Abstract
The expression of receptors for cholecystokinin (CCK) and other similar acting Ca2+-mobilizing hormones was studied in Xenopus laevis oocytes. Poly(A)+ RNA was prepared from pancreatic AR42J cells, which normally express receptors for CCK and bombesin and the RNA injected into oocytes. The presence of these pancreatic receptors on the oocytes was then demonstrated by hormone-induced mobilization of 45Ca2+. CCK receptors were present 1 day (maximum, 2 days) after injection of RNA and were generally proportional to the amount of poly(A)+ RNA injected (1-50 ng). Oocyte CCK receptors retained selectivity for CCK analogs (CCK8 greater than unsulfated CCK8 greater than CCK4) and were blocked by the specific CCK receptor antagonist CR 1409. When poly(A)+ RNA was subjected to size fractionation on sucrose gradients, activity-inducing CCK receptors showed a single peak centered at 3 kilobases. The generality of this oocyte system for expressing Ca2+-mobilizing hormone receptors was further shown by expression of a response to bombesin after injection of AR42J cell RNA and a response to vasopressin and angiotensin II when poly(A)+ RNA from rat liver was injected. No response to CCK was demonstrable after injection of liver RNA, demonstrating the specificity of this assay.
Full text
PDF
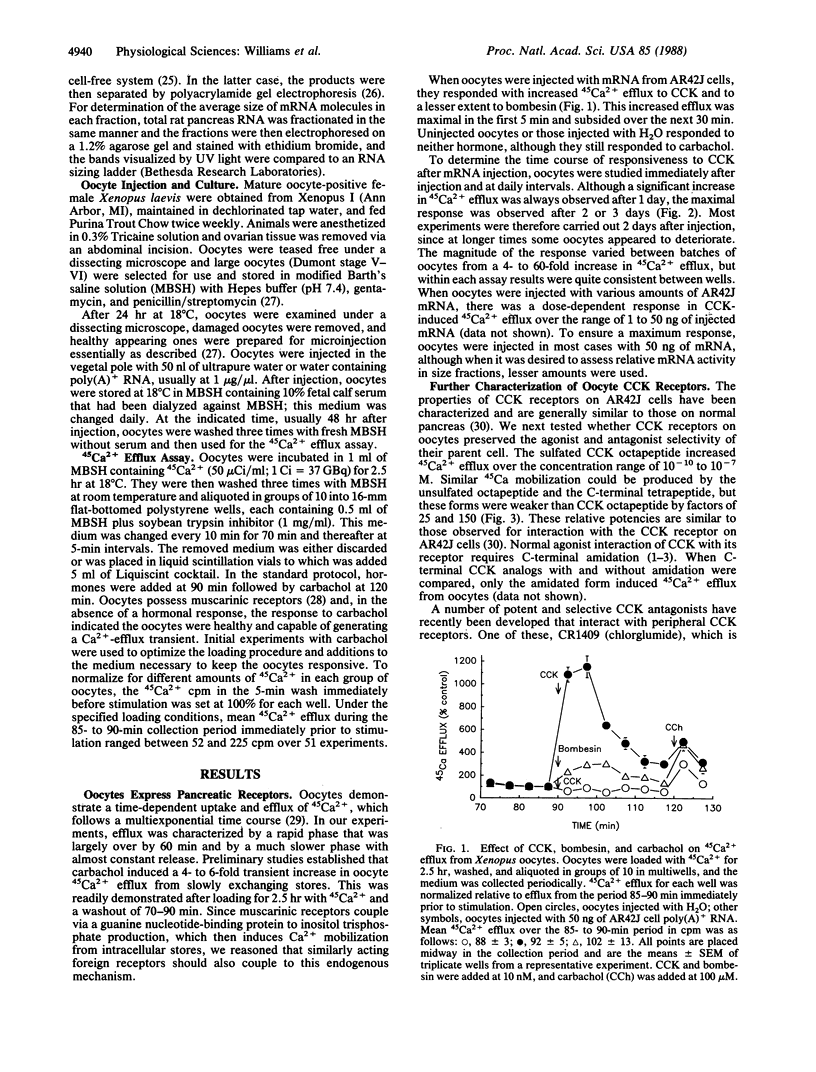
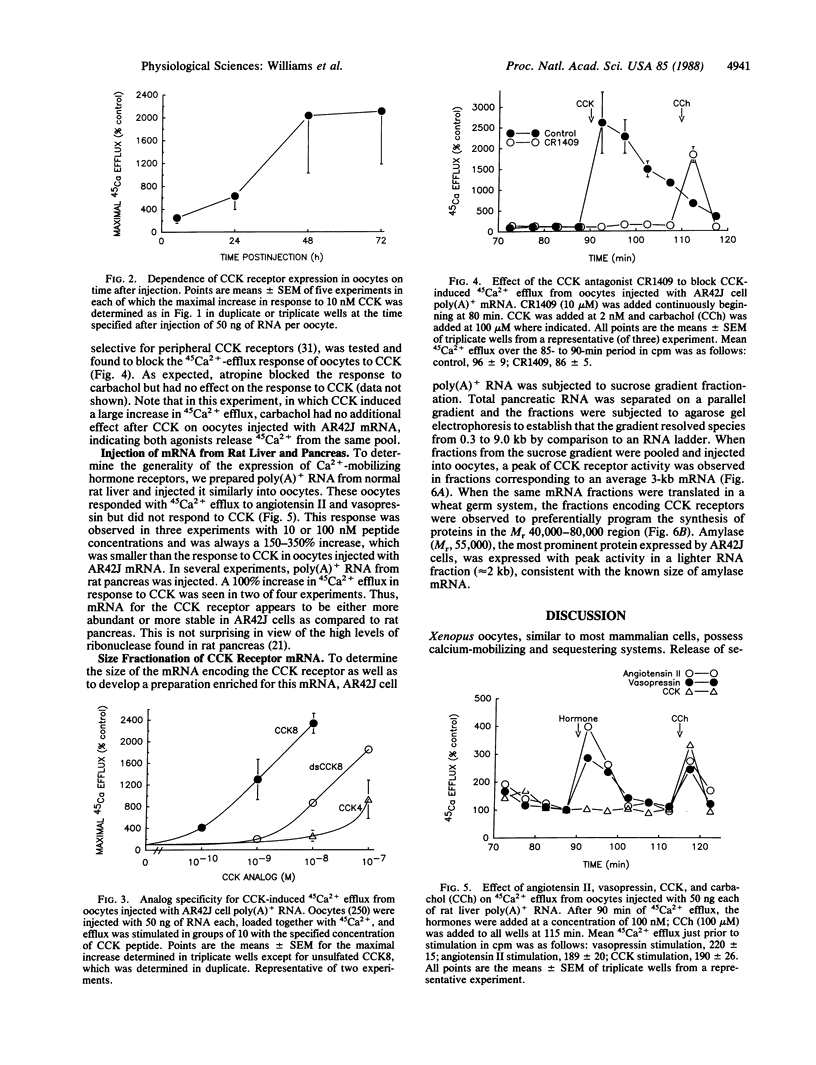


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinfeld M. C. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983 Oct;3(6):411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Busa W. B., Ferguson J. E., Joseph S. K., Williamson J. R., Nuccitelli R. Activation of frog (Xenopus laevis) eggs by inositol trisphosphate. I. Characterization of Ca2+ release from intracellular stores. J Cell Biol. 1985 Aug;101(2):677–682. doi: 10.1083/jcb.101.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Edery M., Ellis L., Standring D., Beaudoin J., Roth R. A., Rutter W. J. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8014–8018. doi: 10.1073/pnas.82.23.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Properties of human brain glycine receptors expressed in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):235–244. doi: 10.1098/rspb.1984.0032. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane C. D. The fate of genes, messengers, and proteins introduced into Xenopus oocytes. Curr Top Dev Biol. 1983;18:89–116. doi: 10.1016/s0070-2153(08)60580-3. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D. Glucocorticoids increase cholecystokinin receptors and amylase secretion in pancreatic acinar AR42J cells. J Biol Chem. 1986 Feb 15;261(5):2096–2101. [PubMed] [Google Scholar]
