Abstract
MDMA (3,4-methylenedioxymethamphetamine; Ecstasy) is a popular recreational drug that produces long-lasting serotonin (5-HT) neurotoxicity consisting of reductions in markers for 5-HT axons. 5-HT innervates cortical and subcortical brain regions mediating motor function, predicting that MDMA users will have altered motor system neurophysiology. We used functional magnetic resonance imaging (fMRI) to assay motor task performance-associated brain activation changes in MDMA and non-MDMA users. 24 subjects (14 MDMA users and 10 controls) performed an event-related motor tapping task (1, 2 or 4 taps) during fMRI at 3 T. Motor regions of interest were used to measure percent signal change (PSC) and percent activated voxels (PAV) in bilateral motor cortex, sensory cortex, supplementary motor area (SMA), caudate, putamen, pallidum and thalamus. We used SPM5 to measure brain activation via three methods: T-maps, PSC and PAV. There was no statistically significant difference in reaction time between the two groups. For the Tap 4 condition, MDMA users had more activation than controls in the right SMA for T-score (p = 0.02), PSC (p = 0.04) and PAV (p = 0.03). Lifetime episodes of MDMA use were positively correlated with PSC for the Tap 4 condition on the right for putamen and pallidum; with PAV in the right motor and sensory cortex and bilateral thalamus. In conclusion, we found a group difference in the right SMA and positive dose–response association between lifetime exposure to MDMA and signal magnitude and extent in several brain regions. This evidence is consistent with MDMA-induced alterations in basal ganglia–thalamocortical circuit neurophysiology and is potentially secondary to neurotoxic effects on 5-HT signaling. Further studies examining behavioral correlates and the specific neurophysiological basis of the observed findings are warranted.
Keywords: Drug abuse, Neuroimaging, Serotonin, Movement, Toxicity
Introduction
Understanding MDMA (3,4-methylenedioxymethamphetamine; Ecstasy) effects on human brain function is of considerable neurobiological and public health importance. MDMA is a widely used recreational drug that acutely alters synaptic concentrations of multiple monoamine neurotransmitters (Bankson and Cunningham, 2001) and produces long-lasting serotonin (5-HT) neurotoxicity (Green et al., 2003). After chronic and sufficiently high exposures, MDMA neurotoxicity manifests as a dose-dependent selective functional or structural central nervous system (CNS) 5-HT denervation in most animal models (Green et al., 2003). This leads to shifts in 5-HT influences on cellular neurophysiology and cellular structure (Cowan et al., 2008). Estimates from health and law enforcement agencies indicate ongoing widespread use of Ecstasy, especially in North America, Western Europe and Oceania (United Nations, 2008) After a brief period of decline from peak levels, use among young adults in the United States has been increasing since 2005 and estimates of lifetime use of Ecstasy topped 12 million people in the most recent U.S. surveys (Johnston et al., 2007; Substance Abuse and Mental Health Services Administration, 2008). In addition to its widespread recreational use as Ecstasy, MDMA is currently under evaluation in human clinical trials as an adjunct to psychotherapy for severe post-traumatic stress disorder (PTSD) and anxiety in advanced stage cancer (NIH, 2008a,b). In this context, additional studies of CNS effects of MDMA use are essential to inform potential MDMA users and to guide public policy with regard to the potential risks and benefits of the drug.
MDMA use is associated with a broad range of neuropsychiatric sequelae, but there has not been consensus regarding MDMA effects on brain neurophysiology, with functional neuroimaging studies reporting increased, decreased, or mixed activation differences in MDMA user groups versus comparison groups (Cowan et al., 2006, 2007a,b; Cowan, 2007; Daumann et al., 2003a,b, 2004a; Jacobsen et al., 2004; Jager et al., 2007a,b, 2008b; Moeller et al., 2004, 2007; Valdes et al., 2006). Examining the specific neurophysiological consequences of MDMA use is complicated by several factors, including the possibility that brain differences that may be associated with a tendency for MDMA or polydrug use pre-date MDMA use and the fact that contemporary MDMA users are largely polydrug users. Many functional neuroimaging paradigms rely on standard statistical parametric mapping approaches that use statistical models to compare group differences in mean activation maps (commonly as T- or z-scores). This approach, while a powerful method of detecting between-group differences in brain activation, may be less appropriate in drug abuse research. Since drug use may have a graded effect on the outcome of interest (and thus produce greater variation about the mean), increasing levels of drug exposure may be associated with incremental influences on the variable under study that are not well reflected in average differences. In support of this line of reasoning, in an analysis of visual system activation in MDMA users, we (Cowan et al., 2006) reported no group differences in activation for MDMA polydrug users versus non-MDMA polydrug users, but a within-group analysis of the degree of MDMA exposure on brain activation revealed a specific positive correlation between MDMA use and spatial extent of brain activation in visual cortex.
Although probing the neurophysiological underpinnings of neurocognitive functions, such as verbal memory, that have been repeatedly documented to be impaired in MDMA users may appear to be the most obvious approach to examining altered brain function, a strong argument can also be made for probing primary sensory or motor system function in MDMA users. A compelling case can be made that MDMA-induced 5-HT toxicity is likely manifest in motor system function. 5-HT axons arising from the brainstem raphe nuclei innervate motor areas, including basal ganglia, thalamus, and cortex as part of the basal ganglia–thalamocortical circuit (DeVito et al.,1980; Lavoie and Parent, 1990; McQuade and Sharp, 1997; Wilson and Molliver, 1991). This circuit includes supplementary motor area (SMA), precentral gyrus (primary motor cortex; MC), caudate and putamen, globus pallidus, and motor thalamus (Alexander et al., 1990; Herrero et al., 2002). The dorsal raphe nucleus, which is thought to give rise to the bulk of serotonergic fibers susceptible to MDMA toxicity (Wilson et al., 1989), has a major role in motor system neurophysiology (Jacobs and Fornal, 1997). MDMA administration in rats produces chronic alterations in motor responses (Balogh et al., 2004; Gyongyosi et al., 2008). Studies of resting brain activity in human MDMA users demonstrate MDMA effects on some motor regions with increased regional blood volume in the globus pallidus (Reneman et al., 2000) and decreased metabolic rate in the caudate and putamen (Obrocki et al., 2002a,b). Additional evidence supports structural changes in the 5-HT system in MDMA users with lower levels of the 5-HT reuptake transporter (located on 5-HT axon terminals) in some motor areas (McCann et al., 2008).
Study approach and hypothesis
It is critically important to directly examine task-evoked motor system function in MDMA users because of the compelling pre-clinical rationale and evidence for MDMA effects on 5-HT reuptake transporter levels and resting physiology in motor regions in humans. Using functional magnetic resonance imaging (fMRI) during performance of a parametrically modulated slow event-related finger tapping task, we compared motor tap-induced regional brain activation in MDMA users and non-MDMA users. We also conducted an analysis of dose–response effects of prior recreational MDMA exposure on brain activation measures. For the reasons reviewed earlier, we did not predict that MDMA users would show differences in the between-group comparison but that the degree of prior MDMA use would correlate with brain activation measures in motor regions. In general support of our hypotheses, we found a single region that differed between groups and multiple brain regions in which activation was positively correlated with the degree of MDMA use. These results suggest that MDMA use is associated with long-lasting neurophysiological changes in multiple cortical and subcortical brain regions mediating voluntary motor function.
