Abstract
Two anti-tumor monoclonal antibodies, L6 (anticarcinoma) and 1F5 (anti-B lymphoma), were covalently linked to alkaline phosphatase (AP), forming conjugates that could bind to the surface of antigen-positive tumor cells. The conjugates were capable of converting a relatively noncytotoxic prodrug, etoposide phosphate (EP), into etoposide--a drug with significant antitumor activity. In vitro studies with a human colon carcinoma cell line, H3347, demonstrated that while EP was less toxic than etoposide by a factor of greater than 100, it was equally toxic when the cells were pretreated with L6-AP, a conjugate that bound to the surface of H3347 cells. The L6-AP conjugate localized in H3347 tumor xenografts in nude mice and histological evaluation indicated that the targeted enzyme (AP) was distributed throughout the tumor mass. A strong antitumor response was observed in H3347-bearing mice that were treated with L6-AP followed 18-24 hr later by EP. This response, which included the rejection of established tumors, was superior to that of EP (P less than 0.005) or etoposide (P less than 0.001) given alone. The IF5-AP conjugate did not bind to H3347 cells and did not enhance the toxicity of EP on these cells in vitro. In addition, IF5-AP did not localize to H3347 tumors in nude mice and did not demonstrate enhanced antitumor activity in combination with the prodrug.
Full text
PDF

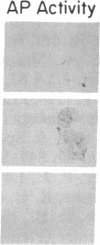
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo N., Kato Y., Takeda Y., Saito M., Umemoto N., Kishida K., Hara T. In vitro cytotoxicity of a human serum albumin-mediated conjugate of methotrexate with anti-MM46 monoclonal antibody. Cancer Res. 1987 Feb 15;47(4):1076–1080. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gallego J., Price M. R., Baldwin R. W. Preparation of four daunomycin-monoclonal antibody 791T/36 conjugates with anti-tumour activity. Int J Cancer. 1984 Jun 15;33(6):737–744. doi: 10.1002/ijc.2910330605. [DOI] [PubMed] [Google Scholar]
- Hellström I., Horn D., Linsley P., Brown J. P., Brankovan V., Hellström K. E. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986 Aug;46(8):3917–3923. [PubMed] [Google Scholar]
- Lambert J. M., Senter P. D., Yau-Young A., Blättler W. A., Goldmacher V. S. Purified immunotoxins that are reactive with human lymphoid cells. Monoclonal antibodies conjugated to the ribosome-inactivating proteins gelonin and the pokeweed antiviral proteins. J Biol Chem. 1985 Oct 5;260(22):12035–12041. [PubMed] [Google Scholar]
- O'Dwyer P. J., Leyland-Jones B., Alonso M. T., Marsoni S., Wittes R. E. Etoposide (VP-16-213). Current status of an active anticancer drug. N Engl J Med. 1985 Mar 14;312(11):692–700. doi: 10.1056/NEJM198503143121106. [DOI] [PubMed] [Google Scholar]
- Ohkawa K., Tsukada Y., Hibi N., Umemoto N., Hara T. Selective in vitro and in vivo growth inhibition against human yolk sac tumor cell lines by purified antibody against human alpha-fetoprotein conjugated with mitomycin C via human serum albumin. Cancer Immunol Immunother. 1986;23(2):81–86. doi: 10.1007/BF00199811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland G. F., Simmonds R. G., Gore V. A., Marsden C. H., Smith W. Drug localisation and growth inhibition studies of vindesine-monoclonal anti-CEA conjugates in a human tumour xenograft. Cancer Immunol Immunother. 1986;21(3):183–187. doi: 10.1007/BF00199359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Yeh M. Y., Hellström I., Hellström K. E. Clonal variation in expression of a human melanoma antigen defined by a monoclonal antibody. J Immunol. 1981 Apr;126(4):1312–1317. [PubMed] [Google Scholar]