Abstract
Objective
The 5,10-methylenetetrahydrofolate reductase gene (MTHFR) has been linked to unipolar major depressive disorder (MDD) and MRI hyperintensities. We examined the relationship between the MTHFR C677T polymorphism (C677T) and a) geriatric depression, b) MRI hyperintense lesion volume, and c) neurocognitive test performance.
Design
Cross-sectional.
Setting
Duke University Medical Center.
Participants
Depressed (N=178) and comparison (N=85) elderly subjects.
Measurements
Subjects had blood drawn to assess MTHFR genotype, were imaged by MRI to determine their white (WML) and gray (GML) matter hyperintense lesion volume, and assessed using a comprehensive neurocognitive battery evaluating multiple domains of function. Linear regression models were fit to test the effect of genotype, a depression by genotype interaction, and an age by genotype interaction on both hyperintense lesion volume measures and neurocognitive task performance.
Results
The MTHFR C677T genotype by age interaction term was significantly associated with MRI WML volume (p=0.0175), however this relationship was no longer statistically significant when WML volumes underwent a log transformation to produce a more normal distribution. The 677T allele was neither more frequent in depressed subjects nor associated with either gray matter hyperintensity volume or neurocognitive test performance.
Conclusions
MTHFR genotype affects the relationship between age and WML volume, where individuals who carry the 677T allele exhibit greater WML volume by age, although this relationship should be verified given the failure to replicate the finding using transformed WML volumes. Genotype was not related to GML volume, cognitive function, or presence of depression, although demographic differences could account for this negative finding.
Keywords: MTHFR polymorphism, depression, white matter lesion, folate, homocysteine, elderly
INTRODUCTION
Several epidemiological studies have implicated reduced folate levels and elevated homocysteine levels as risk factors for depression.1,2 Both are involved in a 1-carbon cycle important for central nervous system function. Folate, a B vitamin, is an important co-factor in the synthesis of several neurotransmitters, including serotonin. S-adenosylmethionine, formed by this pathway, is a coenzyme with antidepressant properties3 which is the sole methyl donor in the nervous system. Homocysteine may accumulate in folate deficiency and it exerts an excitotoxic effect on N-methyl-D-aspartate gluate receptors and inhibits methylation processes.4 The enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR) plays a significant role in folate metabolism by converting 5,10-methylene tetrahydrofolate to 5-methyltetrahydrofolate, the predominant and active form of folate needed for the generation of S-adenosylmethionine.5 As a result, individuals deficient in MTHFR activity often have impaired 1-carbon metabolism with resultant hyperhomocysteinemia. Alterations to this enzyme’s activity may be one mechanism through which deficits in 1-carbon metabolism can influence the pathogenesis of conditions such as depression and schizophrenia.6
One common genetic variant of the MTHFR gene, the MTHFR C677T polymorphism, leads to an amino acid change from alanine to valine, which results in a thermolabile variant of the enzyme with approximately 30% of the wild-type enzyme activity.7 Although it has been associated with a number of psychiatric disorders, including depression, schizophrenia, anxiety disorders, and bipolar disorder,8 ,9 many studies have also found no significant role for the MTHFR C677T polymorphism in conferring susceptibility to unipolar depression.10 ,11 These findings are complicated by methodological problems, such as small sample sizes and heterogeneous populations, and even meta-analyses of previous studies have arrived at conflicting conclusions. One meta-analysis by Gilbody et al. of 10 studies investigating a correlation between MTHFR and non-bipolar depression demonstrated that the MTHFR TT homozygous genotype increases the risk for depression.12 However, this result is inconsistent with two other meta-analyses carried out by Zintzaras and Gaysina et al., which both found no association with the MTHFR gene and major depressive disorder.13 ,14 All of the above meta-analyses, however, included subjects of all ranges rather than the geriatric population examined in this study. In older, depressed subjects, it appears TT homozygous individuals have both elevated homocysteine levels and increased risk of depression.15 This relationship in older individuals is further complicated by potential associations between this polymorphism and age related cerebral lesions.