Methods
Methods format, including section headings, corresponds where appropriate to guidelines for reporting MRI studies as suggested by Carter et al. (2008) and Poldrack (2007). The study used a case-control parametric measures design to assess BOLD signal as a marker for brain function during a motor task. The between-subjects factor was group (MDMA users and non-MDMA users). The within-subjects factor was level of motor activity for several tapping conditions.
Human subjects
Participants
Participants were recruited for this report as part of a larger ongoing observational study examining MDMA effects on structural, functional, and spectroscopic indices of brain function using magnetic resonance imaging. Each participant took part in various components of the overall study. Twenty-seven right-handed participants [16 MDMA polydrug users (4 females) and 11 non-MDMA using controls (5 females), age range of 18–35 years (mean 25.2, SD = 5.6)] participated in the motor function portion of the study. Data from 24 participants having useable data are reported (excluded participants are described below). Participants were recruited via advertisements in local news media, email, flyers in dance venues, and by word of mouth. The advertisements stated that Ecstasy or other drug users ages 18–35 were needed for a study of drug effects on the brain and indicated that participants would be compensated for their time. Participants were provisionally screened by phone for inclusion/exclusion criteria. To avoid participant sharing of perceived inclusion/exclusion criteria participants admitted or excluded from the study were not told which criteria were used to permit or exclude enrollment. This approach was adopted to help prevent participants from misrepresenting clinical or drug use history in order to gain study entry. Participants were blind to all inclusion/exclusion criteria other than the requirement for Ecstasy or other drug use.
Entry criteria
Study inclusion criteria were: age 18–35 with self-reported history of prior MDMA or other drug use. Exclusion criteria were: 1) MDMA use history—self-reported lifetime use of less than 5 tablets of MDMA (for MDMA user group to exclude participants with very low-level MDMA use), 2) psychiatric history—history of current or past substance or alcohol dependence, history of current or past DSM-IV (as determined by Structured Clinical Interview for Diagnosis; SCID-C) (First et al., 1997). Axis 1 psychiatric disorder (except substance-induced mood disorder or substance abuse), 3) medication use—taking psychoactive medications whether prescription or over-the-counter within six weeks prior to the study (defined as: antihypertensives, antiepileptic, antianxiety, antipsychotic, antidepressant, psychostimulants, inkgo biloba, ephedra, St. John’s Wort), 4) systemic medical illness—e.g. chronic hepatitis, seizure disorder, HIV, diabetes, thyroid or parathyroid disorder, Lupus, cerebral vasculitis, hypertension, cancer (other than skin cancer), 5) head injury—history of loss of consciousness for over 30 min, 6) contraindications to MR scanning—claustrophobia, implanted medical devices, pacemaker, aneurysm clips, non-removable metallic piercings, other possible metal in the body including shrapnel, sheet metal filings, pregnancy, and 7) positive urine drug screen or alcohol breathalyzer screen on the scan day.
Abstinence criteria
Participants were asked to abstain from alcohol and cannabis use for at least 48 h prior to the study day and to abstain from all other drugs for at least 14 days. To encourage participants to remain honest about drug use occurring between screen day and MRI scanning days, participants were asked to contact study staff if any interim drug use occurred so that the study could be rescheduled without penalty to the subject.
Screening
No participants had a lifetime Axis I psychiatric diagnosis. Participants also completed detailed substance abuse questionnaires using a time-line follow back method (Fals-Stewart et al., 2000). The questionnaires probed drug use history in alcohol, cannabis, stimulants, hallucinogens, opiates, sedatives, dissociative anesthetics, anabolic steroids, and inhalants. The questionnaire contains items to indicate when a specific drug was last used, number of total lifetime episodes, episodes in the last month, and approximate amount of the drug used (Cowan et al., 2006, 2007a,b). Subjects updated the drug screening questionnaire on each study day (to ensure accurate assessment of recent use) and were screened on each study day to rule out pregnancy (urine; QuPid One Step Pregnancy Test; Stanbio Laboratory, Inc. San Antonio, TX), recent drug use (urine; amphetamines, methamphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, PCP and tricyclic antidepressants; Triage Drugs of Abuse Panel, Biosite Diagnostics, San Diego, CA), or alcohol use (breath; Alco Sensor III, Intoximeters, St. Louis, MO). Subjects having a positive urine, pregnancy, or alcohol screen were removed from the study prior to MRI scanning.
Confidentiality
To protect participant confidentially, a Certificate of Confidentiality was obtained from the National Institute on Drug Abuse (NIDA) and participants were informed of the Certificate protections in the informed consent document.
Excluded participants
Of the 27 initial participants in the motor study, data from two participants was excluded for achieving less than 70% accuracy on the behavioral tasks. Data from one participant was excluded for excessive head motion in the scanner. The demographics of the final study group are shown in Table 1.
Table 1.
Age and drug use variables for commonly used drug classes by MDMA/non-MDMA user status.
| MDMA |
Non-MDMA |
|||||||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean (S.D.) | Abstinence days mean (S.D.) | Min. | Max. | Mean (S.D.) | Abstinence days mean (S.D.) | |
| Age | 18.0 | 35.0 | 26.0 (5.4) | – | 18.0 | 33.0 | 22.9 (5.4) | – |
| Ecstasy episodes | 8 | 80 | 29.6 (20.1) | 669.4 (664.5) | 0 | 0 | 0 | – |
| Ecstasy milligrams* | 400.0 | 8000.0 | 2365.2 (2018.3) | – | 0 | 0 | 0 | – |
| Alcohol episodes | 12 | 4306 | 845.5 (1149.1) | 22.3 (18.5) | 26.0 | 2143 | 466.6 (651.1) | 23.5 (11.5) |
| Alcohol units* | 24.0 | 12,254.0 | 3353.2 (3909.0) | – | 63.0 | 6243.0 | 1944.0 (2479.2) | – |
| Cannabis episodes | 0 | 2501 | 399.27 (754.4) | 574.5 (1776.3) | 2.0 | 1516 | 202.0 (463.9) | 194.5 (272.5) |
| Cannabis joints* | 0 | 1250 | 229.19 (387.1) | – | 0.5 | 759.0 | 128.6 (233.7) | – |
| Cocaine episodes | 0 | 104 | 26.7 (33.2) | 676.5 (980.1) | 0 | 31 | 3.9 (9.7) | 399.7 (345.0) |
| Cocaine gram | 0 | 100.4 | 15.4 (26.7) | – | 0 | 9.0 | 1.0 (2.8) | – |
| Methamphetamine episodes | 0 | 85 | 10.9 (24.0) | 1655.9 (1364.0) | 0 | 17 | 1.7 (5.4) | 193.0 (N/A) |
| Methamphetamine milligrams | 0 | 2950.0 | 377.3 (862.3) | – | 0 | 610.0 | 61.0 (192.9) | – |
The standardized drug values are 50 mg per tablet MDMA; a unit of alcohol is 14 g pure alcohol (12 oz. beer or standard drink equivalent); a joint consists of 10 puffs of cannabis.
Days since last dose. All participants used alcohol. All participants except for one MDMA user used cannabis. In the control group; 2 used amphetamines, 1 used methamphetamine, and 3 used cocaine. In the MDMA group; 4 used amphetamines, 7 used methamphetamine and 9 used cocaine.
Ethics approval
Research participants provided written informed consent for study participation. The study was approved by the Vanderbilt University Institutional Review Board and conformed to the World Medical Association Declaration of Helsinki.
