Abstract
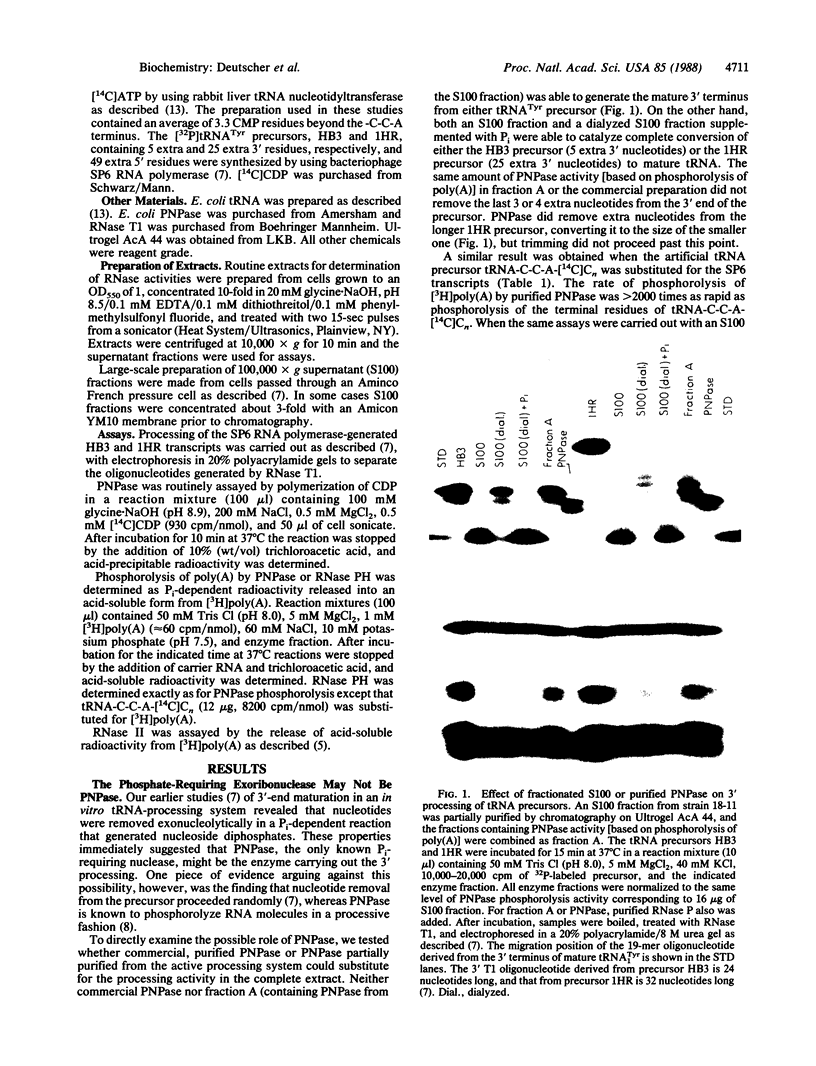
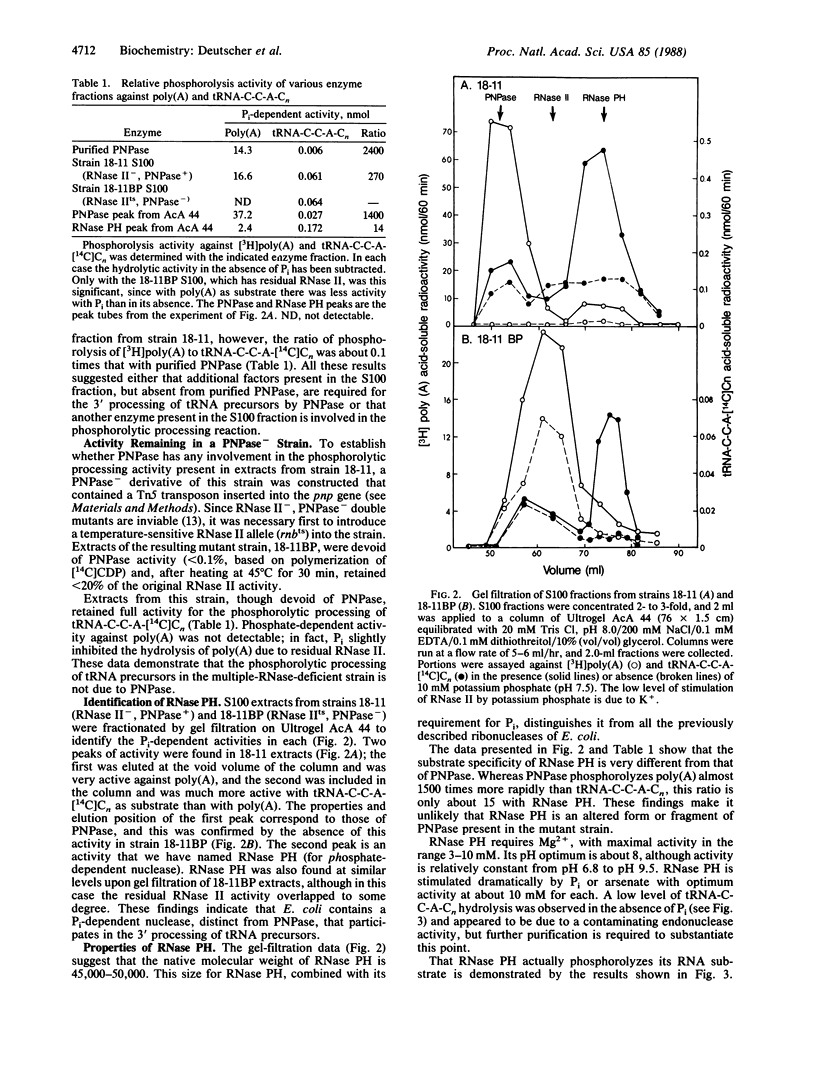
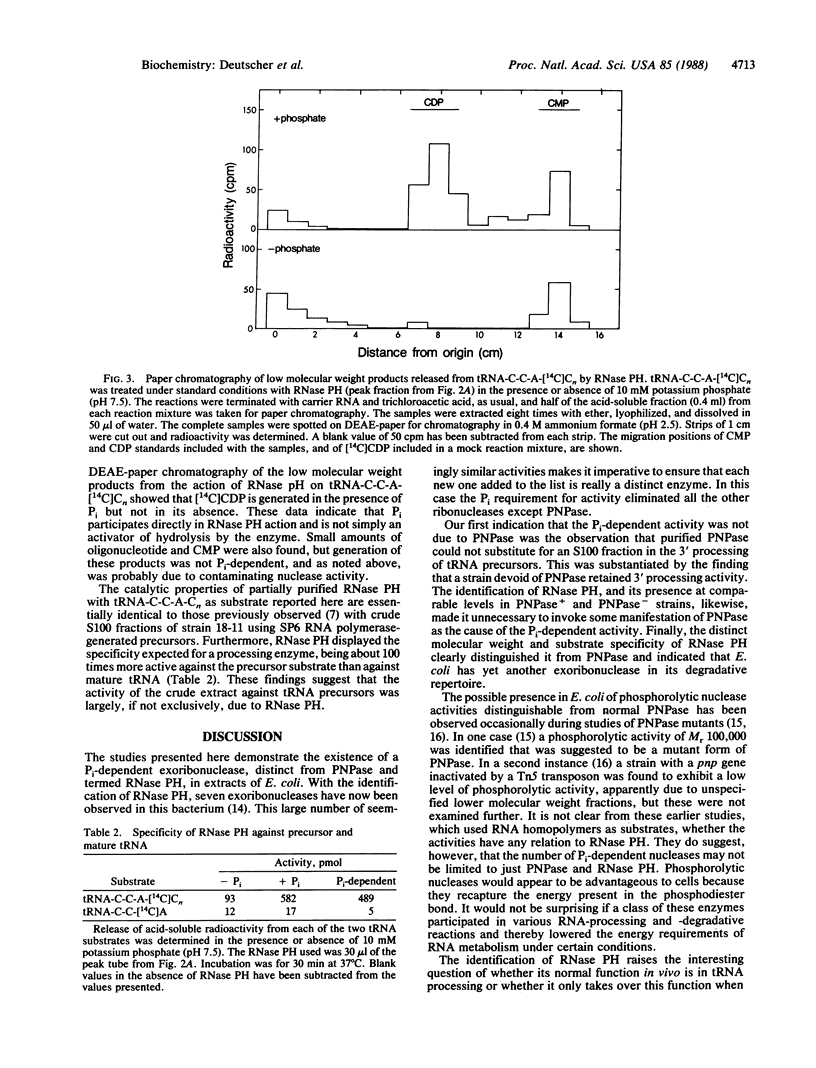
Final trimming of the 3' terminus of tRNA precursors in Escherichia coli is thought to proceed by an exonucleolytic mechanism. However, mutant strains lacking as many as four exoribonucleases known to act on tRNA still grow normally and process tRNA normally. Extracts from such a multiple-RNase-deficient strain accurately mature tRNA precursors exonucleolytically in vitro in a reaction that requires inorganic phosphate. Here we show that this reaction is not due to polynucleotide phosphorylase (PNPase) but, rather, that it is mediated by a phosphate-requiring exonuclease that we have named RNase PH. Purified PNPase is incapable of completely processing tRNA precursors, and extracts from a PNPase- strain retain full activity for phosphorolytic processing. Although both PNPase and RNase PH act in a phosphorolytic manner, they differ substantially in size and substrate specificity. RNase PH has a molecular mass of 45-50 kDa and favors tRNA precursors as substrates. The possible physiological role of RNase PH and the advantages of phosphorolytic processing are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asha P. K., Blouin R. T., Zaniewski R., Deutscher M. P. Ribonuclease BN: identification and partial characterization of a new tRNA processing enzyme. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3301–3304. doi: 10.1073/pnas.80.11.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin R. T., Zaniewski R., Deutscher M. P. Ribonuclease D is not essential for the normal growth of Escherichia coli or bacteriophage T4 or for the biosynthesis of a T4 suppressor tRNA. J Biol Chem. 1983 Feb 10;258(3):1423–1426. [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. 3' processing of tRNA precursors in ribonuclease-deficient Escherichia coli. Development and characterization of an in vitro processing system and evidence for a phosphate requirement. J Biol Chem. 1988 Jan 25;263(3):1518–1523. [PubMed] [Google Scholar]
- Cudny H., Deutscher M. P. Apparent involvement of ribonuclease D in the 3' processing of tRNA precursors. Proc Natl Acad Sci U S A. 1980 Feb;77(2):837–841. doi: 10.1073/pnas.77.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. E. coli RNases: making sense of alphabet soup. Cell. 1985 Apr;40(4):731–732. doi: 10.1016/0092-8674(85)90330-7. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Ghosh R. K. Preparation of synthetic tRNA precursors with tRNA nucleotidyltransferase. Nucleic Acids Res. 1978 Oct;5(10):3821–3829. doi: 10.1093/nar/5.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. RNase T is responsible for the end-turnover of tRNA in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6427–6430. doi: 10.1073/pnas.82.19.