-exponential phase cells by the action of Spo0A~P, which represses the gene for AbrB and induces the synthesis of an antirepressor that inhibits AbrB protein (Banse et al., 2008, Strauch et al., 1990). Lastly, the action of SinI derepresses slrR, which is also under the direct negative control of SinR (Chu et al., 2008, Kobayashi, 2008). SlrR is a regulatory protein that contains regions of similarity to both SinI and SinR (Chu et al., 2008). The SlrR protein stimulates transcription of yqxM (but not eps nor slrR) and represses genes that mediate cell separation following cytokinesis (hence promoting cell chaining) (Chu et al., 2008, Kobayashi, 2008). Mutants of sinI and slrR are severely impaired for biofilm formation whereas a sinR mutant forms hyper robust biofilms (Branda et al., 2006, Chu et al., 2008, Kearns et al., 2005, Kobayashi, 2007, Kobayashi, 2008).
Recently, Kobayashi (Kobayashi, 2008) reported an additional regulatory gene, slrA, that contributes to biofilm formation. The slrA gene is a paralog of sinI and is under the negative control of a TetR-like repressor YwcC (Kobayashi, 2008). The author reached two principal conclusions about slrA. First, slrA can substitute for sinI when its repressor gene ywcC is deleted. That is, when SlrA synthesis is derepressed, SlrA bypasses the requirement for sinI in biofilm formation. Second, SlrA protein works by directly stimulating the activity of SlrR. That is, SlrA acts downstream of SinI and SinR, promoting matrix production via direct interaction with SlrR.
Here we challenge both conclusions and propose a simple alternative model for how SlrA acts and its significance in biofilm formation. First, as might have been expected from its high similarity to SinI, SlrA is an antirepressor that binds to, and inactivates, SinR. Thus, SinR is subject to inhibition by two paralogous antirepressors, SinI and SlrA. Second, SlrA does not stimulate the activity of SlrR with respect to matrix gene expression. To the contrary, our evidence shows that SlrR binds to, and antagonizes, SlrA. Thus, SlrA, SinR, and SlrR constitute a negative feedback loop that reverses the effect of SlrA. Third, SlrA does not substitute for SinI in biofilm formation. Even under conditions in which SlrA synthesis is derepressed by mutation of ywcC, a sinI mutant remains blocked in biofilm formation (although, as we show, such ywcC sinI double mutants readily acquire suppressor mutations in sinR). Fourth, under standard laboratory conditions, SlrA plays only a minor role in biofilm formation. A slrA mutant is only slightly depressed for eps and yqxM expression and exhibits only a subtle defect in biofilm formation. Instead, we suggest that YwcC and SlrA constitute a supplemental pathway for augmenting the action of SinI and transiently (due to the feedback loop) stimulating matrix production in response to an unknown signaling molecule.
Results and Discussion
Mutations of ywcC and slrA mildly impair biofilm formation
We created null mutations in ywcC (ΔywcC) and slrA (ΔslrA) and tested their effects on colony and pellicle architecture in the wild strain 3610 grown on MSgg medium. The ΔywcC mutant formed somewhat more robust biofilms than did the 3610 parent strain (Fig. 1). Meanwhile, the ΔslrA mutant was mildly impaired in biofilm formation; it was modestly defective in colony morphology and formed a thick, but less architecturally complex pellicle than did the wild type (Fig. 1).
Figure 1. Mutations of ywcC and slrA mildly influence biofilm formation.
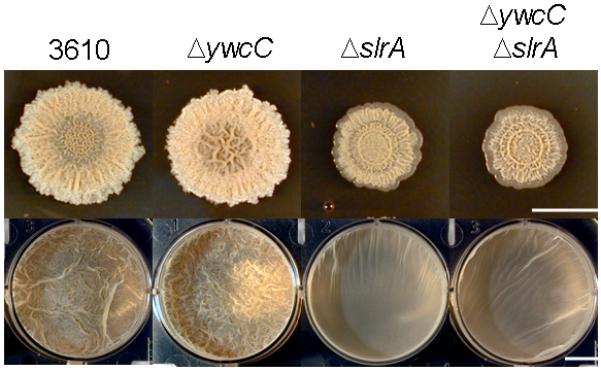
Colonies (upper panels) and pellicles (lower panels) were formed by 3610, ΔywcC (YC295), ΔslrA (YC294), and ΔywcC ΔslrA double mutant (YC296) strains grown on MSgg medium. Bar, 1 cm.
We also constructed a ΔywcC ΔslrA double mutant. Its phenotype was indistinguishable from that of a ΔslrA single mutant (Fig. 1), a finding consistent with the view that YwcC is a repressor of slrA and that slrA acts downstream of ywcC (Kobayashi, 2008). Reinforcing the view that YwcC is a repressor of slrA, the ΔywcC mutation strongly derepressed expression of a lacZ fusion to the slrA promoter (PslrA-lacZ) (Fig. 2A).
Figure 2. An slrA mutation mildly impairs transcription of SinR-controlled genes.