Experimental design
Task design
We chose a randomized event-related parametric (i.e. 1, 2 or 4 taps) design to minimize habituation, permit the removal of error trials, and assess the relationship between activation and motor effort (Fig. 1). The instructions and stimuli were projected onto a screen placed just behind the subject’s head using an Avotec projector. Participants viewed the task via a mirror attached to the head coil. Participants were instructed to tap their right index finger as quickly and accurately as possible according to the number presented. A five-button keypad (Rowland Institute of Science, Boston, MA) was used to collect response time data. The stimuli were presented for one s and responses were recorded using E-Prime Version 2.0 (Psychology Software Tools, Inc. Pittsburgh, PA, USA; http://www.pstnet.com/index.htm). The stimuli presented were: “1”, “2”, or “4” as white numbers on a black background. The onset time for each stimulus is presented in Fig. 1. The intertrial interval was jittered and ranged from 11 to 15 s, with a mean intertrial interval of 13.12 s. There were three conditions measured: Tap 1 (one tap), Tap 2 (two taps), and Tap 4 (four taps). This protocol was executed for each subject and each study run had the same stimulus presentation sequence. Two functional task runs were acquired for each participant except for four participants who had one functional run (due to time constraints or scanner error). Errors were defined as non-completion of the task.
Fig. 1.
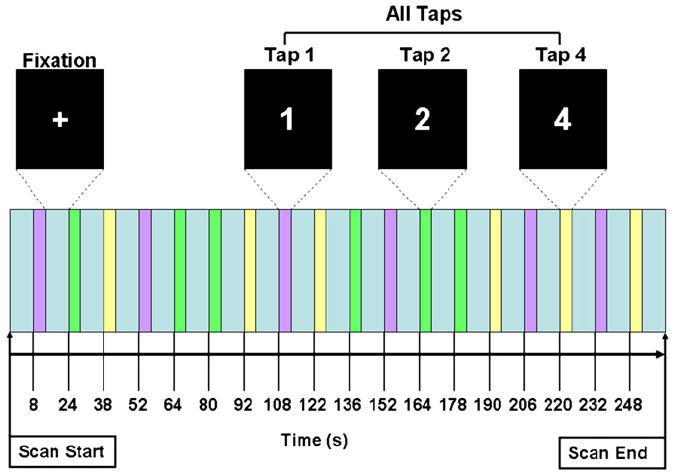
Task design demonstrating visual stimuli and stimulus onset times. Not to scale. Scan time 266 s.
fMRI acquisition
Imaging was performed using a Philips 3 T Intera Achieva MRI scanner (Philips Medical Systems, Andover, MA, USA). In each 266 second functional run, 28 field echo EPI (130 dynamics, 4.50 mm slice thickness with 0.40 or 0.45 mm gap, 2 s TR, 35 ms TE, 79° flip angle, FOV = 240, matrix = 128 × 128) scans were acquired. For coregistration of the functional data, 170 whole-brain 3-D anatomical T1-weighted/TFE (with SENSE coil) images were taken for each subject (4.6 s TE, 8° flip angle, FOV = 256, matrix = 256 × 256, voxel size = 1 × 1 × 1 mm).
fMRI preprocessing
Data were analyzed using SPM5 (Wellcome Department of Cognitive Neuroscience, London, UK) utilizing the General Linear Model (GLM). The functional data was temporally processed using the first slice as a reference for order. Each individual’s functional data was spatially realigned (to mean) to correct for motion and then coregistered to their anatomical image. Next, the anatomical and functional images were normalized into stereotactic space using SPM’s 152 average T1 template (Montreal Neurological Institute). The normalized functional images were then smoothed with a full-width half maximum (FWHM) 8 mm Gaussian kernel.
fMRI data analysis
Regions of interest
Because of the a priori interest in motor-associated regions of the brain, an explicit mask was used to include only motor-related regions of interest and increase statistical power by reducing the number of statistical comparisons. Motor-relevant regions were selected as according to the basal ganglia–thalamocortical circuits described by Alexander et al. (1990) and the explicit mask was created using MarsBaR (Brett et al., 2002). The masking image was a combination of seven bilateral motor regions found in the aal toolbox for SPM (Tzourio-Mazoyer et al., 2002). The specific bilateral regions of interest used to create the masking image were as follows: supplementary motor area (SMA), precentral gyrus (primary motor cortex; MC), caudate, putamen, pallidum, thalamus, and postcentral gyrus (primary sensory cortex).
Between-groups contrasts
Contrast images comparing each tap condition to baseline were created for each subject. These contrast images were then entered into a second level random effects analysis for between-group contrasts using a two-sample T-test (MDMA users versus non-MDMA users). The statistical parametric maps (SPMs) generated from the between-group comparison were thresholded at a whole-brain cluster-corrected alpha level of p ≤ 0.05 for a voxel-wise alpha of p = 0.001 (cluster size 26 voxels).
Within-group contrasts
Since the degree of MDMA use has been associated with altered spatial extent of regional brain activation (Cowan et al., 2007a,b), we analyzed regional brain activation as both mean percent BOLD signal change and the spatial extent (number of suprathreshold voxels) of activation per ROI. Mean BOLD percent signal change was calculated for the ROI using the MarsBaR toolbox (Brett et al., 2002) for SPM. The spatial extent of activation was measured by determining the percent of suprathreshold voxels (p < 0.001) within an ROI.
Statistical methods
Statistical analyses for descriptive data, behavioral task data, and for fMRI measures (percent BOLD signal change and percent activated voxels) were analyzed using SPSS (SPSS for Windows version 15.0 software; SPSS Inc.). Descriptive statistical summaries presented are means and standard deviations unless otherwise specified. Reaction time data were normally distributed, thus analysis of the within-subjects tap condition and between-groups MDMA use condition was conducted using multivariate mixed-effects analysis of variance. This enabled us to both control Type I error rates and to look for a possible interaction effect of tap condition (1, 2, or 4 taps) and MDMA use (dose) on reaction times. Activation data were not normally distributed, therefore univariate Mann–Whitney tests were used to assess MDMA use group differences in those values.
Since we were primarily interested in long-term cumulative effects of MDMA exposure within the MDMA use group, drug use variables were summarized as lifetime episodes (defined as each discrete 24-hour period). However, because both MDMA and non-MDMA user groups had exposure to other drugs, we also analyzed the possible covarying association of lifetime episodes of drug use for the drugs most commonly used by our participants (alcohol, cannabis, cocaine, and methamphetamine) and outcomes of interest. As expected, drug exposure was not normally distributed; thus, bivariate associations between drug exposure and fMRI measures were quantified using Spearman correlation coefficients based on ranks; multivariate methods of association (canonical correlation) incorporating drug exposure also used ranked data as the basis for analysis. We acknowledge that a large number of tests were conducted in this study. Tests were considered statistically significant on 2-tailed analysis if p ≤ 0.05. Because we expected that outcome variables across motor system would be intercorrelated to some degree, we estimated the statistical threshold for significance after accounting for multiple comparisons using the method of Sidak (1967) and Holland and Copenhaver (1987). When possible, multivariate methods (e.g., mixed-effects multivariate ANOVA, canonical correlation) were used to provide some control of the possible, study-wise Type I error rate.
Results
Participant characteristics by group are summarized in Table 1. There were 14 MDMA polydrug users (4 females) and 10 non-MDMA users (5 females). MDMA users had overall greater exposure to cocaine than their non-MDMA counterparts (Mann–Whitney test; p = 0.022) but there were no differences for use levels of other drugs.