MRI white matter hyperintense lesions (WMLs) are associated with geriatric depression, an observation that led to hypotheses that they may precipitate or affect the course of geriatric depression.16 The relationship among MTHFR genotype, WMLs, and MDD has not been well described. Geriatric depression is associated with elevated plasma homocysteine levels,15 a relationship independent of hyperintensities,11 although elevated homocysteine levels and hypofolatemia are associated with greater WML volumes.17 A Japanese study demonstrated that the prevalence of MRI WMLs was greater in subjects with the MTHFR TT genotype as opposed to the TC or CC genotype, especially in older subjects.18 Similarly, few studies have examined the relationship between MTHFR genotype and cognitive function in geriatric depression. In geriatric samples not specifically examining depressed populations, some studies have linked the MTHFR TT genotype with lower scores on various cognitive tests 19 while others have not.20
The goals of the present study were: 1) to examine MTHFR C677T allele frequency in older depressed and nondepressed subjects and 2) to test for an association between MTHFR genotype and both MRI hyperintensity volume and neurocognitive task performance. We hypothesized that T alleles would more frequently occur in depressed subjects than nondepressed subjects. We further hypothesized that individuals who carried one or more T alleles would exhibit greater hyperintensity volume and poorer performance on neurocognitive tests.
METHODS
Subjects
Subjects were enrolled in the Neurocognitive Outcomes of Depression in the Elderly (NCODE) study, a study of older adults with and without depression at Duke University Medical Center. Details of methods used in the NCODE study have been reported previously.21 Briefly, depressed subjectswere enrolled in the study if they met criteria for a currentepisode of unipolar major depression and were age 60 and older. Subjects were referred to the study from the Duke inpatientand outpatient psychiatry services and from the Duke GeneralInternal Medicine Clinic. Exclusion criteria included presenceof another major psychiatric illness, including schizophrenia, schizoaffective disorder, bipolar disorder, lifetime alcoholor substance dependence, and dementia. As part of screening for dementia, all subjects completed a Mini-Mental State Exam (MMSE) and individuals with a MMSE of 24 or less were excluded. Subjects with psychotic depression or comorbid anxiety disorders were included, as longas major depression was assessed by the study psychiatrist tobe the primary psychiatric disorder. Aside from dementia, otherneurological illnesses that could affect structural brain MRIscans were excluded, including stroke, Parkinson disease, multiplesclerosis, and seizure disorder. Subjects with contraindicationsto brain MRI were also excluded. Community-dwelling nondepressed comparison subjects were recruited from Duke’s Aging Center Subject Registry. Eligible comparison subjects had a non-focal neurological examination, no self-report of neurologic or psychiatric illness, no evidence of a psychiatric diagnosis based on the structured interview, and no contraindication to MRI.
For this study, only Caucasian subjects were used to reduce heterogeneity in gene frequencies among different racial populations. After complete descriptionof the study to the subjects, written informed consent was obtained. The study was approved by the Institutional Review Board atDuke University Medical Center.
Structured Interview and Depression Assessment
A study geriatric psychiatrist interviewed eachdepressed subject and completed standardized clinical assessments including the Montgomery-Asberg Depression Rating Scale (MADRS).22 A trained intervieweradministered the Duke Depression Evaluation Schedule (DDES),23 which assesses depression using National Institute of MentalHealth Diagnostic Interview Schedule,24 as well as cognitivestatus and physical health (two questions asking about diagnosisof "high blood pressure" and "heart trouble”).
Genotyping
DNA was extracted from fresh and frozen blood and stored according to previously reported methods.25 An aliquot of DNA was used for MTHFR genotyping using polymerase chain reaction (PCR) amplification with a Taqman by-design assay (Applied Biosystems) that recognized the single nucleotide polymorphism (SNP) which defines the C677T polymorphism (rs1801133, reference sequence AAAGCTGCGTGATGATGAAATCG [a/g] CTCCCGCAGACACCTTCTCCTTCAAG on the forward strand).26 The samples were examined with an ABI7900 DNA analyzer and the genotypes determined with the SDS software package (Applied Biosystems). Greater than 95% genotyping efficiency was required before data were submitted for further analysis. Blinded duplicates that were also used in the genotyping analyses and they were required to match 100% before the data was accepted for analysis.
MRI Acquisition
Subjects were imaged with a 1.5-Tesla whole-body MRI system (Signa, GE Medical Systems, Milwaukee, WI) using a standard head (volumetric) radiofrequency coil. A dual-echo fast spin-echo acquisition was obtained in the axial plane for morphometry. The pulse sequence parameters were as follows: repetition time, 4000 msec; echo time, 30, 135 msec, ±16 kHz full imaging bandwidth; echo train length, 16, 256 × 256 matrix, contiguous 3-mm section thickness, 1 excitation, and a 20-cm field of view.