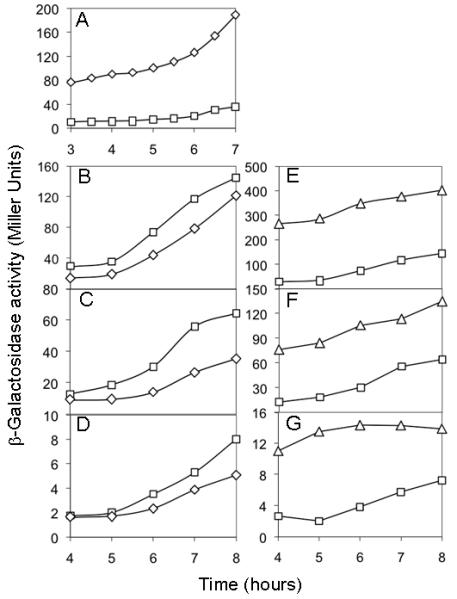
(A) Expression of PslrA-lacZ in a ΔywcC mutant (YC527, diamonds) and in the wild type (YC526, squares). (B-G) Expression of PyqxM-lacZ (panels B and E), PepsA-lacZ (panels C and F), and PslrR-lacZ (panels D and G) in a ΔslrA mutant (strains YC501, YC502, and YC503; diamonds in panels B-D), a ΔywcC mutant (strains YC505, YC506, and YC507; triangles in panels E-G), and the wild type (strains FC134, YC130, and YC122; squares in panels B-G). An ΔepsH mutation was introduced into some of the reporter strains in this experiment and experiments described below. The ΔepsH mutation prevented formation of bundled cell chains but was known to have little effect on the expression of SinR-controlled genes (Chu et al., 2008, Chu et al., 2006, Kearns et al., 2005).
Mutation of slrA mildly impairs the transcription from SinR-controlled promoters
Next, we measured the effect of the ΔywcC and ΔslrA mutations on the expression of lacZ fusions to the promoters of three transcription units (the yqxM and epsA-O operons and the slrR gene), known to be under the direct negative control of SinR. The results of Fig. 2 show that expression of the PyqxM-lacZ (panel B), PepsA-lacZ (panel C), and PslrR-lacZ (panel D) fusions was only mildly impaired in the ΔslrA mutant. These results are consistent with, and reinforce the findings of Fig. 1, indicating that SlrA plays a minor role in biofilm formation under the conditions tested.
In contrast to the mild effect of ΔslrA, the ΔywcC mutation had a major effect, markedly increasing the expression of all three lacZ fusions above that of the wild type (Fig. 2, panels E-G). This effect was almost completely dependent on slrA as demonstrated by the fact that expression of PyqxM-lacZ and PepsA-lacZ was significantly lower in a ΔywcC ΔslrA double mutant than in the ΔywcC single mutant but similar to that seen in the ΔslrA single mutant (Fig. 3A).
Figure 3. An slrA mutation is epistatic to a ywcC mutation and a sinR mutation is epistatic to a slrA mutation.
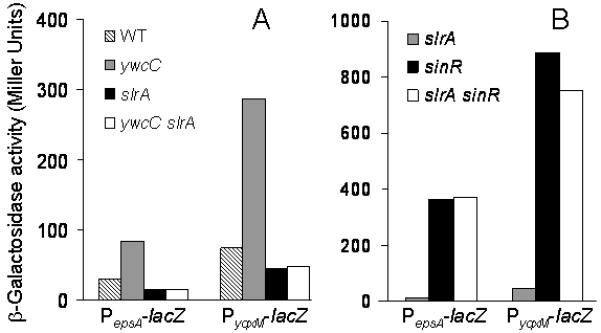
Shown are the expression of PepsA-lacZ and PyqxM-lacZ. Panel A shows expression of the fusions in wild type, ΔywcC, ΔslrA, and ΔywcC ΔslrA double mutant strains (from left to right, the strains are YC130, YC506, YC502, YC510, FC134, YC505, YC501, and YC509, respectively) cultured in MSgg medium to early stationary phase. Panel B shows expression of the reporters in ΔslrA, ΔsinR, and ΔslrA ΔsinR double mutant strains grown similarly (from left to right, the strains are YC502, YC133, YC569, YC501, FC135, and YC568, respectively).
Because SlrA is a paralog of SinI and because SinI is known to be an antirepressor of SinR, the repressor for PyqxM, PepsA, and PslrR, we hypothesized that SlrA, like SinI, is an antirepressor of SinR. Under normal conditions in cells wild type for ywcC, slrA is expressed at only low levels and hence does not contribute greatly to biofilm formation. However, when the YwcC repressor is absent, SlrA accumulates to high levels, substantially reversing SinR-mediated repression.
As a first test of this hypothesis, we created a ΔsinR ΔslrA double mutant. The results of Fig. 3B show that the effect of the ΔsinR mutation was epistatic to that of ΔslrA. That is, expression of the PyqxM-lacZ and the PepsA-lacZ fusions in the ΔsinR ΔslrA double mutant was similar to that seen in a ΔsinR single mutant. Thus, the results so far are consistent with the hypothesis that SlrA acts upstream of SinR in the pathway controlling biofilm matrix gene expression.
SinI functionally substitutes for SlrA
If SlrA is simply an alternative SinI-like antirepressor, then it should be possible to replace SlrA with SinI without altering biofilm formation or the expression of SinR-controlled genes. Accordingly, we replaced the slrA coding sequence, leaving the slrA regulatory sequences intact, with the coding sequence of sinI. To do this, we created a strain that was deleted for slrA (ΔslrA) at its native locus and contained a copy of sinI under the control of the slrA promoter (PslrA-sinI) integrated at amyE. As a control, we created a strain that lacked slrA at its native locus but contained an intact copy of slrA (PslrA-slrA) at amyE. The results of Fig. 4 show that the (mild) effects on colony architecture and pellicle formation caused by ΔslrA were complemented as effectively by PslrA-sinI as by PslrA-slrA. We conclude that SlrA is functionally equivalent to its paralog SinI.
Figure 4. SinI functionally substitutes SlrA.
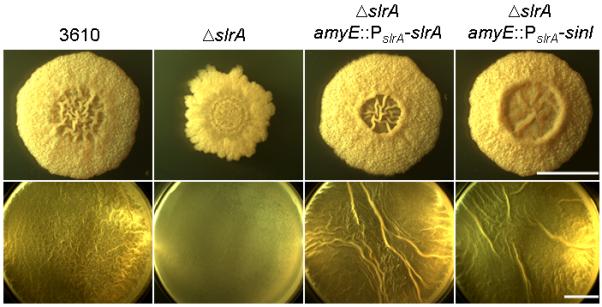
Shown are colony architecture (upper panels) and pellicle formation (lower panels) for 3610, a ΔslrA mutant (YC294), a ΔslrA mutant complemented with PslrA-slrA (YC563), and a ΔslrA mutant complemented with PslrA-sinI (YC564) grown on MSgg medium. Bar, 1 cm.
