Abstract
Opium poppy produces a diverse array of pharmaceutical alkaloids, including the narcotic analgesics morphine and codeine. The benzylisoquinoline alkaloids of opium poppy accumulate in the cytoplasm, or latex, of specialized laticifers that accompany vascular tissues throughout the plant. However, immunofluorescence labeling using affinity-purified antibodies showed that three key enzymes, (S)-N-methylcoclaurine 3′-hydroxylase (CYP80B1), berberine bridge enzyme (BBE), and codeinone reductase (COR), involved in the biosynthesis of morphine and the related antimicrobial alkaloid sanguinarine, are restricted to the parietal region of sieve elements adjacent or proximal to laticifers. The localization of laticifers was demonstrated using antibodies specific to the major latex protein (MLP), which is characteristic of the cell type. In situ hybridization showed that CYP80B1, BBE, and COR gene transcripts were found in the companion cell paired with each sieve element, whereas MLP transcripts were restricted to laticifers. The biosynthesis and accumulation of alkaloids in opium poppy involves cell types not implicated previously in plant secondary metabolism and dramatically extends the function of sieve elements beyond the transport of solutes and information macromolecules in plants.
INTRODUCTION
The opium poppy is an ancient medicinal plant and the only commercial source for the narcotic analgesics morphine and codeine. Global production of morphine for licit pharmaceutical applications is ∼150 tons annually (United Nations, 2002a). However, the estimated illicit production of morphine for the synthesis of heroin is at least 10-fold higher and contributes to numerous social and political problems throughout the world (United Nations, 2002b). Despite the widespread significance of opium poppy, many basic aspects of morphine and codeine metabolism are poorly understood, including the cellular localization of their biosynthesis in the plant.
Morphine is a major component of the alkaloid-rich latex in opium poppy. Latex is the cytoplasm of specialized cells, or laticifers, that form an internal secretory system associated with phloem tissues of the vascular system throughout the plant (Thureson-Klein, 1970). The manual lancing of unripe seed capsules is the traditional method for the collection of opium poppy latex. The air-dried latex, or opium, then is extracted with solvents to isolate morphine. When damaged, laticifers release a copious volume of latex because their cellular contents are under positive turgor pressure similar to sieve elements of the phloem. Moreover, despite the compound origin of opium poppy laticifers, perforations develop between the lateral walls of adjacent cells, ensuring a contiguous network of latex vessels (Nessler and Mahlberg, 1977). Opium poppy latex is characterized by an abundance of major latex proteins (MLPs), which constitute a family of highly conserved, low molecular weight polypeptides found exclusively in laticifers (Nessler et al., 1985).