MRI Processing
Volume measurements were performed with a modified version of MrX software, which was created by GE Corporate Research and Development (Schenectady, NY) and originally modified by Brigham and Women’s Hospital for image segmentation (Boston, MA). The segmentation protocol used has been previously described along with illustrations demonstrating how image intensity is converted to segmented tissue types.27 This supervised, semiautomated method uses the multiple magnetic resonance contrasts available to identify different tissue classifications through a “seeding” process wherein a trained analyst manually selects pixels in each tissue type to be identified (such as gray matter, white matter, cerebrospinal fluid, lesions, or background).
Lesion areas were then selected based upon explicit rules developed from neuroanatomical guidelines, consultation with a neuroradiologist, and knowledge of the neuropathology of lesions. WMLs on the segmented image included both periventricular lesions and lesions in the deep white matter. Subcortical gray matter lesions (GMLs) were defined as lesions within the caudate nucleus, putamen, globus pallidus or thalamus. Regions likely to be partially volumed cerebrospinal fluid rather than lesions were excluded. The final step was to run a summarizing program that calculated the volume of each tissue type.
All technicians received extensive training by experienced volumetric analysts. Reliability was established by repeated measurements on sixteen MR scans by each rater before raters were approved to process study data. Intraclass correlation coefficients (ICC’s) were: left cerebral gray matter lesions = 0.995, right cerebral gray matter lesions = 0.996, left cerebral white matter lesions = 0.988, and right cerebral white matter lesions = 0.994.
Neurocognitive Tests
Although a small number of subjects (N=15) elected to complete only MRI, most participants completed neurocognitive measures assessing multiple domains of function, based on a battery developed for epidemiological and clinical research purposes designed for the NCODE study. The domains and tasks include: 1) timed attentional processing as assessed by Parts A and B of the Trail Making Test28 and the Symbol-Digit Modalities Test;29 2) attention/working memory assessed by Digit Span from the Wechsler Adult Intelligence Scale-Revised;30 3) verbal memory assessed by the Logical Memory I (immediate recall) and Logical Memory II (delayed recall) subtests from the Wechsler Memory Scale-Revised;30 4) visual memory assessed by correct responses on the Benton Visual Retention Test;31 5) lexical fluency assessed by the Controlled Oral Word Association Test from the Multilingual Aphasia Examination;32 6) semantic fluency was assessed with a one-minute Animal Naming test,33 and 7) constructional praxis assessed by performance copying the Rosen figures.34 All tests were administered according to published guidelines by a psychometric technician who was trained and supervised by a licensed clinical neuropsychologist.
Statistical Analysis
All statistical analyses were conducted using SAS 9.1 (Cary, NC). Initial comparisons of demographic, neuroimaging, and cognitive measures were performed using pooled, two-tailed t-tests for continuous variables and chi-square tests for categorical variables, including examination of MTHFR allele frequency between depressed and nondepressed subjects. The MTHFR variable was dichotomized so that TT and TC were one group and CC was the comparison group.
General linear models were fit to test for the effect that the subjects’ status (depressed or nondepressed) and MTHFR genotype had on the total white and gray matter lesion volumes as outcome variables. The predictor variables for the models with lesion volumes as outcomes included the main effects of patient status, MTHFR genotype, a depression status by MTHFR gene effect interaction term, and an age by MTHFR gene interaction term. Age and sex were included as additional demographic independent variables.
The predictor variables for the models with neurocognitive tests as outcomes were constructed with the main effects status (patient versus control) and MTHFR genotype as well as an interaction term of these two variables. The models were fit including age, education, and gender as covariates. Similar to the lesion models, we tested for a depression status by gene interaction effect and an age by gene interaction effect. Subjects who did not complete all neurocognitive tasks were included in analyses of those tasks they did complete.
RESULTS
Table 1 summarizes the background characteristics, MTHFR genotype, and MRI hyperintensity data in the two groups. Of the 263 Caucasian participants included in this study, 178 were depressed and 85 were nondepressed comparison subjects. There were no significant group differences in mean age or sex representation, but the comparison subjects exhibited significantly higher MMSE scores and were more highly educated. In contrast to our published results in a larger sample,35 in this subsample with MTHFR genotype data, there were not consistent significant differences in hyperintensity lesion volume between depressed and nondepressed subjects.
Table 1.