SlrA binds to SinR and inhibits SinR from binding to its operators
Next, we investigated whether SlrA interacts with SinR and whether this interaction inhibits SinR from binding to DNA. To do this, we appended a histidine tag at the N-terminus of SlrA (His6-SlrA) and produced the His-tagged protein in E. coli. We incubated a cleared lysate containing His6-SlrA from E. coli with a cleared lysate from a B. subtilis strain mutated for slrR (ΔslrR) and then applied the mixture to Ni-NTA agarose beads (Qiagen). The reason that we used a ΔslrR mutant was to exclude the possibility that SlrA interacts with SinR but that the interaction is indirect and is mediated by SlrR. As a negative control, we used a lysate from E. coli lacking His6-SlrA. Using anti-SinR antibodies, we detected SinR in the eluate from the His6-SlrA-containing beads but not from the beads lacking His6-SlrA (Fig. 5A). Interestingly, SinR was not the only protein that adhered to the beads in a His6-SlrA-dependent manner; at least one other as yet unidentified protein that reacted with the anti-SinR antibodies was detected (asterisk in Fig. 5A).
Figure 5. SlrA binds to SinR and SlrR.
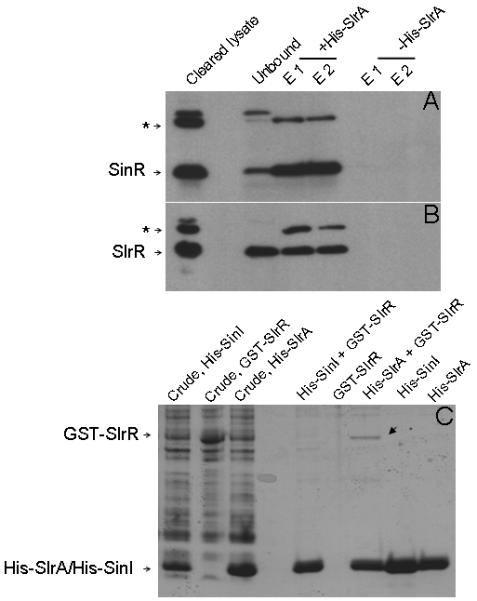
Panels A and B show immuno-detection of SinR (A) and SlrR (B) in the eluate from Ni-NTA agarose beads upon applying mixed lysates containing His6-SlrA and B. subtilis total protein prepared from ΔslrR (A) or ΔsinR (B) mutant cells. Asterisks in A and B indicate an unknown protein that reacts with the antibodies. Unbound represents samples from flow-through fractions. E1 and E2 are samples from two elution fractions from the affinity column. Panel C shows affinity purification of GST-SlrR. Crude lysates were prepared from E. coli cells engineered to produce His6-SinI, GST-SlrR, or His6-SlrA. Each lysate (or mixed lysates) was applied to Ni-NTA agarose beads. The arrow identifies GST-SlrR retained by His6-SlrA.
We conducted electrophoretic mobility shift assays (EMSA) to investigate whether the binding of SlrA to SinR inhibits the capacity of SinR to bind to its operator. We used purified histidine-tagged forms of SinR, SlrA, and SinI proteins as described above. SinI was used as a positive control as it was previously been shown to inhibit SinR from binding to DNA (Kearns et al., 2005). As shown in Fig. S2A, the presence of His6-SinR alone markedly retarded the electrophoretic mobility of a DNA probe containing the SinR operator in the eps operon. This retardation was reversed by the addition of increasing amounts of His6-SinI (Fig. S2B) or His6-SlrA (Fig. S2C). It can be seen that SlrA was a less potent anti-repressor of SinR, than was SinI. Nonetheless, SlrA was clearly effective in reversing binding of SinR to the DNA probe.
The ywcC mutation does not bypass the requirement for SinI in biofilm formation
It is known that the SinI antirepressor is required to derepress SinR-controlled genes and that a ΔsinI mutant is profoundly blocked in biofilm formation (Kearns et al., 2005). However, a recent report (Kobayashi, 2008) claimed that in the absence of YwcC, SinI is no longer required for pellicle formation. Specifically, a ΔywcC ΔsinI double mutant was reported to form robust pellicles (Kobayashi, 2008). We attempted to reproduce this result by creating a ΔywcC ΔsinI double mutant and testing its capacity to form biofilms in both MSgg and 2X SGG media. The results of Fig. 6A show that the double mutant was blocked in biofilm formation when grown on either medium as judged in both the colony and pellicle assays (Fig. 6A). Thus, the absence of YwcC does not bypass the requirement for SinI, even though SlrA is overproduced in a ΔywcC mutant. Evidently, either not enough SlrA antirepressor is made under these circumstances to bypass the requirement for SinI or SlrA is a less efficient antirepressor for SinR than is SinI.
Figure 6. Derepression of slrA does not bypass sinI but does bypass slrR.