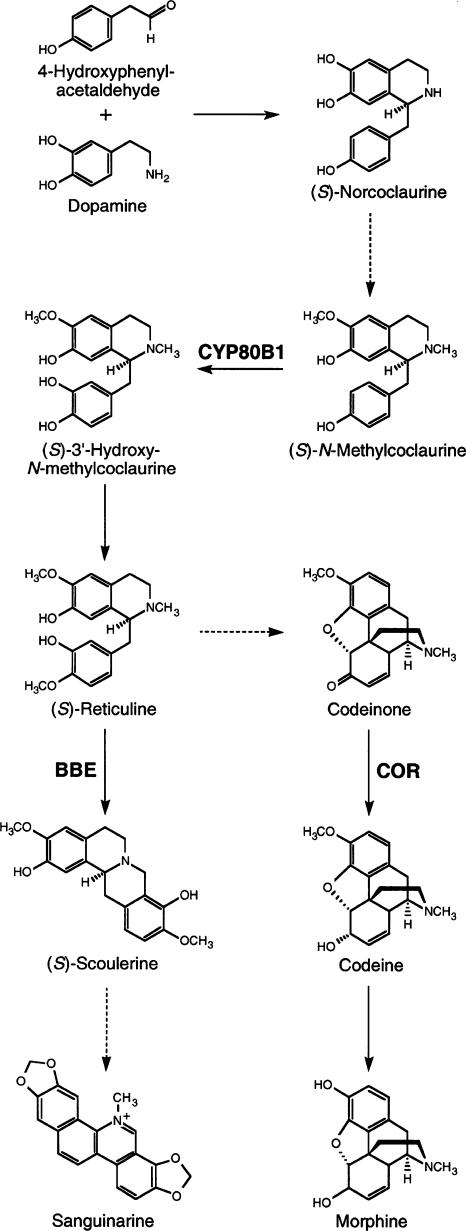
Morphine and codeine are members of the large and diverse group of benzylisoquinoline alkaloids, of which >2500 different structures have been identified in plants. Alkaloids are considered secondary metabolites because they are not essential for normal plant development but often play important ecophysiological roles. Morphine is most abundant in the latex of aerial organs, whereas the antimicrobial agent sanguinarine is the major alkaloid in opium poppy roots (Facchini and De Luca, 1995). Morphine and sanguinarine share a common biosynthetic pathway (Figure 1), beginning with the condensation of two l-Tyr derivatives to produce the central precursor (S)-norcoclaurine (Facchini, 2001). Specific O- and N-methyltransferases convert (S)-norcoclaurine to (S)-N-methylcoclaurine. A cytochrome P450–dependent monooxygenase [(S)-N-methylcoclaurine 3′-hydroxylase (CYP80B1)] catalyzes the 3′-hydroxylation of (S)-N-methylcoclaurine (Pauli and Kutchan, 1998). The subsequent 4′-O-methylation of (S)-3′-hydroxy-N-methylcoclaurine yields (S)-reticuline, the last common intermediate in the biosynthesis of both sanguinarine and morphine. Berberine bridge enzyme (BBE) catalyzes the conversion of (S)-reticuline to (S)-scoulerine, the first committed step in the sanguinarine pathway (Facchini et al., 1996). Alternatively, (S)-reticuline can be isomerized to its (R)-epimer as the first step in the formation of morphine. The NADPH-dependent enzyme codeinone reductase (COR) converts (−)-codeinone to (−)-codeine as the penultimate step in morphine biosynthesis (Unterlinner et al., 1999).
Figure 1.
Sites of Action of (S)-N-Methylcoclaurine-3′-Hydroxylase (CYP80B1), Berberine Bridge Enzyme (BBE), and Codeinone Reductase (COR) in the Biosynthesis of Morphine and Sanguinarine.
Dashed arrows indicate multiple enzymatic steps.
The accumulation of morphine and related secondary metabolites in the large, membranous vesicles of opium poppy latex has contributed to the long-standing assumption that alkaloids are synthesized in laticifers (Fairbairn and Wassel, 1964; Fairbairn et al., 1968; Wilson and Coscia, 1975; Roberts et al., 1983). However, several key enzymes involved in morphine biosynthesis have not been detected in latex (Gerardy and Zenk, 1993a, 1993b), suggesting that although alkaloids accumulate in laticifers, their synthesis occurs elsewhere. Using immunofluorescence labeling and in situ hybridization to identify the cellular localization of alkaloid biosynthesis in opium poppy, we show here that key alkaloid biosynthetic enzymes and gene transcripts are found in sieve elements and companion cells, respectively. These two phloem cell types have not been implicated previously in plant secondary metabolism. The implication of sieve elements in the biosynthesis of complex alkaloids dramatically extends the function of the phloem beyond the transport of solutes and information macromolecules in plants.