Univariate Analysis for Demographic, Clinical Features, MTHFR Genotype, and MRI Hyperintensities
| Depressed Subjects (N=178) | Comparison Subjects (N=85) | Test Statistic | df | p value | |
|---|---|---|---|---|---|
| Age | 69.9 (7.7) | 70.0 (5.7) | t = 0.10 | 216 | 0.9214 |
| Sex (% Female) | 116 (65.2%) | 58 (68.2%) | X2 = 0.24 | 1 | 0.6230 |
| Education | 13.7 (2.9) | 15.6 (1.7) | t = 6.50 | 254 | <.0001 |
| MMSE | 28.3 | 29.1 | t = 4.47 | 261 | <.0001 |
| MADRS | 8.9 (8.5) | - | - | - | - |
| MTHFR genotype | X2 = 0.53 | 2 | 0.766 | ||
| CC; N(%) | 75 (42.1%) | 32 (37.7%) | |||
| CT; N(%) | 84 (47.2%) | 44 (51.8%) | |||
| TT; N(%) | 19 (10.7%) | 9 (10.6%) | |||
| MRI Hyperintensities | |||||
| WML Volume | 7.02 (11.23) | 5.32 (7.14) | t = −1.49 | 240 | 0.1381 |
| GML Volume | 0.27 (0.50) | 0.18 (0.26) | t = −1.88 | 260 | 0.0619 |
Values are expressed as mean (standard deviation) or N (%). Age and education displayed in years, MRI volumes as milliliters. MTHFR: methylenetetrahydrofolate reductase; MRI: magnetic resonance imaging; WML: white matter lesion; GML: gray matter lesion
In the total sample, there was a 34.98% frequency of mutant alleles. With an expected frequency of 35.00% among North American Caucasians, the distribution of genotype frequencies of the C677T polymorphism did not deviate from Hardy-Weinberg equilibrium (χ2 = 0.16, 1 df, p >0.95). There was no significant difference in MTHFR allele frequency between depressed and nondepressed subjects (Table 1).
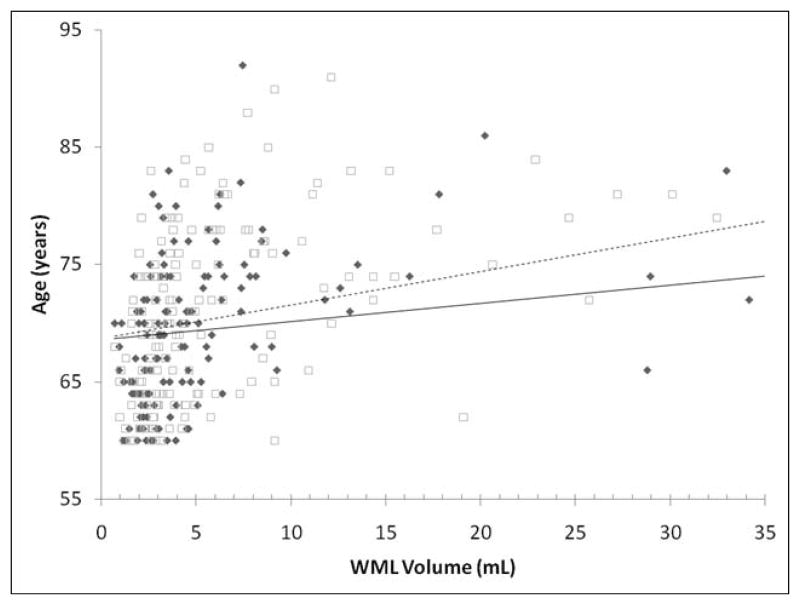
After testing for unadjusted group differences in hyperintensity volume by MTHFR genotype (Table 2), we examined these relationships in general linear models controlling for presence of depression, age, and sex. We found no statistically significant relationship between genotype and total WML volume (F1,262 = 0.57, p=0.4522). Next, we investigated whether a depression by genotype interaction term was significantly associated with WML volume and found no statistically significant interaction (F1,262 = 0.00, p=0.9861). Finally, we tested for an association between WML volume and an age by genotype interaction term. In this model, there was a main effect of genotype on WML volume. The age by genotype interaction term was also significantly associated with total WML volume (Table 3), where the slope of the line (beta = 0.450, F1,262 = 5.72, p = 0.0175) describing the relationship between age and lesion volume was steeper in the cohort of 677T allele carriers than 677C allele homozygotes (Figure 1). In separate models, we found that GML volumes were not significantly associated with MTHFR genotype (F1,262 = 0.04, p=0.8493), the depression by genotype interaction (F1,262 =0.50, p = 0.4792), or the age by genotype interaction (F1,262 = 0.86, p = 0.3549).
Table 2.