- Luthe D. S. A simple technique for the preparation and storage of sucrose gradients. Anal Biochem. 1983 Nov;135(1):230–232. doi: 10.1016/0003-2697(83)90755-8. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Snutch T. P., Dascal N., Lester H. A., Davidson N. Rat brain 5-HT1C receptors are encoded by a 5-6 kbase mRNA size class and are functionally expressed in injected Xenopus oocytes. J Neurosci. 1987 Apr;7(4):1159–1165. doi: 10.1523/JNEUROSCI.07-04-01159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Niederau M., Williams J. A., Grendell J. H. New proglumide-analogue CCK receptor antagonists: very potent and selective for peripheral tissues. Am J Physiol. 1986 Jun;250(6 Pt 1):G856–G860. doi: 10.1152/ajpgi.1986.250.6.G856. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Robinson K. R., Smith L. D. Calcium, potassium, and sodium exchange by full-grown and maturing Xenopus laevis oocytes. Dev Biol. 1977 Nov;61(1):28–40. doi: 10.1016/0012-1606(77)90339-6. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Parker I., Miledi R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1986 Aug 22;228(1252):307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Neurotensin and substance P receptors expressed in Xenopus oocytes by messenger RNA from rat brain. Proc R Soc Lond B Biol Sci. 1986 Nov 22;229(1255):151–159. doi: 10.1098/rspb.1986.0079. [DOI] [PubMed] [Google Scholar]
- Rosenzweig S. A., Miller L. J., Jamieson J. D. Identification and localization of cholecystokinin-binding sites on rat pancreatic plasma membranes and acinar cells: a biochemical and autoradiographic study. J Cell Biol. 1983 May;96(5):1288–1297. doi: 10.1083/jcb.96.5.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto C., Goldfine I. D., Williams J. A. Characterization of cholecystokinin receptor subunits on pancreatic plasma membranes. J Biol Chem. 1983 Oct 25;258(20):12707–12711. [PubMed] [Google Scholar]
- Sankaran H., Goldfine I. D., Deveney C. W., Wong K. Y., Williams J. A. Binding of cholecystokinin to high affinity receptors on isolated rat pancreatic acini. J Biol Chem. 1980 Mar 10;255(5):1849–1853. [PubMed] [Google Scholar]
- Simmen F. A., Schulz T. Z., Headon D. R., Wright D. A., Carpenter G., O'Malley B. W. Translation in Xenopus oocytes of messenger RNA from A431 cells for human epidermal growth factor receptor proteins. DNA. 1984 Oct;3(5):393–399. doi: 10.1089/dna.1984.3.393. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Szecòwka J., Goldfine I. D., Williams J. A. Solubilization and characterization of CCK receptors from mouse pancreas. Regul Pept. 1985 Mar;10(2-3):71–83. doi: 10.1016/0167-0115(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Pinto L. H., O'Connor C. M., Smith L. D. Progesterone induces a rapid increase in [Ca2+]in of Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1534–1536. doi: 10.1073/pnas.77.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]