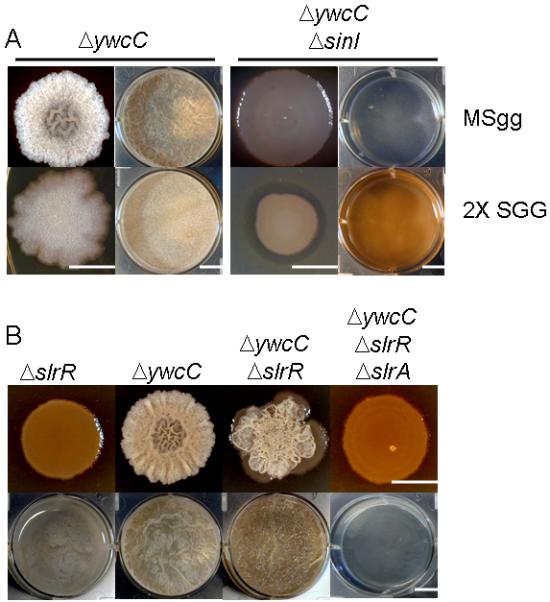
Panel A shows colonies (first and third columns from the left) and pellicles (second and fourth columns) formed by a ΔywcC mutant (YC295) and a ΔywcC ΔsinI double mutant (YC298) on MSgg (upper panels) and 2X SGG (lower panels) media. Panel B shows colonies (upper panels) and pellicles (lower panels) formed by ΔslrR (YC131), ΔywcC (YC295), ΔywcC ΔslrR (YC297), and ΔywcC ΔslrR ΔslrA mutants (YC530) on MSgg medium. Bar, 1 cm.
How then are we to explain the results obtained by Kobayashi (Kobayashi, 2008)? Conceivably, the discrepancy is due to an unknown difference in the strain backgrounds used by the two laboratories. Alternatively, however, the robust biofilms seen previously may have been due to a suppressor mutation. As reported previously, upon prolonged incubation ΔsinI mutant cells readily give rise to suppressor mutants that are restored for biofilm formation (Kearns et al., 2005). Such mutants can arise by loss-of-function mutations of sinR (Kearns et al., 2005). We wondered whether this might have been the case for the ΔywcC ΔsinI double mutant in the work of Kobayashi. We therefore tested the double mutant for pellicle formation in 2X SGG medium by carrying out six independent inoculations with the ΔywcC ΔsinI double mutant and incubating the cultures at 23 °C for three days (Fig. S1A). Three out of six cultures eventually formed robust pellicles (sup1 and sup3) or began to do so (sup2). We purified cells from the three putative suppressor mutants and confirmed that they were capable of forming robust biofilms (Fig. S1B). Evidently, all three strains had acquired suppressor mutations. We found that at least one of the suppressor mutants contained a mutation (sup3) that mapped to the sinR coding region, which we identified as a missense mutation (Ala28 → Glu). We conclude that a ΔywcC mutation (at least in our strain background) does not bypass the requirement for SinI in biofilm formation, but that as reported previously in the absence of SinI suppressor mutations readily take over the population in pellicle cultures.
Expression of slrA and of genes derepressed by SlrA is unimodal
Expression of sinI and of downstream genes activated by the action of SinI is known to exhibit cell population heterogeneity (Chai et al., 2008, Vlamakis et al., 2008). That is, the sinI gene itself and genes that are derepressed by the action of SinI are expressed at a high level in some cells in the population (ON cells) and at a low level in other cells (OFF cells) (Fig. 7, column B) (Chai et al., 2008, Vlamakis et al., 2008). In light of this bimodality, we decided to investigate the distribution of slrA expression in the cell population. Accordingly, we fused the slrA promoter to the fluorescent reporter gene gfp, creating PslrA-gfp. Fluorescence microscopy revealed that the PslrA-gfp fusion was expressed more or less uniformly throughout the population (Fig. 7, column A). Thus, unlike sinI, slrA is not bimodal in its expression.
Figure 7. Unimodal expression of slrA.
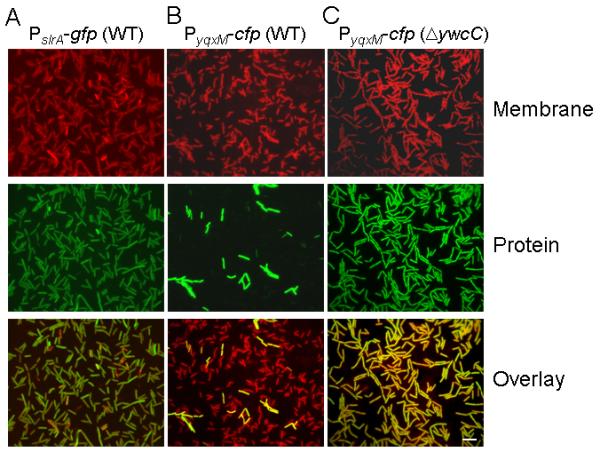
Shown are fluorescence micrographs of wild type cells harboring a PslrA-gfp fusion (YC567, column A), and of wild type cells (YC189, column B) and ΔywcC mutant cells (YC540, column C) harboring a PyqxM-cfp. Membranes were strained with FM4-64 (red; upper panels). Fluorescence was false-colored green (middle panels). Bar, 5 μm.
Next, we examined the effect of over expressing slrA on the cell population distribution of the expression of a promoter (that for the yxqM operon) under SinR control by using a PyqxM-cfp fusion. Fluorescence microscopy revealed that in cells mutant for ywcC, the PyqxM-cfp fusion was expressed more or less uniformly by all cells (Fig. 7C). For comparison, and as reported previously (Chai et al., 2008, Vlamakis et al., 2008), expression of PyqxM-cfp was limited to only a small number of cells in the population in wild-type (ywcC+) bacteria (Fig. 7B).
We interpret these findings as follows. We surmise that all cells in the population, both sinI ON cells and sinI OFF cells, must have at least a low level of SinI. (Recall that a ΔywcC mutation does not bypass sinI and hence the effect of ΔywcC is dependent on SinI.) When YwcC is absent, SlrA is over produced in all cells in the population, supplementing SinI (even in cells with a low level of SinI) and causing derepression of SinR-controlled genes throughout the population. In other words, the YwcC-SlrA pathway is an override that could in principle allow all cells in the population to produce matrix even under nutrient conditions in which Spo0A activity and hence SinI levels are low.