Univariate Analysis for Lesion Volume by MTHFR genotype
| CC (N=107) | CT or TT (N=156) | Test Statistic | df | p-value | |
|---|---|---|---|---|---|
| WML Volume | 5.45 (7.80) | 7.17 (11.4) | 1.45 | 261 | 0.1478 |
| GML Volume | 0.22 (0.34) | 0.26 (0.50) | 0.71 | 260 | 0.4376 |
Volumes are in mLs, expressed as mean (standard deviation). All were calculated using the Satterthwaite t-test for unequal variances except the total and right WML volume tests in the comparison group which used a two-tailed pooled t-test.
Table 3.
Regression Model Results for WML volume with an Age by Genotype Interaction Term
| Variable | F1,262 Value | P value |
|---|---|---|
| MTHFR genotype | 5.35 | 0.0215 |
| Age | 18.20 | <.0001 |
| Sex | 0.06 | 0.8068 |
| Depressed | 1.26 | 0.2618 |
| Age – MTHFR Interaction | 5.72 | 0.0175 |
MTHFR: methylenetetrahydrofolate reductase; WML: white matter lesion
Figure 1. Relationship between age and WML volume by MTHFR genotype.
Black diamonds and the solid line represent subjects with the CC genotype. Open squares and the dashed line represent subjects with the CT or TT genotype.
We conducted two sets of analyses to better characterize the data. First, we examined models of WML and GML volumes including MTHFR genotype as a trichotomous variable (CC, CT, TT) and found our conclusions did not change. MTHFR genotype continued to have no significant association with GML volumes, and the relationships detailed in Table 3 remained consistent (MTHFR: F2,262 = 5.37, p = 0.0052; age-MTHFR interaction: F2, 262 = 6.07, p = 0.0027). Second, due to the observation that WML volumes did not appear normally distributed, we conducted a log-transformation on the WML volume data. In these models, neither MTHFR nor the age-MTHFR interaction reached statistical significance.
Of the 248 subjects who completed neurocognitive tests, depressed patients performed significantly poorer on all neurocognitive tests than comparison subjects (data not shown). Performance averages for all tests dichotomized by MTHFR genotype are shown in Table 4, with significant univariate differences only for MMSE total, COWA and praxis.
Table 4.
Neurocognitive Test Performance in MTHFR genotypes
| CC (N=105) | CT or TT (N=143) | Test Statistic | df | p value | |
|---|---|---|---|---|---|
| MMSE | 27.7 (2.7) | 28.4 (2.0) | T = 2.03 | 183 | 0.0442 |
| Controlled Oral Word Association Test1 | 35.0 (12.7) | 38.9 (12.6) | t = −2.40 | 245 | 0.0173 |
| Symbol-Digit Modalities Test2 | 38.3 (11.3) | 40.2 (11.7) | t = −1.21 | 239 | 0.2288 |
| Trail Making Test (A)3 | 50.2 (41.1) | 44.0 (33.0) | t = 1.31 | 179 | 0.1931 |
| Trail Making Test (B)4 | 125.1 (75.2) | 117.2 (74.9) | t = 0.80 | 239 | 0.4233 |
| Logical Memory I (immediate verbal recall)3 | 24.8 (8.9) | 25.5 (8.4) | t = −0.64 | 245 | 0.5214 |
| Logical Memory II (Delayed verbal recall)3 | 20.8 (9.8) | 21.7 (9.5) | t = −0.73 | 245 | 0.4648 |
| Semantic Fluency Test | 16.5 (5.2) | 17.7 (5.5) | t = −1.67 | 246 | 0.0959 |
| Benton Visual Retention Test5 | 5.7 (2.0) | 6.0 (2.1) | t = −1.29 | 242 | 0.1996 |
| Constructional Praxis Test3 | 9.8 (1.3) | 10.1 (1.0) | t = −2.03 | 177 | 0.0434 |
| WAIS-R Digit Span Test6 | 15.5 (4.2) | 15.6 (4.2) | t = −.030 | 211 | 0.7630 |
Values are expressed as mean (standard deviation) or N (%). All comparisons used Satterthwaite t-tests for unequal variances, except for the Controlled Oral Word Association test, the semantic fluency test, and the WAIS-R Digit Span Test, which used pooled, two-tailed t-tests.
Due to missing values, CT or TT N=142;
Due to missing values, CC N=101; CT or TT N=140;
Due to missing values, CC N=104;
Due to missing values, CC N=100; CT or TT N=141;
Due to missing values, CC N=103; CT or TT N=141;
Due to missing values, CC N=87; CT or TT N=126
General linear models were fit to test an association between the MTHFR genotype and performance. Interaction terms between MTHFR genotype and depression diagnosis and genotype and age were also examined in models, but these terms did not reach statistical significance for any neurocognitive task. In models with only primary effects included as independent variables, MTHFR genotype was not significantly associated with performance on any neurocognitive task, including COWA (F1,247 = 2.19, p = 0.1403) or praxis (F1,247 = 2.89, p = 0.0905). The relationship between genotype and global cognitive function, as assessed by MMSE, approached but did not achieve statistical significance (F1,246 = 3.83, p = 0.0514). Education level and a diagnosis of depression accounted for most of the variability in these variables, while age was also significantly associated with MMSE score.