RESULTS
Key Alkaloid Biosynthetic Enzymes Generally Are Located in All Plant Organs
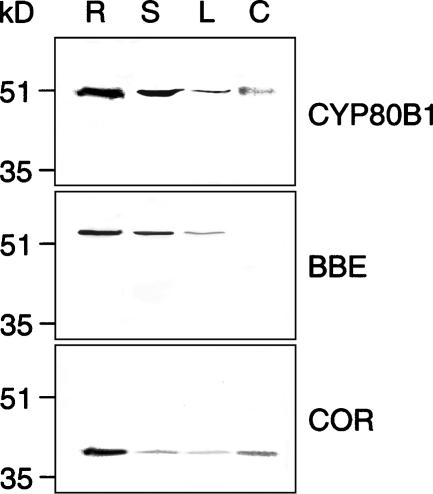
Polyclonal antibodies were raised against recombinant CYP80B1, BBE, and COR in both mice and rabbits. Immunoblot analysis using affinity-purified IgG fractions demonstrated the specificity of the antibodies. One band was detected in each lane of an immunoblot containing crude protein extracts from different opium poppy organs using affinity-purified mouse IgGs raised against CYP80B1, BBE, and COR (Figure 2). Identical results were obtained using affinity-purified rabbit IgGs (data not shown). The immunoreactive proteins were consistent with the expected molecular masses of CYP80B1 (54 kD), BBE (57 kD), and COR (36 kD). All three enzymes were present in each organ except for BBE, which was not detected in the carpel (Figure 2). The highest level of each protein was found in roots. No signals were detected on immunoblots probed with preimmune IgG fractions.
Figure 2.
Immunospecificity of Affinity-Purified Antibodies.
Immunoblot showing the levels of CYP80B1, BBE, and COR in crude protein extracts of various opium poppy organs. Protein blots were probed with affinity-purified polyclonal antibodies. Data are representative of three independent experiments. R, root; S, stem; L, leaf; C, carpel.
Immunolocalization Identifies a Distinct Cell Type Involved in Alkaloid Biosynthesis
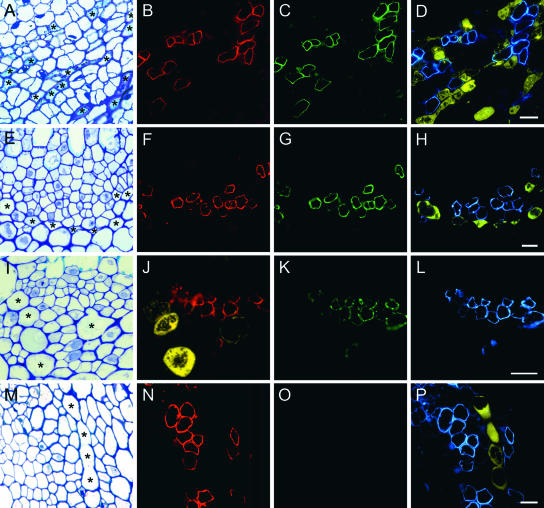
Immunofluorescence labeling using resin-embedded cross-sections of various opium poppy organs showed the colocalization of CYP80B1, BBE, and COR to a specific cell type associated with vascular tissue throughout the plant (Figure 3). In roots, bundles composed of sieve element/companion cell pairs and laticifers were interspersed among parenchyma tissue throughout the secondary phloem, which surrounds a core of secondary xylem (Figure 3A). The use of affinity-purified antibodies showed that CYP80B1 (Figure 3B), BBE (Figure 3C), and COR (Figure 3D) were localized to the same cells in the root vascular tissue. Colocalization using affinity-purified mouse anti-COR and rabbit anti-MLP IgGs, which specifically labeled latex proteins, showed that laticifers were adjacent or proximal to cells containing CYP80B1, BBE, and COR (Figure 3D).
Figure 3.
Alkaloid Biosynthetic Enzymes Are Localized to a Specific Cell Type Adjacent or Proximal to Laticifers in Opium Poppy.
(A) to (D) Anatomical staining and immunofluorescence localization of CYP80B1 (red), BBE (green), COR (blue), and MLP (yellow) in the phloem of serial root cross-sections.
(E) to (H) Phloem of serial stem cross-sections.
(I) to (L) Phloem of serial leaf cross-sections.
(M) to (P) Phloem of serial carpel cross-sections.
Asterisks show the locations of several laticifers in sections (A), (E), (I), and (M) stained with toluidine blue O. Bars = 25 μm.