Global task effects on brain activation
To confirm task-related activation in motor brain regions, we first examined activation during task performance across all task conditions (Tap 1, Tap 2, and Tap 4) and all subjects, irrespective of group status (Table 2; Fig. 2). The peak T-score is a useful index of maximal activation in the ROI, the percent activated voxels measure is an indicator of the spatial extent of activation or proportion of the ROI activated by the task, and the percent BOLD signal change reflects the mean signal change across the entire ROI. All fourteen regions of interest were activated by the task compared to baseline (fixation). The most intense activations were on the left side in supplemental motor area (SMA), precentral gyrus (primary motor cortex) and postcentral gyrus (primary sensory cortex). The basal ganglia (caudate, putamen, and pallidum) and thalamus generally showed less intense activations than cortex, but the pattern for greater left sided activation was still largely evident.
Table 2.
Activated voxels in anatomical regions of interest.
| Global task activation (Tap 1, Tap 2, Tap 4) | ||||||
|---|---|---|---|---|---|---|
| Anatomical area | Peak T | Percent BOLD signal change | Percent activated voxels | MNI coordinates {mm} |
||
| x | y | z | ||||
| Supp motor area L | 11.91 | 0.42 | 40.5 | 0 | 9 | 45 |
| Supp motor area R | 7.04 | 0.31 | 30.9 | 9 | −9 | 60 |
| Precentral gyrus L | 10.67 | 0.36 | 33.5 | −30 | −21 | 63 |
| Precentral gyrus R | 7.94 | 0.22 | 13.2 | 39 | 0 | 48 |
| Caudate L | 8.60 | 0.13 | 9.3 | −12 | 9 | 6 |
| Caudate R | 7.18 | 0.13 | 9.6 | 12 | 3 | 9 |
| Putamen L | 7.23 | 0.26 | 21.1 | −30 | 6 | 0 |
| Putamen R | 6.07 | 0.22 | 17.3 | 27 | 6 | 3 |
| Pallidum L | 9.34 | 0.28 | 16.1 | −18 | 3 | 3 |
| Pallidum R | 7.41 | 0.24 | 12.4 | 18 | 6 | 0 |
| Thalamus L | 8.9 | 0.23 | 20.6 | −12 | −15 | 3 |
| Thalamus R | 8.45 | 0.21 | 17.2 | 9 | −15 | 3 |
| Postcentral gyrus L | 10.54 | 0.35 | 35.4 | −45 | −18 | 54 |
| Postcentral gyrus R | 9.20 | 0.12 | 8.8 | 51 | 6 | 27 |
Peak T is for activated voxel group at statistical threshold of p < 0.05 FWE correction and extent threshold = 50 voxels. Percent BOLD signal change is for the entire anatomical region. Percent activated voxels is percent of total ROI volume. MNI coordinates are for peak T value.
Fig. 2.
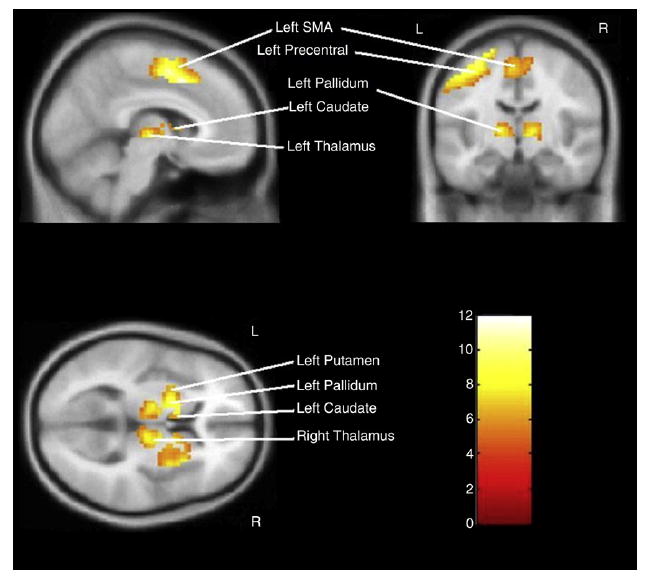
Global task activation (Tap 1 + Tap 2 + Tap 4) versus baseline showing activation and T-score intensities in anatomically selected motor regions.
Global task effects and task performance
To examine the relationship between task performance and brain activation measures, we summarized the associations between reaction time and activation as percent BOLD signal change and percent activated voxels for each ROI across all participants regardless of group status (reaction times and activation measures were averaged across all subjects for each condition). Overall, there was a general tendency for both activation measures to show positive correlations with reaction time for Tap 1, with mostly negative, but non-significant associations for Tap 2, and with stronger negative, correlations for Tap 4. Correlations of reaction time (using a p value of ≤ 0.05 uncorrected for multiple comparisons) with activation measures reached statistical significance for right precentral gyrus (rs = 0.580, p = 0.030) and bilateral thalamus (right: rs = 0.668, p = 0.009; left: rs = 0.575, p = 0.032) for percent activated voxels and in right putamen (rs = 0.546, p = 0.044) and pallidum (rs = 0.634, p = 0.015) for the percent BOLD signal change measure.
Between-group effects
Prior to conducting group comparisons, we tested for effects of age and gender on the behavioral and fMRI outcome variables. There was no statistically significant association of age or gender with reaction time, nor with percent BOLD signal change or percent activated voxels for either task or brain region. Therefore, age and gender were not further considered as possible confounders in subsequent analyses.
Task performance
Reaction time data are presented in Fig. 3. The reaction times used for the analysis were the average of each subject’s responses per condition. Analysis of tapping stimulus condition by group indicated a statistically significant linear effect on reaction time of tap condition (p < 0.001), however there was no statistically significant difference in that linear pattern with MDMA use (p = 0.644; mixed-effect, multivariate analysis of variance). Similarly, post-hoc tests indicated no statistically significant differences in reaction time between MDMA users and non-users within any of the three tap stimuli (Table 3).
Fig. 3.
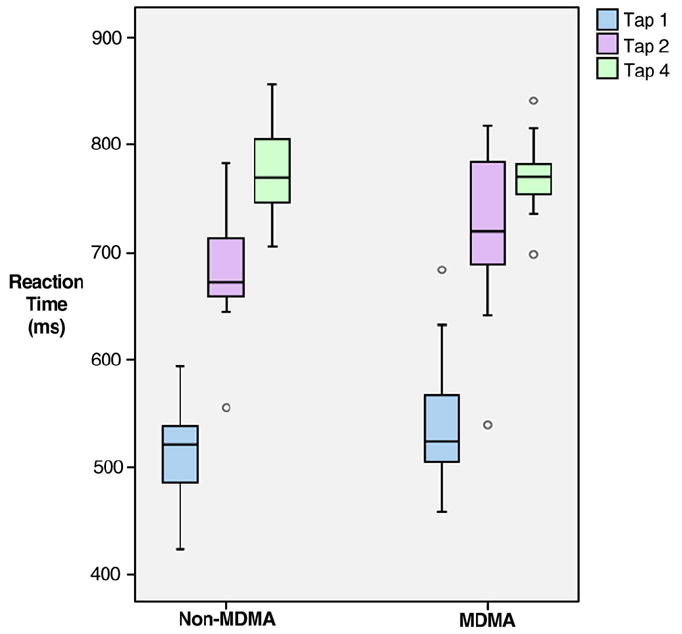
Reaction time (ms) grouped according to MDMA user status and stimulus.
Table 3.