DISCUSSION
The major finding of this study in older Caucasian adults is that there is an interaction between MTHFR C677T genotype and age that predicts WML volume, wherein individuals who are T allele carriers tend to have greater lesion volume at a given age than do individuals who are C allele homozygous. We found no association between the MTHFR genotype and depression, GML volume, or neurocognitive task performance. Although many studies have observed that hyperintense lesion volume increases as age increases, we found the C677T genotype affects this relationship characterized by genotype-related differences in the slope of the line describing the age-WML volume relationship. When compared with 677C homozygous individuals, individuals who carry one or more 677T alleles exhibit a steeper slope, so advanced age is associated with greater WML volumes.
Meta-analyses have shown an inconsistent relationship between the MTHFR genotype and major depression.12–14 Gilbody et al.12 described an association between the C677T polymorphism and major depression with an odds ratio of 1.36. However, the ten studies included in the meta-analysis included patients of both European and Asian origin, and depression was diagnosed by a variety of means including self-report. Another meta-analysis14 that limited its analysis to case-control studies with subjects of European origin and DSM-IV diagnosed depression reported that there was no significant association between the 677T allele and depression. Corroborating some meta-analyses,13,14 we did not find a significant association between the C677T polymorphism and depression. Our results are in disagreement with other studies,8,9 including a recent Australian study of late-life depression which found a greater risk of depression in individuals with the TT genotype.15
We approached the study with the hypothesis that MTHFR genotype may be related to MRI hyperintensities, possibly through homocysteine elevation. In previous studies, the C677T MTHFR polymorphism has been associated with higher homocysteine levels.36 An association between high homocysteine levels and WMLs has also been reported, which may be a plausible mechanism by which the MTHFR polymorphism causes WMLs.37 Homocysteine may contribute to the development of WMLs by a variety of mechanisms. It is heavily involved in the one-carbon cycle, which is responsible for the synthesis of methyl groups used by S-adenosylmethionine (SAMe) to synthesize monoamine neurotransmitters.3 Elevated homocysteine may disrupt this production these neurotransmitters, which may be a contributing factor to the development of depression.38 Tolmunen et al.39 conducted a large scale study finding that hyperhomocysteinemia in men was associated with a greater risk of depression, even after controlling for vascular risk factors. Other studies did not find elevated homocysteine to be associated with depression after controlling for medical comorbidity.40
Although our study would have been strengthened by obtaining homocysteine levels, a possible direct association between MTHFR genotype and hyperintense lesions has not been studied extensively. A smaller study in late-onset depression found no direct relationship between genotype and lesions,11 but a large community-based study demonstrated that the 677T allele was associated with silent brain infarcts in Japanese subjects age 60 years or older.18 These studies used visual scoring systems to rate hyperintensity severity while we used a semi-automated volumetric method. However, this suggests that the gene may have a small effect on hyperintensity severity that could be missed in smaller samples. Our finding of an age-gene interaction suggests that the 677T allele only has an effect on WML volume in context of other aging-related phenomenon. Possible candidates include age-related factors that affect absorption of folate and vitamin B12, such as atrophic gastritis. Atrophic gastritis is common in the elderly, increases in prevalence with advanced age, and is characterized by a reduction in gastric acid secretion, which can lead to small intestinal bacterial overgrowth and reduced absorption of several micronutrients.41 ,42
We also found that after controlling for relevant covariates, the C677T MTHFR polymorphism was not significantly associated with neuropsychological task performance. Thus, although Table 4 appears to show that T allele carriers exhibit better task performance, these may represent false positive results due to the large number of comparisons, as subsequent analyses conducted in complete models found that genotype was not significantly related to task performance. Instead, variation in task performance was strongly associated with education level and depression, and age. This finding is consistent with other previous studies which did not identify a relationship between cognition and MTHFR genotype in older subjects,10,20 although one large epidemiological study in women found that the TT genotype was associated with poorer performance on the Digit Symbol Substitution and Trails B tasks.19
Limitations of our study include a relatively small sample size compared with other genetic studies, although this was a reasonable size for a neuroimaging study. Further, the sample included both depressed and nondepressed individuals, and this population stratification may have affected our results given our observations in differences in education between the groups and previous reports associating geriatric depression with greater medical comorbidity. In addition, we examined only the MTHFR genotype, and did not assess folate status or homocysteine levels. Although the 677T allele is associated with elevated homocysteine, this finding may be more pronounced in individuals with low folate levels.7 Future studies need to combine these clinical data with the genetic data. Finally, a particular statistical limitation is that when we transformed the WML volume data to fit a normal distribution, neither genotype nor the age-genotype interaction were statistically significant. This approach has not been routinely used in the analysis of cerebral hyperintense lesion volume data, and such transformations create problems with data interpretation and risk losing the characteristics of the data that may be needed to fully understand their effects. However, the inability to replicate the relationship in the transformed data lowers confidence in these findings, which should be viewed with caution.