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P., Marlor C. W., Zaniewski R. Ribonuclease T: new exoribonuclease possibly involved in end-turnover of tRNA. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4290–4293. doi: 10.1073/pnas.81.14.4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. Processing of tRNA in prokaryotes and eukaryotes. CRC Crit Rev Biochem. 1984;17(1):45–71. doi: 10.3109/10409238409110269. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Jan;83(1):120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L. M., Levy S. B. Tn5 insertion in the polynucleotide phosphorylase (pnp) gene in Escherichia coli increases susceptibility to antibiotics. J Bacteriol. 1987 Mar;169(3):1321–1324. doi: 10.1128/jb.169.3.1321-1324.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier C. Isolation of a polynucleotide phosphorylase mutant using a kanamycin resistant determinant. Mol Gen Genet. 1980;178(2):343–349. doi: 10.1007/BF00270482. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., Grunberg-Manago M. Structure et activité de la polynucléotide phosphorylase d'Escherichia coli: une espèce à faible poids moléculaire. Eur J Biochem. 1969 Apr;8(4):577–590. doi: 10.1111/j.1432-1033.1969.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Zaniewski R., Petkaitis E., Deutscher M. P. A multiple mutant of Escherichia coli lacking the exoribonucleases RNase II, RNase D, and RNase BN. J Biol Chem. 1984 Oct 10;259(19):11651–11653. [PubMed] [Google Scholar]