Reaction times by group and tap condition.
| Stimulus condition | MDMA mean (S.D.) | Non-MDMA mean (S.D.) | Effect size | pa |
|---|---|---|---|---|
| Tap 1 | 523.07 (63.93) | 498.91 (67.22) | 0.36 | 0.381 |
| Tap 2 | 706.09 (71.53) | 671.02 (59.08) | 0.49 | 0.217 |
| Tap 4 | 773.76 (33.37) | 768.19 (31.66) | 0.17 | 0.685 |
Analysis of stimulus condition by MDMA group indicated a statistically significant linear effect of tap condition (p < 0.001), however no statistically significant difference in that linear pattern with MDMA use (p = 0.644) (multivariate mixed-effects analysis of variance).
Post-hoc univariate tests of MDMA group differences within each stimulus condition (independent T-test).
Statistical activation maps
There were no differences in activation between MDMA users and non-MDMA users for the Tap 1 and Tap 2 conditions. For the Tap 4 condition, MDMA users demonstrated greater activation in the right supplementary motor area (peak T-score 5.18, z-score 4.20; Fig. 4).
Fig. 4.
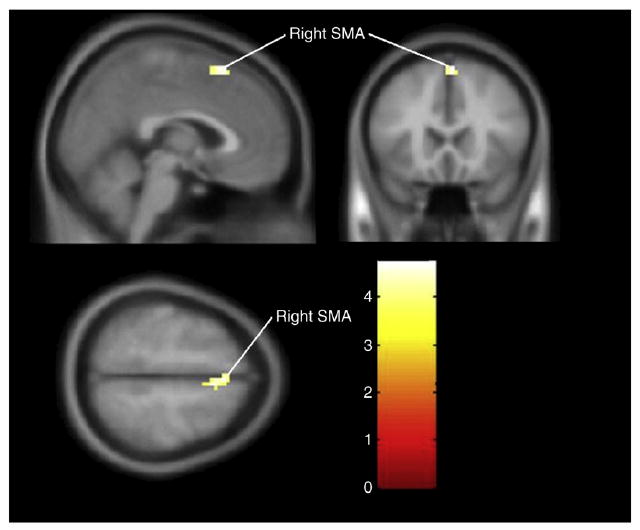
Group differences (MDMA users – non-MDMA users) for Tap 4. Cluster-level significance (p = 0.024, cluster size 38 voxels) in the right supplementary motor area (SMA), (MNI coordinates: 6, 12, 57) with a maximum T-score of 5.18.
We compared the magnitude of regional BOLD signal change (percent BOLD signal change) and the spatial extent of activation (percent activated voxels) by group and region for the MDMA use groups.
Percent BOLD signal change
There were no statistically significant differences for percent BOLD signal change between groups for either region on the Tap 1 or Tap 2 tasks. However, consistent with the findings from the T-score analysis, percent BOLD signal change for the Tap 4 condition showed greater activation in the MDMA, versus non-MDMA group for the right SMA (Mann–Whitney test; 0.41 ± 0.14 versus 0.29 ± 0.28, p = 0.035).
Percent activated voxels
There were no statistically significant differences for the percent of activated voxels between groups for either region on the Tap 1 and Tap 2 tasks. However, consistent with the percent BOLD signal change findings, for the Tap 4 condition we found a statistically significant difference between MDMA users and non-MDMA users for percent activated voxels in the right SMA (Mann–Whitney test; 27.05 ± 15.4 versus 22.45 ± 25.5, p = 0.026).
MDMA dose effects on brain activation
Because of the between-group differences found in the Tap 4 condition, we restricted our subsequent within-group analyses of drug effects to that condition. We investigated the associations of drug dose with task performance, percent BOLD signal change, and percent activated voxels across all subjects reporting exposure to a particular drug (i.e. we did not restrict this analysis to the MDMA users only, except for the analysis of MDMA effects).
Task performance
The degree of drug use as lifetime episodes was not correlated with task performance (p > 0.05).
Percent BOLD signal change
Bivariate associations of MDMA use with regional percent BOLD signal change for the Tap 4 condition are shown in Table 4. The strongest correlations of lifetime episodes of MDMA use were in the right putamen (rs = 0.546, p = 0.044) and right pallidum (rs = 0.634, p = 0.015). There were no statistically significant correlations between lifetime use episodes of alcohol, cannabis, cocaine or methamphetamine and percent BOLD signal change.
Table 4.
Regional brain activation correlation with MDMA use.
| Regions of interest | Percent BOLD signal change |
Percent activated voxels |
||
|---|---|---|---|---|
| (rs) | p | (rs) | p | |
| SMA L | 0.517 | 0.058 | 0.343 | 0.230 |
| SMA R | 0.380 | 0.180 | 0.370 | 0.193 |
| Precentral L | 0.241 | 0.406 | 0.341 | 0.232 |
| Precentral R | 0.477 | 0.084 | 0.580 | 0.030 |
| Caudate L | 0.411 | 0.144 | 0.343 | 0.230 |
| Caudate R | 0.405 | 0.151 | 0.332 | 0.246 |
| Putamen L | 0.376 | 0.185 | 0.248 | 0.393 |
| Putamen R | 0.546 | 0.044 | 0.354 | 0.214 |
| Pallidum L | 0.453 | 0.104 | 0.280 | 0.333 |
| Pallidum R | 0.634 | 0.015 | 0.480 | 0.082 |
| Thalamus L | 0.418 | 0.137 | 0.575 | 0.032 |
| Thalamus R | 0.458 | 0.100 | 0.668 | 0.009 |
| Postcentral L | −0.034 | 0.908 | 0.262 | 0.365 |
| Postcentral R | 0.230 | 0.428 | 0.642 | 0.013 |
Correlation of percent BOLD signal change and percent activated voxels with lifetime episodes of MDMA use according to pre-defined anatomical region of interest for Tap 4 condition. rs = Spearman’s rho.
Bolded = statistically significant (p < 0.05; two-tailed).
Percent activated voxels
Bivariate associations of MDMA use with percent activated voxels for the Tap 4 condition are shown in Table 4. Lifetime episodes of MDMA use were positively correlated with percent activated voxels in the right precentral cortex (rs = 0.580, p = 0.030), left thalamus (rs = 0.575, p = 0.032), right thalamus (rs = 0.668, p = 0.009) and right postcentral cortex (rs = 0.642, p = 0.013).
Analysis of the associations of other drug use with percent activated voxels revealed a statistically significant correlation of episodes of alcohol use with percent activated voxels in left postcentral (rs = 0.422, p = 0.045), and left precentral (rs = 0.442, p = 0.035) cortex. There were no statistically significant associations of lifetime episodes of cannabis, cocaine, or methamphetamine use in any region for the Tap 4 condition. As reported above, neither of these regions had statistically significant associations with MDMA use, thus no further analysis for possible co-varying effects was conducted.
Discussion
We hypothesized that recreational MDMA use would be associated with altered regional brain activation in motor regions during task performance. We tested this hypothesis using a translational research design based upon prior reports of MDMA-induced 5-HT neurotoxicity and the role of 5-HT in motor function. In addition to the increased activation in right supplementary motor area (SMA) in the MDMA group, greater MDMA use predicted increased regional brain activation across multiple regions mediating motor function, in agreement with our hypothesis. The results for the correlation of MDMA use with activation held for both ipsilateral (with reference to left primary motor cortex) and contralateral motor areas. This consistency suggests a general graded effect of MDMA exposure on brain activation during voluntary motor processing, likely reflecting altered regional neurophysiology and possibly due to MDMA effects on 5-HT signaling.