SlrR is an antagonist of SlrA
A fourth and critical member of the SinI and SinR family of proteins in B. subtilis is SlrR, a SinR-like protein that is required both for biofilm formation and efficient transcription of the yqxM operon (Chu et al., 2008, Kobayashi, 2008). It was previously proposed that the chief function of SlrA is to bind to, and stimulate the activity of, SlrR (Kobayashi, 2008). As we have seen, a ΔywcC mutant (in which slrA is over expressed) exhibits somewhat enhanced biofilm formation compared to the wild type. If SlrA is an activator of SlrR, then a ΔslrR mutation ought to eliminate this phenotype. To test this prediction, we constructed a ΔywcC ΔslrR double mutant and examined its capacity to form biofilms. Contrary to the prediction, the biofilms formed by the double mutant exhibited a hyper wrinkly phenotype (Fig. 6B), a phenotype similar to that seen for a ΔsinR mutant (Kearns et al., 2005). Thus, the double mutant was more, rather than less, robust in biofilm formation than was the ΔywcC single mutant.
Prompted by this observation, we examined transcription from the SinR-controlled promoters PyqxM-lacZ (panel A), PepsA-lacZ (panel B), and PslrR-lacZ (panel C) in the ΔywcC ΔslrR double mutant, as shown in the experiment of Fig. 8. Remarkably, in all cases transcription was higher in the double mutant than in a ΔywcC single mutant, in which all three fusions are already expressed at a higher level than the wild type (Fig. 2E-G). Furthermore, the remarkably high levels of expression caused by ΔslrR depended on SlrA, because further introduction of a ΔslrA mutation abolished both robust biofilm formation (Fig. 6B) and the elevated gene expression (Fig. 8C). We conclude that SlrA is not a positive regulator of SlrR with respect to matrix gene expression. (We do not exclude the possibility that SlrA stimulates the activity of SlrR with respect to other downstream genes in the biofilm pathway, such as those involved in cell chaining.) To the contrary, because the absence of SlrR elevated SlrA-mediated antirepression of SinR controlled genes, we infer that SlrR acts negatively on SlrA, which, as we have seen, is an antagonist of SinR.
Figure 8. An slrR mutation markedly derepresses the expression of SinR-controlled fusions when slrA is derepressed.
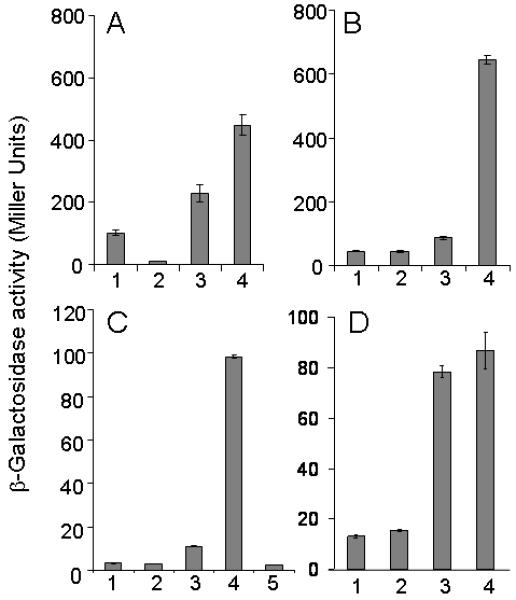
Shown are the expression of PyqxM-lacZ (panel A), PepsA-lacZ (panel B), PslrR-lacZ (panel C), and PslrA-lacZ (panel D) in the wild type and various mutants. The strains are as follows: (1) wild type; (2) ΔslrR; (3) ΔywcC; (4) ΔslrR ΔywcC; (5) ΔywcC ΔslrR ΔslrA.
We considered two models for the effect of SlrR on SlrA: (1) SlrR is a repressor of the slrA gene and (2) SlrR binds to SlrA protein and blocks SlrA’s activity as an anti-SinR repressor. We eliminated the former possibility by showing that ΔslrR had only a small effect on the expression of a PslrA-lacZ fusion in an otherwise wild type background or in a ΔywcC mutant (Fig. 8D). To investigate whether SlrR binds to SlrA, we incubated the His6-SlrA-containing lysate described above with a cleared lysate from a B. subtilis mutant lacking sinR (ΔsinR) and then applied the mixture to Ni-NTA agarose beads (Qiagen). Using anti-SinR antibodies, which react with both SinR and SlrR (Chu et al., 2008), we detected SlrR in the eluate from the His6-SlrA-containing beads but not from the beads lacking His6-SlrA (Fig. 5B). (This interaction did not depend on SinR because the B. subtilis lysate had been prepared from a ΔsinR mutant). Thus, SlrR apparently interacts with SlrA.
As a further test that SlrR and SlrA interact, we engineered the production of a GST-SlrR fusion protein and a His6-SlrA in E. coli. We then asked whether GST-SlrR would adhere to His6-SlrA bound to Ni-NTA agarose beads. As a control, we produced lysate with His6-SinI. As shown in Fig. 5C, a low but significant level of GST-SlrR adhered to the beads in the presence of His6-SlrA but not in the presence of His6-SinI nor in the absence of both His-tagged proteins. These findings reinforce the conclusion that SlrR binds to SlrA and that it does so in a specific manner.
In toto, these results suggest that SlrA, SinR, and SlrR form a negative feedback loop, as we explain. Production of SlrA, which is an antirepressor for SinR, causes derepression of genes under SinR control, including the slrR gene. As a consequence, production of SlrR protein is stimulated by the action of SlrA. SlrR, in turn, binds to, and inhibits the activity of, SlrA. Thus, the action of SlrA is self-limiting.