In summary, in a North American-based Caucasian population, the MTHFR C677T polymorphism was not associated with geriatric depression or cognition. The MTHFR CT and TT genotypes are associated with greater WML volume at a given age, suggesting that the genotype’s effect on WML volume may be related to other aging-related changes and may help explain some of the variance in WML volumes across populations. Further studies are needed to investigate the precise relationship among the MTHFR genotype, homocysteine levels, and MRI hyperintensities, as well as physiological consequences of the above factors as one ages, and also to determine if interventions aimed at improving folate status and reducing homocysteine levels may reduce this age-related genotype effect on cerebral hyperintensities.
Acknowledgments
Supported by NIMH grants P50 MH60451, R01 MH54846, K24 MH70027, and K23 MH65939.
We would like to thank Denise Messer, MA for her work in developing the MRI analysis method and processing MRI scans.
Footnotes
No futher disclosures to report.
References
- 1.Kim JM, Stewart R, Kim SW, et al. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–274. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- 2.Mischoulon D, Raab MF. The role of folate in depression and dementia. J Clin Psychiatr. 2007;68:28–33. [PubMed] [Google Scholar]
- 3.Bottiglieri T, Hyland K, Reynolds EH. The clinical potential of ademetionine (S-adenosylmethionine) in neurological disorders. Drugs. 1994;48:137–152. doi: 10.2165/00003495-199448020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Obeid R, McCaddon A, Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med. 2007;45:1590–1606. doi: 10.1515/CCLM.2007.356. [DOI] [PubMed] [Google Scholar]
- 5.Cantoni GL, Mudd SH, Andreoli V. Affective disorders and S-adenosylmethionine: a new hypothesis. Trends Neurosci. 1989;12:319–324. doi: 10.1016/0166-2236(89)90038-6. [DOI] [PubMed] [Google Scholar]
- 6.Frankenburg FR. The role of one-carbon metabolism in schizophrenia and depression. Harv Rev Psychiatry. 2007;15:146–160. doi: 10.1080/10673220701551136. [DOI] [PubMed] [Google Scholar]
- 7.Ueland PM, Hustad S, Schneede J, et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 8.Lewis SJ, Lawlor DA, Davey Smith G, et al. The thermolabile variant of MTHFR is associated with depression in the British Women’s Heart and Health Study and a meta-analysis. Mol Psychiatry. 2006;11:352–360. doi: 10.1038/sj.mp.4001790. [DOI] [PubMed] [Google Scholar]
- 9.Bjelland I, Tell GS, Vollset SE, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–626. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- 10.Almeida OP, Flicker L, Lautenschlager NT, et al. Contribution of the MTHFR gene to the causal pathway for depression, anxiety and cognitive impairment in later life. Neurobiol Aging. 2005;26:251–257. doi: 10.1016/j.neurobiolaging.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Chen CS, Tsai JC, Tsang HY, et al. Homocysteine levels, MTHFR C677T genotype, and MRI hyperintensities in late-onset major depressive disorder. Am J Geriatr Psychiatry. 2005;13:869–875. doi: 10.1176/appi.ajgp.13.10.869. [DOI] [PubMed] [Google Scholar]
- 12.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 13.Zintzaras E. C677T and A1298C methylenetetrahydrofolate reductase gene polymorphisms in schizophrenia, bipolar disorder and depression: A meta-analysis of genetic association studies. Psychiatr Genet. 2006;47:589–593. doi: 10.1097/01.ypg.0000199444.77291.e2. [DOI] [PubMed] [Google Scholar]
- 14.Gaysina D, Cohen S, Craddock N, et al. No association with the 5,10-methylenetetrahydrofolate reductase gene and major depressive disorder: Results of the depression case control (DeCC) study and a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2007;147B:699–706. doi: 10.1002/ajmg.b.30665. [DOI] [PubMed] [Google Scholar]