Global task effects
The global task effects analysis demonstrated that the slow event-related tap design produced detectable activation in the a priori chosen motor regions. Slow event-related designs show linear summation with increasing stimulus frequency (Fransson et al., 1999) and may provide a more accurate reflection of activation measures in circumstances associated with potentially altered neurovascular coupling, as in the case of MDMA effects on 5-HT innervation of cerebral blood vessels. The largely inverse relationship between tap reaction time and activation is somewhat consistent with earlier findings, in which reaction time was inversely correlated with activation in some cortical regions, but not in SMA (Mohamed et al., 2004). The exact mechanism for reduced BOLD signal with increased reaction time is unclear, but may reflect altered cerebral neurophysiology necessary for planning and execution of motor tasks.
Between-group effects
Notably, the MDMA versus non-MDMA group comparison in this study found the greatest difference in activation in the SMA, with greater activation in MDMA users. Because there was no effect of MDMA use on task performance, this suggests that motor reaction time as measured by simple cued tap is not associated with MDMA use. Potential neurophysiological explanations for this finding may include increased synaptic input from other brain regions afferent to SMA, greater local synaptic activity, or a combination of the two. The greater SMA activation in MDMA users in the between-groups analysis could be interpreted at one level as a requirement for increased neuronal or metabolic resources in MDMA users to maintain task performance. This interpretation would be in line with the normal task performance in the MDMA group, and is consistent with increased activation but preserved task performance seen in individuals at risk for or progressing to Alzheimer’s disease (Bondi et al., 2005; Vannini et al., 2007). Because SMA activation did not correlate with levels of MDMA use, it is possible that SMA effects are a trait difference pre-dating MDMA exposure or that MDMA affects SMA function at very low exposure levels, producing a ceiling effect.
MDMA dose effects
The degree of prior MDMA use was positively associated with the degree of BOLD signal change and the spatial extent of activation in multiple pre-defined cortical and subcortical anatomical regions. While the corticostriatal system consists of complexly interconnected loops and parallel circuits, voluntary motor output can be reasonably construed as initiated in SMA and feeding forward to relevant cortical and subcortical regions. Therefore, the increased activation in SMA, as discussed above, would be consistent with loss of a net inhibitory 5-HT input to SMA with subsequent increases in feed-forward excitation to motor cortex and subcortical putamen and pallidum. However, despite the between-group differences in right SMA activation, we did not find a corresponding significant effect in right SMA in the correlational analysis examining associations between the degree of MDMA use and activation measures. This suggests that increased right SMA activation does not follow into subcortical regions in a clear-cut manner.
Serotonin (5-HT) and motor function
Two nuclear groups, the dorsal raphe and median raphe nuclei provide the bulk of 5-HT innervation to the cerebral cortex and basal ganglia in primates (Hornung, 2003). There is some overlap between regions innervated by dorsal raphe and median raphe (Hornung, 2003), and the fiber specificity of MDMA effects is not known in humans. Because the preponderance of available evidence seems to favor a specific effect of MDMA on fine-diameter dorsal raphe fibers, we will refer generally to “loss” or “reduction” in 5-HT innervation in relation to the dorsal raphe.
As depicted in Fig. 5, dorsal raphe 5-HT axons innervate basal ganglia–thalamocortical structures at all levels. 5-HT receptors of varying subtypes and affecting multiple neurotransmitters are present in motor regions (Fink and Gothert, 2007). Because 5-HT release has both direct (via heteroreceptors on neurons comprising the basal ganglia–thalamocortical circuit) and indirect effects on non-5-HT axons innervating those neurons, the consequences of MDMA effects on 5-HT are not easily predicted. However, the observed findings are consistent with a net increase in circuit activation with increasing exposure to MDMA.
Fig. 5.
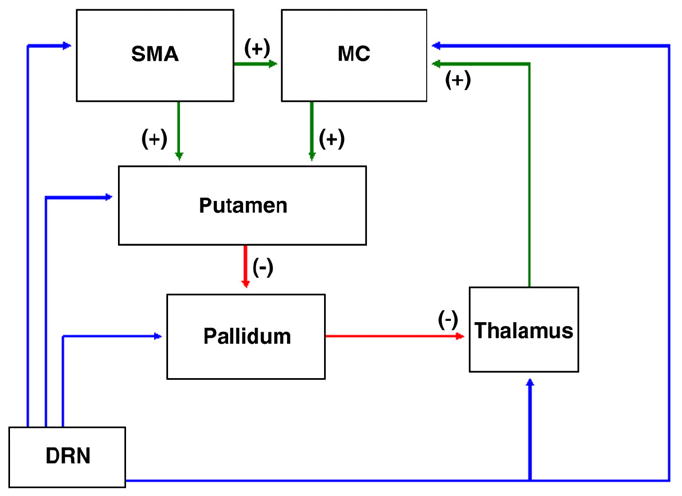
Simplified basal ganglia–thalamocortical circuit. Motor circuit paths begin with input arriving to the putamen from the supplementary motor area (SMA), and primary motor cortex (MC). Output from the putamen (and parallel flow from cortex as well) then continues via direct or indirect paths (not shown). The direct path consists of an inhibitory gamma-amino-butyric-acid (GABA) projection from the putamen to the globus pallidus internal segment (GPi) and substantia nigra pars reticulata (SNr). GPi and SNr send inhibitory GABAergic projections to the ventrolateral (VL) nucleus of the thalamus. VL thalamic projections to neocortex are glutamatergic and project to MC, including the hand region in primates (Holsapple et al., 1991). The simplified result of stimulation of the direct path in the basal ganglia–thalamocortical loop is excitatory input to the cortex. The indirect path of the basal ganglia–thalamocortical loop involves GABA inputs from the putamen to the globus pallidus external segment (GPe) which in turn provides a GABAergic projection to the subthalamic nucleus (STN). The STN receives direct input from cortex as well (Alexander et al. 1990) and projects to the GPi and SNr via excitatory glutamate axons. GPI/SNr innervate the VL via GABAergic axons. The simplified result of cortical input to the indirect pathway is inhibition of excitatory thalamic input to the cortex.
MDMA and motor function
Animal models of MDMA administration have suggested that MDMA produces chronic effects such as diminished motor response to MDMA administration or decreased effects of a 5-HT1B agonist (Balogh et al., 2004; Gyongyosi et al., 2008). To our knowledge, prior studies have not used functional neuroimaging to probe task-evoked motor function in human MDMA users. Studies using fMRI to investigate other cognitive processes have found either no (Jager et al., 2007b) or mixed task-dependent effects for prospective use (Jager et al., 2008a). Cross-sectional fMRI studies have found task and region dependent effects. In a study comparing occipital cortical activation to photic stimulation in MDMA users and controls, we found no between-group differences in occipital cortical activation but a positive correlation between lifetime MDMA use and the spatial extent of occipital activation (Cowan et al., 2006). Moeller et al. (2004) found increased activation in medial frontal cortex, thalamus, putamen, and hippocampus during performance of a delayed, but not an immediate memory task. A subsequent analysis examining impulsivity scores (Valdes et al., 2006) in the same cohort found that motor impulsivity scores correlated positively with dorsolateral prefrontal cortical activation but this finding was not related to MDMA use. In a series of cross-sectional studies (Daumann et al., 2004a,b), MDMA users displayed a general trend for increased parietal activation during working memory performance. Despite no detected effects on memory, MDMA users had reduced activation and reduced spatial extent of activation in hippocampus during an episodic memory retrieval task (Daumann et al., 2005). Conversely, Jacobsen et al. (2004) found longer reaction times and impaired hippocampal activation in a group of MDMA-exposed adolescents.