Summary and Perspectives
The chief contribution of this investigation is the demonstration that SlrA, a SinI paralog, is, like SinI itself, an antirepressor for the SinR master regulator for biofilm formation. Thus, SinR is subject to the action of two antirepressors.
SlrA is part of a pathway that augments matrix production in B. subtilis. The pathway consists of two proteins in addition to SlrA: YwcC and SlrR (Fig. 9). As reported previously, YwcC is a TetR-type repressor that inhibits transcription of the slrA gene (Kobayashi, 2008). When YwcC is absent or inactivated by the action of an unknown ligand (Ramos et al., 2005), slrA is derepressed (Kobayashi, 2008). The resulting SlrA protein binds to, and inactivates, the SinR repressor, thereby contributing to derepression of the yqxM and eps matrix operons and the slrR regulatory gene. Importantly, constitutive synthesis of SlrA (at least in our strain background) does not substitute for SinI; a ΔsinI mutant was severely impaired in biofilm formation even in the absence of YwcC. Instead, production of SlrA augments SinI-mediated antagonism of SinR.
Figure 9. Model for the circuitry governing biofilm formation.
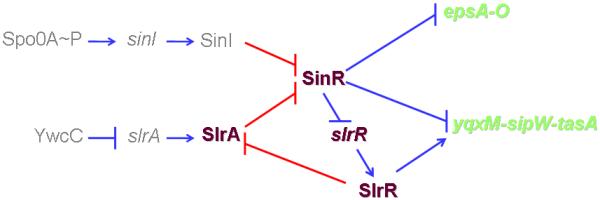
The model proposes that SinR is the target of two parallel pathways and that the YwcC-SlrA-SlrR pathway contains a negative feedback loop, in which SlrR binds to and inhibits SlrA. Under standard laboratory conditions, biofilm formation is principally governed by the Spo0A pathway and that the YwcC pathway, which is activated by an unknown signal, is supplementary to the Spo0A pathway. (For simplicity AbrB is omitted from the diagram). Transcriptional regulation in the circuitry was indicated as blue bars or arrows while protein-protein interaction was indicated as red bars or arrows.
A striking feature of the YwcC pathway is that it sets in motion a negative feedback loop (Fig. 9). The DNA-binding protein SlrR is a downstream regulator in the circuitry controlling matrix production; its synthesis is under the direct negative control of the SinR master regulator, and SlrR, in turn, stimulates transcription of the yqxM operon and promotes cell chaining (Chu et al., 2008, Kobayashi, 2007, Kobayashi, 2008). Importantly, SlrR, as we have shown, also has the capacity to bind to, and inhibit, SlrA. Thus, SlrA both stimulates the synthesis of SlrR (indirectly, by contributing to the inactivation of SinR) and is inhibited by SlrR. We propose, therefore, that the YwcC pathway is self-limiting. Turning on SlrA synthesis by inactivation of YwcC is expected to result in a burst of matrix production that would then shut itself off as SlrR accumulates. Thus, SlrA differs from SinI in one remarkable respect: it is capable of binding to either of the two partner proteins: SinR, for which it is an antirepressor, and SlrR, which inhibits SlrA.
We conclude that matrix production is subject to two intersecting pathways: the Spo0A pathway and the YwcC pathway (Fig. 9). Under standard laboratory conditions, the YwcC pathway plays a minor role in biofilm formation. Mutants of slrA have only a mild effect on biofilm formation and on transcription of SinR-controlled genes and operons. Only under the artificial circumstance in which YwcC was removed by mutation could a substantial effect be seen on transcription of downstream matrix genes. And even then the effect remained dependent on SinI. Thus, under laboratory conditions the principal pathway for matrix production is the Spo0A pathway, which is triggered by nutrient limitation and the quorum sensing molecule surfactin (Ireton et al., 1993, López et al., 2009a, López et al., 2009c, Sonenshein, 2000).
A key challenge for the future is to identify the environmental signal (ligand) to which YwcC responds (Ramos et al., 2005). We propose that even when the Spo0A pathway is largely silent, the presence of this signaling molecule would cause a rapid burst in matrix production. Unlike the Spo0A pathway, which exhibits cell population heterogeneity (Chai et al., 2008, Vlamakis et al., 2008), the YwcC pathway harnesses all cells in the population to produce matrix. This might be expected to cause a severe drain on resources if the signal persisted in the environment. However, the self-limiting effect of the SlrR-mediated negative feedback loop ensures that this production period would only be transitory.
Experimental procedures
Strains and media
Bacillus subtilis PY79, 3610 and other derivatives were grown in Luria-Bertani (LB), MSgg (Branda et al., 2001), or 2X SGG medium (Kobayashi, 2007), at 37°C or 22°C as indicated. Escherichia coli strains were grown in LB medium at 37°C for general purposes or at 30°C for protein expression. All strains used in this study were listed in Table S1. Solid agar medium contained 1.5% Bacto agar. When appropriate, antibiotics were added at the following concentrations for growth of B. subtilis: 10 μg per ml of tetracycline, 100 μg per ml of spectinomycin, 10 μg per ml of kanamycin, 5 μg per ml of chloramphenicol, and 1 μg per ml of erythromycin. For growth of E. coli, the concentrations of added antibiotics were indicated elsewhere.
Strain construction
To construct strains with promoter-lacZ fusions integrated into the chromosome at the amyE locus, the promoter sequences of the genes were amplified by Polymerase-Chain-Reaction (PCR). B. subtilis 3610 chromosomal DNA was used as the template in PCR reactions, and oligonucleotides PslrA-F1 and PslrA-R1, and PslrR-F1 and PslrR-R1 were used for amplification of the promoter sequences of slrA and slrR respectively. PCR products were cloned into the plasmid pDG268 (Antoniewski et al., 1990). The resulting recombinant plasmids were then transformed into PY79 following the standard transformation protocol for B. subtilis (Gryczan et al., 1978). Transformants were selected for a double crossover recombination at the amyE locus on the chromosome of PY79. The promoter-lacZ fusions at the amyE locus were then transferred from the PY79 background into the 3610 background using SPP1 phage mediated transduction as described previously (Chu et al., 2006, Yabsin & Young, 1974).
