Abstract
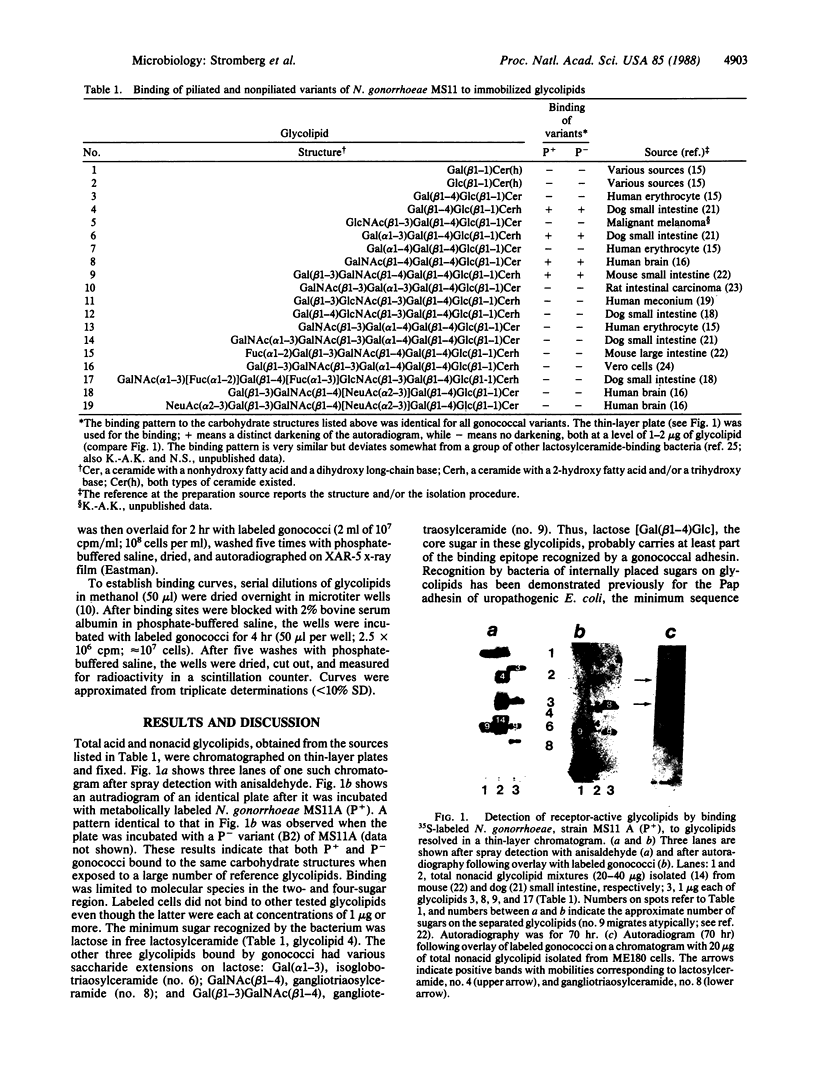
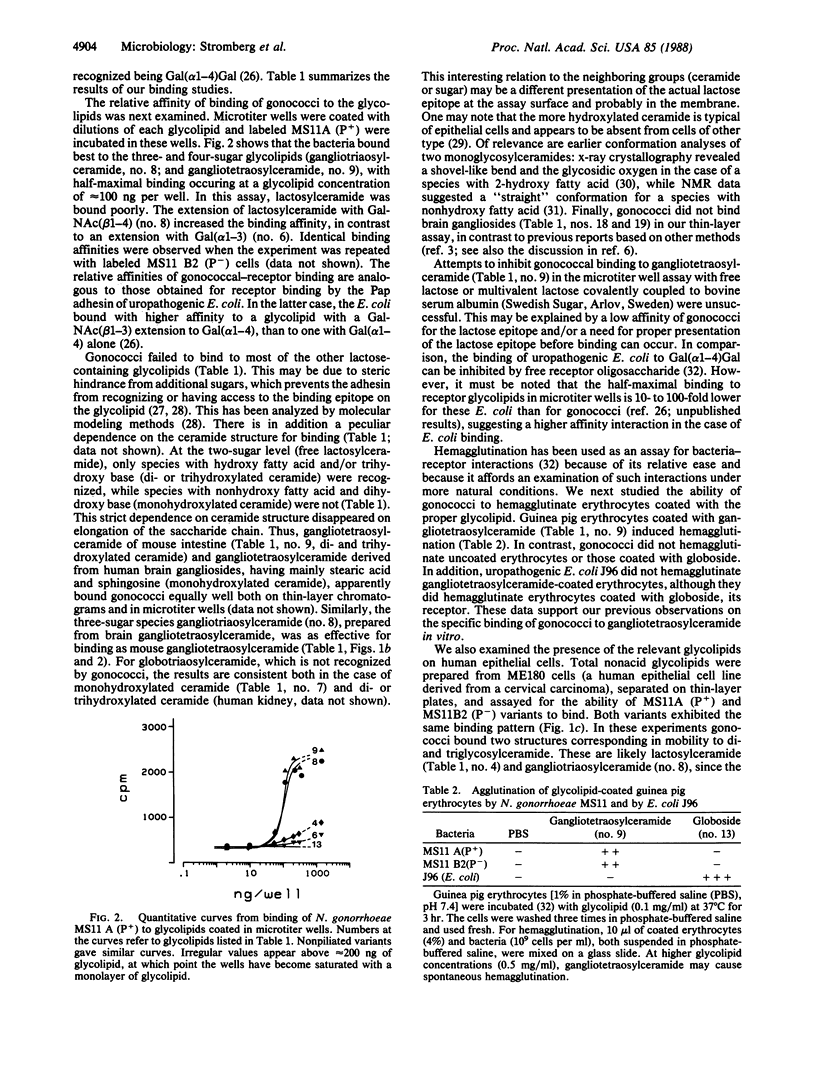
Different strains and isogenic variants of Neisseria gonorrhoeae were assayed for their ability to bind glycolipids extracted from various sources. Among a large number of reference glycolipids, binding was observed only to lactosylceramide [Gal(beta 1-4)Glc(beta 1-1)Cer], isoglobotriaosylceramide [Gal(alpha 1-3)Gal(beta 1-4)Glc(beta 1-1)Cer], gangliotriaosylceramide [GalNAc(beta 1-4)Gal(beta 1-4)Glc(beta 1-1)Cer], and gangliotetraosylceramide [Gal(beta 1-3)GalNAc(beta 1-4)Gal(beta 1-4)Glc(beta 1-1)Cer]. The latter two glycolipids bound gonococci with the highest affinity. Lactosylceramide and gangliotriaosylceramide were found in glycolipid preparations from ME180 cells, an epithelial cell line derived from a human cervical carcinoma, and thus are possible receptors for gonococci. The gonococcal surface component that bound the above glycolipids is a protein distinct from pilin and protein II.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomberg J., Breimer M. E., Karlsson K. A. Glycosphingolipids of a green monkey kidney cell line (GMK AH-1). Evidence for a novel pentaglycosylceramide based on globotetraosylceramide. Biochim Biophys Acta. 1982 Jun 11;711(3):466–477. doi: 10.1016/0005-2760(82)90061-3. [DOI] [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Cannon J. G., Sparling P. F. The genetics of the gonococcus. Annu Rev Microbiol. 1984;38:111–133. doi: 10.1146/annurev.mi.38.100184.000551. [DOI] [PubMed] [Google Scholar]
- Falk P., Holgersson J., Jovall P. A., Karlsson K. A., Strömberg N., Thurin J., Brodin T., Sjögren H. O. An antigen present in rat adenocarcinoma and normal colon non-epithelial stroma is a novel Forssman-like glycolipid based on isoglobotetraosylceramide. Biochim Biophys Acta. 1986 Sep 12;878(2):296–299. doi: 10.1016/0005-2760(86)90161-x. [DOI] [PubMed] [Google Scholar]
- Gubish E. R., Jr, Chen K. C., Buchanan T. M. Attachment of gonococcal pili to lectin-resistant clones of Chinese hamster ovary cells. Infect Immun. 1982 Jul;37(1):189–194. doi: 10.1128/iai.37.1.189-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., McKibbin J. M., Strömberg N., Thurin J. Isoglobotriaosylceramide and the Forssman glycolipid of dog small intestine occupy separate tissue compartments and differ in ceramide composition. Biochim Biophys Acta. 1983 Jan 7;750(1):214–216. doi: 10.1016/0005-2760(83)90224-2. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., Strömberg N., Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985 Apr;146(1):158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Leffler H., Strömberg N. Gangliotetraosylceramide is a major glycolipid of epithelial cells of mouse small intestine. FEBS Lett. 1982 Mar 22;139(2):291–294. doi: 10.1016/0014-5793(82)80873-9. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycolipids as attachment sites for microbes. Chem Phys Lipids. 1986 Dec 15;42(1-3):153–172. doi: 10.1016/0009-3084(86)90050-2. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Larson G. Structural characterization of lactotetraosylceramide, a novel glycosphingolipid isolated from human meconium. J Biol Chem. 1979 Sep 25;254(18):9311–9316. [PubMed] [Google Scholar]
- Karlsson K. A. Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Maurer L., Orndorff P. E. Identification and characterization of genes determining receptor binding and pilus length of Escherichia coli type 1 pili. J Bacteriol. 1987 Feb;169(2):640–645. doi: 10.1128/jb.169.2.640-645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKibbin J. M., Spencer W. A., Smith E. L., Mansson J. E., Karlsson K. A., Samuelsson B. E., Li Y. T., Li S. C. Lewis blood group fucolipids and their isomers from human and canine intestine. J Biol Chem. 1982 Jan 25;257(2):755–760. [PubMed] [Google Scholar]
- Momoi T., Ando S., Magai Y. High resolution preparative column chromatographic system for gangliosides using DEAE-Sephadex and a new porus silica, Iatrobeads. Biochim Biophys Acta. 1976 Sep 27;441(3):488–497. [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Fernandez R., Tai J. Y., Rothbard J., Gotschlich E. C. Gonococcal pili. Primary structure and receptor binding domain. J Exp Med. 1984 May 1;159(5):1351–1370. doi: 10.1084/jem.159.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E., Billyard E., So M., Storzbach S., Meyer T. F. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell. 1985 Feb;40(2):293–300. doi: 10.1016/0092-8674(85)90143-6. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance studies of N-palmitoylglucosylceramide (cerebroside) head group structure. Biochemistry. 1982 Jun 22;21(13):3154–3160. doi: 10.1021/bi00256a019. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Jernstrom P., Nolan J. F., Byatt P. Some properties of a new epithelial cell line of human origin. J Natl Cancer Inst. 1970 Jul;45(1):107–122. [PubMed] [Google Scholar]
- Uhlin B. E., Norgren M., Båga M., Normark S. Adhesion to human cells by Escherichia coli lacking the major subunit of a digalactoside-specific pilus-adhesin. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1800–1804. doi: 10.1073/pnas.82.6.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]