Other research has suggested that MDMA use is associated with altered brain structure or function in subcortical motor areas. Studies of resting brain activity in human MDMA users (Reneman et al., 2000) found increased regional blood volume in the globus pallidus while de Win et al. (2008a) reported altered blood volume in globus pallidus, putamen and the thalamus after low-dose MDMA use (de Win et al., 2008b). Obrocki et al. (2002a,b) found decreased metabolic rate in caudate and putamen of MDMA users. Further structural changes in the 5-HT system in MDMA users include reduced markers of 5-HT reuptake transporter binding in the thalamus (Buchert et al., 2003, 2004; de Win et al., 2008b; McCann et al., 1998, 2005), caudate (Buchert et al., 2003, 2004; McCann et al., 1998, 2005), and putamen (McCann et al., 1998, 2005). De Win et al. (2008a,b) used diffusion tensor imaging (DTI) and found indirect evidence for altered axonal structure in thalamus and putamen.
Limitations
Several theoretical and practical considerations limit our ability to conclude that MDMA causes altered regional brain activation during motor performance via a 5-HT mechanism. First, we do not have empirical verification that our test subjects were actually exposed to MDMA. While the participants self-reported use of Ecstasy, we do not have analyses of the contents of the drugs they ingested. Available estimates for pill purity in the US and Western Europe indicate, however, 80% or more of Ecstasy pills contained MDMA by the late 1990s (Parrott, 2004).
Second, our cohort of MDMA users, as with other MDMA users worldwide, had higher levels of polydrug exposure than the control group (Wish et al., 2006). This limits the utility of a cross-sectional between-groups comparison, and we believe the more viable approach for cross-sectional studies of MDMA effects is to examine dose–response associations. This may be particularly important when polydrug effects influence the mean signal in a direction opposite that of MDMA and when MDMA use is associated in a dose–response manner with outcomes.
Third, there is not sufficient supporting neurophysiological data linking BOLD function to MDMA effects in non-human primates to permit us to specifically test the hypothesis that MDMA-induced 5-HT toxicity leads to the observed effects on BOLD signal. The potential differences between experimental models and human users are considerable and the neural circuitry involved, with combinations of excitatory and inhibitory neurons and feed-forward and feedback connections at every level of basal ganglia–thalamocortical processing means that any attempt to interpret these findings must be considered at a very general level. Nonetheless, we believe that an attempt to understand MDMA effects in the context of a translational approach based on basic neuroscience is warranted and necessary.
Fourth, sample size limitations may have limited our ability to detect case-control differences in the random effects model and Type II experimental error may explain the absence of differences in additional motor regions. However, as noted in the Introduction, comparisons of mean differences may not be sensitive to MDMA effects if MDMA produces a dose–response effect on activation measures, such that the variation about the mean is expected to be a function of dose, and not group status per se. Since the within-group correlation analysis reveals consistent associations between the degree of MDMA use and outcome variables, we believe the dose effects analysis is the most relevant to potential MDMA toxicity.
Fifth, in reporting our results, we did not fully adjust our statistical threshold for the potential effects of multiple statistical comparisons. The between-groups comparison of statistical activation maps, performed within SPM5, used a cluster-corrected threshold of p ≤ 0.05. The between-groups analysis performed on the BOLD signal change and activated voxels did not employ corrected p-values and the estimated corrected thresholds were p ≤ 0.012 (for BOLD signal change) and p ≤ 0.018 for activated voxels. For the within-group correlation analysis of MDMA effects on outcome variables, the corrected p value threshold for statistical significance was p ≤ 0.012 for the percent signal change measure and p ≤ 0.015 for the percent activated voxels measures. As a result, only the finding for the correlation of MDMA use and percent activated voxels in right thalamus and right postcentral gyrus would have remained significant if these thresholds were applied. While adjusting for repeated statistical comparisons is an important method to avoid Type I error, the consistent direction of the effects across regions and the size of the correlation coefficients suggests that the observed results are not chance findings.
Implications
In the presence of normal task performance, the clinical relevance of the observed findings remains uncertain, but is suggestive of widespread brain effects of MDMA use on motor circuitry. As noted previously (Bondi et al., 2005; Vannini et al., 2007), other researchers have suggested that increased activation in the face of preserved task performance may suggest that additional brain resources are necessary to achieve the same outcome. As such, it is possible that MDMA use may reduce the efficiency of brain function during motor task performance. We have thus far demonstrated a positive correlation with MDMA use and spatial extent of activation in visual cortex (Cowan et al., 2006) and, here, expand that finding to include both spatial extent of activation and magnitude of BOLD signal change in motor regions. Additional studies probing specific neurocognitive aspects of motor function in relation to neurophysiology seem warranted. Inferences regarding the connection between MDMA use, reduced 5-HT signaling, and the observed findings require additional research linking MDMA and 5-HT neurophysiology in non-human primates to non-invasive imaging markers in human MDMA users. Because motor system is intimately connected with 5-HT function, it is possible that alterations in motor function may predict effects elsewhere in the brain, including regions involved in mood and higher cognitive functions. These results raise the concern that recreational MDMA use is associated with long-lasting effects on brain neurophysiology.
Acknowledgments
The authors wish to thank Amy L. Bauernfeind, Kimberly L. Morton, and Christopher J. Cannistraci for their technical advice. We would like to thank the following funding agencies for their generous support of this work: NIDA — DA015137, DA020149 and DA00366 to RLC; NCRR — Vanderbilt CTSA UL1 RR024975; NIMH — K23 MH01828 to RMS; NIMH K01-MH083052 to JUB.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia–thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Balogh B, Molnar E, Jakus R, Quate L, Olverman HJ, Kelly PA, Kantor S, Bagdy G. Effects of a single dose of 3,4-methylenedioxymethamphetamine on circadian patterns, motor activity and sleep in drug-naive rats and rats previously exposed to MDMA. Psychopharmacology (Berl) 2004;173:296–309. doi: 10.1007/s00213-004-1787-9. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. 3,4-Methylenedioxymethamphetamine (MDMA) as a unique model of serotonin receptor function and serotonin–dopamine interactions. J Pharmacol Exp Ther. 2001;297:846–852. [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox 2002 [Google Scholar]
- Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M. Long-term effects of “ecstasy” use on serotonin transporters of the brain investigated by PET. J Nucl Med. 2003;44:375–384. [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, Schulze O, Schmidt U, Clausen M. A voxel-based PET investigation of the long-term effects of “Ecstasy” consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Carter CS, Heckers S, Nichols T, Pine DS, Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol Psychiatry. 2008;64:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Haga E, deB FB, Dietrich MS, Vimal RL, Lukas SE, Renshaw PF. MDMA use is associated with increased spatial BOLD fMRI visual cortex activation in human MDMA users. Pharmacol Biochem Behav. 2006;84:219–228. doi: 10.1016/j.pbb.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007a;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007b;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Roberts DM, Joers JM. Neuroimaging in human MDMA (Ecstasy) users. Ann N Y Acad Sci. 2008;1139:291–298. doi: 10.1196/annals.1432.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Fimm B, Willmes K, Thron A, Gouzoulis-Mayfrank E. Cerebral activation in abstinent ecstasy (MDMA) users during a working memory task: a functional magnetic resonance imaging (fMRI) study. Brain Res Cogn Brain Res. 2003a;16:479–487. doi: 10.1016/s0926-6410(03)00075-2. [DOI] [PubMed] [Google Scholar]