To construct strains with the promoter-gfp fusion for the slrA gene, similar procedures as described above were applied except that the amplified PCR product was cloned into a plasmid pYC121. Plasmid pYC121 contains a promoter-less gfp gene flanked by the amyE sequences (Chai et al., 2008). Construction of the PyqxM-cfp fusion was described previously (Chai et al., 2008).
All insertion deletion mutations were generated by long-flanking homology PCR that has been described previously (Wach, 1996). Primers used for generating those insertion deletion mutations in this work were listed in Table S2.
Genetic swap between slrA and sinI
The regulatory sequence of the slrA gene and the coding sequence of the sinI gene were amplified by PCR using primers PslrA-F1 and PslrA-R2 (for slrA), and primers sinI-F2 and sinI-R2 (for sinI), respectively. The two PCR products were simultaneously cloned into the vector pDG1662 (Guérout-Fleury et al., 1996) in a three-fragment ligation. The recombinant plasmid was used to introduce the PslrA-sinI fusion into the chromosomal amyE locus of a 3610 derivative that is mutant for slrA (ΔslrA) by following the method described above. As a control, the regulatory and the coding sequences of the slrA gene were amplified by PCR using primers PslrA-F1 and slrA-R1. The PCR products were again cloned into the vector pDG1662. Introduction of the PslrA-slrA fusion to the ΔslrA mutant followed the same procedures as described above.
Assays of colony and pellicle formation
For colony architecture analysis on solid media, cells were first grown to exponential growth phase in LB broth and 3-μl of the above cells was spotted to the solid medium containing 1.5% Bacto agar. The plates were incubated at 23°C for three days. Images of the colonies were taken using a Nikon Coolpix 950 digital camera, or using a SPOT camera (Diagnostic Instruments, USA). For pellicle formation in broth media, cells were grown to exponential phase and 9-μl of cells were mixed with 9-ml broth medium in a 6-well plate (VWR). Plates were incubated at 23°C for two or three days. Images of the pellicles were recorded similarly.
β-Galactosidase assays
Cells were cultured in MSgg medium at 37°C in a water bath with shaking. One milliliter of culture was collected at each time point. Cells were spun down and pellets were suspended in 1 ml Z buffer (40 mM NaH2PO4, 60 mM Na2HPO4, 1 mM MgSO4, 10 mM KCl, and 38 mM β-mercaptoethanol) supplemented with 200 μg ml−1 freshly made lysozyme. Resuspensions were incubated at 30°C for 15 min. Reactions were started by adding 200 μl of 4 mg ml−1 ONPG (2-nitrophenyl β-D-galactopyranoside) and stopped by adding 500 μl of 1 M Na2CO3. Samples were briefly spun down. The soluble fractions were transferred to cuvettes (VWR), and OD420 values of the samples were recorded using a Pharmacia ultraspectrometer 2000. The β-galactosidase specific activity was calculated according to the equation [OD420/time x OD600] x dilution factor x 1000. Assays were conducted at least in duplicate.
Protein expression and purification
E. coli strains RL4219, RL4220, YC388, and FC595 were used for production of the recombinant proteins His6-SinI, His6-SinR, His6-SlrA, and GST-SlrR, respectively. 500-ml cultures were grown in LB broth supplemented with 25 μg ml−1 kanamycin and 50 μg ml−1 chloramphenicol (for growth of RL4220), or with 25 μg ml−1 kanamycin (for growth of YC388), or with 100 μg ml−1 ampicillin and 50 μg ml−1 chloramphenicol (for growth of FC595), at 30°C to an OD600 of 0.5. IPTG was then added to a final concentration of 1 mM and cultures were incubated at 30°C for two more hours. Cells were harvested and washed once with 50 ml cold phosphate buffer (20 mM sodium phosphate, 200 mM NaCl, 10% glycerol, 1 mM PMSF, pH 7.4). Cell pellets were suspended in 5 ml of cold phosphate buffer supplemented with 200 μg ml−1 of freshly made lysozyme solution and incubated on ice for 30 min. Lysed cells were further disrupted on ice using sonication. Cell lysates were centrifuged at 5000 rpm for 5 min to remove cell debris and were further centrifuged at 14,000 rpm for 30 min at 4°C. Soluble fractions were transferred to clean cold tubes.
1 ml of Ni-NTA agarose beads (Qiagen) was added to the cleared lysate (or mixed lysates when testing protein-protein interactions) and samples were gently rotated for two hours at 4°C. The lysate/bead mixture was then loaded onto a column and washed five times, each time with two bed volumes of wash buffer (20 mM sodium phosphate, 300 mM NaCl, 10% glycerol, 20 mM imidazole, pH 8.5). The column was eluted with 5 bed volumes of elution buffer (20 mM sodium phosphate, 300 mM NaCl, 10% glycerol, 300 mM imidazole, pH 8.5), and elutes were collected and saved at −80°C.