- 15.Almedia OP, McCaul K, Hankey GJ, et al. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–1294. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- 16.Sneed JR, Rindskopf D, Steffens DC, et al. The vascular depression subtype: Evidence of internal validity. Biol Psychiatry. 2008;64:491–497. doi: 10.1016/j.biopsych.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iosifescu DV, Papakostas GI, Lyoo IK, et al. Brain MRI white matter hyperintensities and one-carbon cycle metabolism in non-geriatric outpatients with major depressive disorder. Psychiatry Res. 2005;140:291–299. doi: 10.1016/j.pscychresns.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Kohara K, Fujisawa M, Ando F, et al. MTHFR gene polymorphism as a risk factor for silent brain infarcts and white matter lesions in the Japanese general population: The NILS-LSA Study. Stroke. 2003;34:1130–1135. doi: 10.1161/01.STR.0000069163.02611.B0. [DOI] [PubMed] [Google Scholar]
- 19.Elkins JS, Johnston SC, Ziv E, et al. Methylenetetrahydrofolate reductase C677T polymorphism and cognitive function in older women. Am J Epidemiol. 2007;166:672–678. doi: 10.1093/aje/kwm140. [DOI] [PubMed] [Google Scholar]
- 20.Bathum L, von Bornemann Hjelmborg J, Christiansen L, et al. Methylenetetrahydrofolate reductase 677C>T and methionine synthase 2756A>G mutations: no impact on survival, cognitive functioning, or cognitive decline in nonagenarians. J Gerontol A Biol Sci Med Sci. 2007;62:196–201. doi: 10.1093/gerona/62.2.196. [DOI] [PubMed] [Google Scholar]
- 21.Steffens DC, Welsh-Bohmer KA, Burke JR, et al. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. J Geriatr Psychiatry Neurol. 2004;17:202–211. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 23.Landerman R, George LK, Campbell RT, et al. Alternative models of the stress buffering hypothesis. Am J Comm Psychol. 1989;17:626–642. doi: 10.1007/BF00922639. [DOI] [PubMed] [Google Scholar]
- 24.Robins LN, Helzer JE, Croughan J, et al. National Institute of Mental Health diagnostic interview schedule. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 25.Rimmler J, McDowell JG, Slotterback BD, et al. Development of a data coordinating center (DCC): data quality control for complex disease studies. Am J Hum Genet. 1998;(suppl 63):A240. [Google Scholar]
- 26.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 27.Payne ME, Fetzer DL, MacFall JR, et al. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002;115:63–77. doi: 10.1016/s0925-4927(02)00009-4. [DOI] [PubMed] [Google Scholar]
- 28.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Tuscon, AZ: Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test-Manual. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- 30.Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 31.Benton A. Revised Visual Retention Test. 4. New York, NY: The Psychological Corporation; 1974. [Google Scholar]
- 32.Benton AL, Hamsher KD, Varney NR, et al. Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 33.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 34.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 35.Taylor WD, MacFall JR, Payne ME, et al. Greater MRI lesion volume in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;30:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Ilhan N, Kucuksu M, Kaman D, et al. The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch Med Res. 2008;39:125–130. doi: 10.1016/j.arcmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Vermeer SE, van Dijk EJ, Koudstaal PJ, et al. Homocysteine, silent brain infarcts, and white matter lesions: the Rotterdam Scan Study. Ann Neurol. 2002;51:285–289. doi: 10.1002/ana.10111. [DOI] [PubMed] [Google Scholar]
- 38.Bottiglieri T, Laundy M, Crellin R, et al. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolmunen T, Hintikka J, Voutilainen S. Association between depressive symptoms and serum concentrations of homocysteine in men: a population study. Am J Clin Nutr. 2004;80:1574–1578. doi: 10.1093/ajcn/80.6.1574. [DOI] [PubMed] [Google Scholar]
- 40.Tiemeier H, van Tuijl HR, Hofman A, et al. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry. 2002;159:2099–2101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 41.Krasinski SD, Russell RM, Samloff IM, et al. Fundic atrophic gastritis in an elderly population. Effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34:800–806. doi: 10.1111/j.1532-5415.1986.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 42.Saltzman JR, Russell RM. The aging gut. Nutritional issues. Gastroenterol Clin North Am. 1998;27:309–324. doi: 10.1016/s0889-8553(05)70005-4. [DOI] [PubMed] [Google Scholar]