- Daumann J, Schnitker R, Weidemann J, Schnell K, Thron A, Gouzoulis-Mayfrank E. Neural correlates of working memory in pure and polyvalent ecstasy (MDMA) users. Neuroreport. 2003b;14:1983–1987. doi: 10.1097/00001756-200310270-00021. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Pilatus U, Thron A, Moeller-Hartmann W, Gouzoulis-Mayfrank E. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett. 2004a;362:113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Daumann J, Hensen G, Thimm B, Rezk M, Till B, Gouzoulis-Mayfrank E. Self-reported psychopathological symptoms in recreational ecstasy (MDMA) users are mainly associated with regular cannabis use: further evidence from a combined cross-sectional/longitudinal investigation. Psychopharmacology (Berl) 2004b;173:398–404. doi: 10.1007/s00213-003-1719-0. [DOI] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Heekeren K, Henke K, Thron A, Gouzoulis-Mayfrank E. Memory-related hippocampal dysfunction in poly-drug ecstasy (3,4-methylenedioxymethamphetamine) users. Psychopharmacology (Berl) 2005;180:607–611. doi: 10.1007/s00213-004-2002-8. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, den Heeten GJ, van den BW. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008a;131:2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, Ramsey NF, Heeten GJ, van den BW. Neurotoxic effects of ecstasy on the thalamus. Br J Psychiatry. 2008b;193:289–296. doi: 10.1192/bjp.bp.106.035089. [DOI] [PubMed] [Google Scholar]
- DeVito JL, Anderson ME, Walsh KE. A horseradish peroxidase study of afferent connections of the globus pallidus in Macaca mulatta. Exp Brain Res. 1980;38:65–73. doi: 10.1007/BF00237932. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. American Psychiatric Press; Washington, D.C: 1997. [Google Scholar]
- Fransson P, Kruger G, Merboldt KD, Frahm J. Temporal and spatial MRI responses to subsecond visual activation. Magn Reson Imaging. 1999;17:1–7. doi: 10.1016/s0730-725x(98)00163-5. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gyongyosi N, Balogh B, Kirilly E, Kitka T, Kantor S, Bagdy G. MDMA treatment 6 months earlier attenuates the effects of CP-94,253, a 5-HT(1B) receptor agonist, on motor control but not sleep inhibition. Brain Res. 2008;1231:34–46. doi: 10.1016/j.brainres.2008.06.099. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nervous System. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Holland BS, Copenhaver MD. An improved sequentially rejective Bonferroni test procedure. Biometrics. 1987;43:417–424. [Google Scholar]
- Holsapple JW, Preston JB, Strick PL. The origin of thalamic inputs to the “hand” representation in the primary motor cortex. J Neurosci. 1991;9:2644–2654. doi: 10.1523/JNEUROSCI.11-09-02644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26:331–343. doi: 10.1016/j.jchemneu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Preliminary evidence of hippocampal dysfunction in adolescent MDMA (“ecstasy”) users: possible relationship to neurotoxic effects. Psychopharmacology (Berl) 2004;173:383–390. doi: 10.1007/s00213-003-1679-4. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, Vervaeke HK, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study. Psychopharmacology (Berl) 2007a;193:403–414. doi: 10.1007/s00213-007-0792-1. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, Vervaeke HK, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Incidental use of ecstasy: no evidence for harmful effects on cognitive brain function in a prospective fMRI study. Psychopharmacology (Berl) 2007b;193:403–414. doi: 10.1007/s00213-007-0792-1. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, van DTI, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008a;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Jager G, de Win MM, van DTI, Schilt T, Kahn RS, van den BW, van Ree JM, Ramsey NF. Assessment of cognitive brain function in ecstasy users and contributions of other drugs of abuse: results from an FMRI study. Neuropsychopharmacology. 2008b;33:247–258. doi: 10.1038/sj.npp.1301415. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future survey, national results on adolescent drug use: overview of key findings. The University of Michigan Institute for Social Research 2007 [Google Scholar]
- Lavoie B, Parent A. Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. J Comp Neurol. 1990;299:1–16. [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine (“ecstasy”) users: relationship to cognitive performance. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Dougherty DM, Narayana PA, Kramer LA, Renshaw PF. Functional MRI study of working memory in MDMA users. Psychopharmacology (Berl) 2004;177:185–194. doi: 10.1007/s00213-004-1908-5. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Lane SD, Buzby M, Swann AC, Hasan KM, Kramer LA, Narayana PA. Diffusion tensor imaging in MDMA users and controls: association with decision making. Am J Drug Alcohol Abuse. 2007;33:777–789. doi: 10.1080/00952990701651564. [DOI] [PubMed] [Google Scholar]
- Mohamed MA, Yousem DM, Tekes A, Browner N, Calhoun VD. Correlation between the amplitude of cortical activation and reaction time: a functional MRI study. AJR Am J Roentgenol. 2004;183:759–765. doi: 10.2214/ajr.183.3.1830759. [DOI] [PubMed] [Google Scholar]
- NIH. NCT00353938: Study of 3,4-methylenedioxymethamphetamine-assisted psychotherapy in people with posttraumatic stress disorder 2008a [Google Scholar]
- NIH. NCT00402298: Randomized placebo-controlled study of MDMA-assisted psychotherapy in people with PTSD — Israel 2008b [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol Lett. 2002a;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Obrocki J, Schmoldt A, Buchert R, Andresen B, Petersen K, Thomasius R. Specific neurotoxicity of chronic use of ecstasy. Toxicol Lett. 2002b;127:285–297. doi: 10.1016/s0378-4274(01)00511-2. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Is ecstasy MDMA? A review of the proportion of ecstasy tablets containing MDMA, their dosage levels, and the changing perceptions of purity. Psychopharmacology (Berl) 2004;173:234–241. doi: 10.1007/s00213-003-1712-7. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Habraken JB, Majoie CB, Booij J, den Heeten GJ. MDMA (“Ecstasy”) and its association with cerebrovascular accidents: preliminary findings. AJNR Am J Neuroradiol. 2000;21:1001–1007. [PMC free article] [PubMed] [Google Scholar]
- Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The national survey on drug use and health (NSDUH) Substance Abuse and Mental Health Services Administration, Office of Applied Studies 2008 [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- United Nations. World drug report. United Nations, Office on Drugs and Crime 2008 [Google Scholar]
- Valdes IH, Steinberg JL, Narayana PA, Kramer LA, Dougherty DM, Swann AC, Barratt ES, Moeller FG. Impulsivity and BOLD fMRI activation in MDMA users and healthy control subjects. Psychiatry Res. 2006;147:239–242. doi: 10.1016/j.pscychresns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Vannini P, Almkvist O, Dierks T, Lehmann C, Whalund LO. Reduced neuronal efficacy in progressive mild cognitive impairment: a prospective fMRI study on visuospatial processing. Psychiatry Research Neuroimaging. 2007;156:43–57. doi: 10.1016/j.pscychresns.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME. The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience. 1991;44:537–553. doi: 10.1016/0306-4522(91)90076-z. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Ricaurte GA, Molliver ME. Distinct morphologic classes of serotonergic axons in primates exhibit differential vulnerability to the psychotropic drug 3,4-methylenedioxymethamphetamine. Neuroscience. 1989;28:121–137. doi: 10.1016/0306-4522(89)90237-6. [DOI] [PubMed] [Google Scholar]
- Wish ED, Fitzelle DB, O’Grady KE, Hsu MH, Arria AM. Evidence for significant polydrug use among ecstasy-using college students. J Am Coll Health. 2006;55:99–104. doi: 10.3200/JACH.55.2.99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]