Immuno-detection of SinR and SlrR
The cleared lysate containing His6-SlrA fusion proteins prepared from the E. coli as described above was mixed in equal volume with cleared lysate from B. subtilis ΔslrR (YC131) or ΔsinR (RL3856) mutant. Preparation of cleared lysate from B. subtilis was as follows: B. subtilis cells were inoculated into 100-ml MSgg broth and grown at 37°C to early stationary phase. Cells were then harvested and washed with 10 ml cold phosphate buffer (20 mM sodium phosphate, 200 mM NaCl, 10% glycerol, 1 mM PMSF, pH 7.4). Cell pellets were suspended in 1 ml of cold phosphate buffer supplemented with 200 μg ml−1 of freshly made lysozyme solution and incubated on ice for 30 min. Lysed cells were further disrupted on ice using sonication. Cell lysates were centrifuged at 5000 rpm for 5 min to remove cell debris and were further centrifuged at 14,000 rpm for 30 min at 4°C. The mixture of the E. coli and the B. subtilis lysates was incubated at 4°C for one hour, applied to Ni-NTA agarose beads, and incubated at 4°C for one more hour. Proteins were eluted afterwards following the same procedures that were described above. Immuno-detection of SinR and SlrR proteins was performed by following a protocol that was described previously (Chai et al., 2008).
Fluorescence microscopy
Cells were grown in MSgg broth to early stationary phase. One milliliter of the culture was harvested and centrifuged. Cells were washed with cold PBS buffer twice and resuspended in 50 μl PBS buffer. 1 μl of diluted membrane staining dye FM4-64 was mixed with resuspended cells. 3-μl of FM4-64 treated cells was dropped on the center of an agar-coated microscopy slide (VWR). Cover slides were pretreated with polyl-lysine (Sigma). Samples were examined using an Olympus workstation BX61. Images were taken using an automated software program SimplePCI and analyzed with program MetaMorph (Universal Imaging Corporation).
Supplementary Material
Acknowledgments
We thank Drs. Kearns D (Indiana University), Vlamakis H and López D for helpful discussions, and members of the Losick and Kolter labs for suggestions and comments during preparation of the manuscript. We also thank Frances Chu for strain construction. This work was supported by NIH grants GM58213 to R.K., GM18546 to R.L., and from BASF to R.K. and R.L. Y.C. is a postdoctoral fellow of the Jane Coffin Childs Foundation.
References
- Aguilar C, Vlamakis H, Losick R, Kolter R. Thinking about Bacillus subtilis as a multicellular organism. Curr. Opin. Microbiol. 2007;10:638–643. doi: 10.1016/j.mib.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniewski C, Savelli B, Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 1990;172:86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- Banse AV, Chastanet A, Rahn-Lee L, Hobbs EC, Losick R. Parallel pathways of repression and antirepression governing the transition to stationary phase in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2008:15547–15552. doi: 10.1073/pnas.0805203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Vik A, Friedman L, Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in Bacillus subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, D. Kearns B, McLoon A, Chai Y, Kolter R, Losick R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- Fujita M, Gonzalez-Pastor JE, Losick R. High- and Low-threshold genes in the Spo0A regulon of Bacillus subtilis. J. Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pastor JE, Hobbs EC, Losick R. Cannibalism by sporulating bacteria. Science. 2003;301:510–513. doi: 10.1126/science.1086462. [DOI] [PubMed] [Google Scholar]
- Gryczan TJ, Contente S, Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J. Bacteriol. 1978;134:318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- Hamon M, N. Stanley R, R. Britton A, A. Grossman D, B. Lazazzera A. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol. Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol. Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- Ireton K, Rudner DZ, Siranosian KJ, Grossman AD. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J. Bacteriol. 2007;189:4920–4931. doi: 10.1128/JB.00157-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol. Microbiol. 2008;69:1399–1410. doi: 10.1111/j.1365-2958.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Lemon KP, Earl AM, Vlamakis HC, Aguilar C, Kolter R. Biofilm development with an emphasis on Bacillus subtilis. Curr. Top. Microbiol. Immunol. 2008;322:1–16. doi: 10.1007/978-3-540-75418-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ, Brannigana JA, Smith I, Wilkinson AJ. Crystallisation of the Bacillus subtilis sporulation inhibitor SinR, complexed with its antagonist, Sinl. FEBS Letters. 1996;378:98–100. doi: 10.1016/0014-5793(95)01432-2. [DOI] [PubMed] [Google Scholar]
- López D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassiumleakage trigger multicellularity in Bacillus subtilis. Proc. Natl. Acad. Sci. USA. 2009a;106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 2009b;33:152–163. doi: 10.1111/j.1574-6976.2008.00148.x. [DOI] [PubMed] [Google Scholar]
- López D, Vlamakis H, Losick R, Kolter R. Paracrine signaling in a bacterium. Genes Dev. 2009c;23:1631–1638. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle V, Fujita M, Jensen ST, Eichenberger P, Gonzalez-Pastor JE, Liu JS, Losick R. The Spo0A regulon of Bacillus subtilis. Mol. Microbiol. 2003;50:1683–1701. doi: 10.1046/j.1365-2958.2003.03818.x. [DOI] [PubMed] [Google Scholar]
- O’Toole G, Kaplan HB. Biofilm formation as microbial development Annu. Rev. Microbiol. 2000;54:49–80. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang X, Gallegos MT, Brennan R, Tobes R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T. Postexponential regulation of sin operon expression in Bacillus subtilis. J. Bacteriol. 2002;184:564–571. doi: 10.1128/JB.184.2.564-571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshein AL. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 2000;3:561–566. doi: 10.1016/s1369-5274(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communites. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Stover AG, Driks A. Regulation of synthesis of the Bacillus subtilis transition-phase, spore-associated antibacterial protein TasA. J. Bacteriol. 1999;181:5476–5481. doi: 10.1128/jb.181.17.5476-5481.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M, Webb V, Spiegelman G, Hoch J. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA. 1990;87:1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamakis H, Aguilar C, Losick R, Kolter R. Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev. 2008;22:945–953. doi: 10.1101/gad.1645008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene sidruptions in Saccharomyces cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yabsin RE, Young FE. Transduction in Bacillus subtilis by bacteriophage SPP1. J. Virol. 1974;14:1343–1348. doi: 10.1128/jvi.14.6.1343-1348.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