Identical results were obtained using sections of stem (Figures 3E to 3H), leaf (Figures 3I to 3L), and carpel (Figures 3M to 3P) from opium poppy. CYP80B1, BBE, and COR antibodies were colocalized to the same cells adjacent or proximal to laticifers, which were identified clearly in stem (Figure 3H), leaf (Figure 3J), and carpel (Figure 3P) using the MLP antibodies. No signals were detected in tissues probed with preimmune IgG fractions. In stems, laticifers generally were larger than those in roots and located closer to the cortex than sieve elements and companion cells (Figure 3F). Similarly, the large laticifers in leaves were abaxial to other phloem tissues (Figure 3I). A cross-section of a vascular bundle in the carpel shows the extensive anastomosis that often occurs between adjacent laticifers (Figure 3M). The size, angular shape, spatial distribution, and ubiquitous occurrence of cells labeled with the CYP80B1, BBE, and COR antibodies are consistent with their identification as sieve elements.
Localization of Alkaloid Biosynthetic Gene Transcripts to Companion Cells
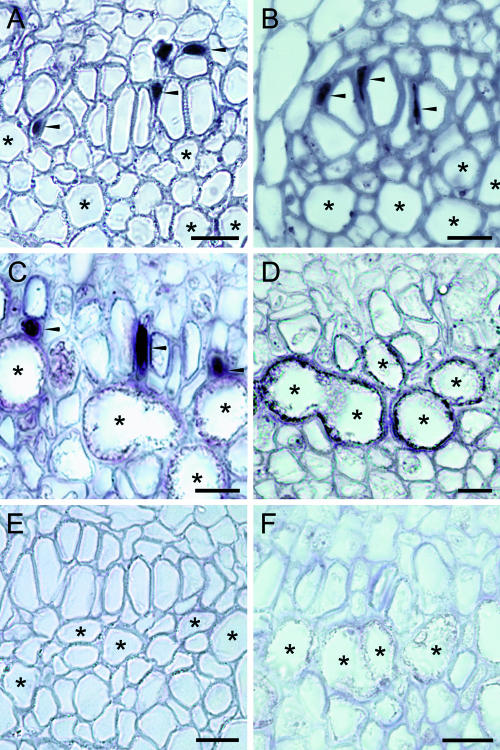
In situ hybridization using digoxigenin (DIG)-labeled antisense RNA probes for CYP80B1 (Figure 4A), BBE (Figure 4B), and COR (Figure 4C) showed the accumulation of transcripts in companion cells adjacent to large, angular sieve elements of the phloem. Transcripts for all three enzymes were detected in most organs, but only CYP80B1 and COR mRNAs were found in carpels. Weak labeling also was detected in xylem parenchyma (data not shown) but was absent from all other tissue in all plant organs. Laticifers in each section could be identified by a relatively thick cell wall, less angular shape, and a diameter generally >30 μm. A DIG-labeled antisense RNA probe for MLP hybridized specifically to laticifers (Figure 4D). MLP transcripts were not detected in other cell types, including companion cells.
Figure 4.
Alkaloid Biosynthetic Gene Transcripts Are Localized to the Companion Cells Paired with Sieve Elements in Opium Poppy.
(A) to (D) In situ hybridization using DIG-labeled antisense probes for CYP80B1 (A), BBE (B), COR (C), and MLP (D) performed on stem ([A] and [B]) and carpel ([C] and [D]) sections.
(E) and (F) In situ hybridization using DIG-labeled sense probes for CYP80B1 (E) and MLP (F) performed on stem (E) and carpel (F) sections.
Asterisks and arrowheads show the locations of several laticifers and labeled companion cells, respectively. Bars = 25 μm.
In situ hybridization was not detected in tissues exposed to sense RNA probes, as shown for CYP80B1 (Figure 4E) and MLP (Figure 4F). Up to fivefold higher concentration of sense RNA probe was used relative to that of the corresponding antisense RNA probe. The lack of signal in sections exposed to sense RNA probes supports the specific hybridization between the antisense RNA probes and gene transcripts localized in either companion cells or laticifers.
Presence of Sieve Plates Confirms the Identity of Sieve Elements
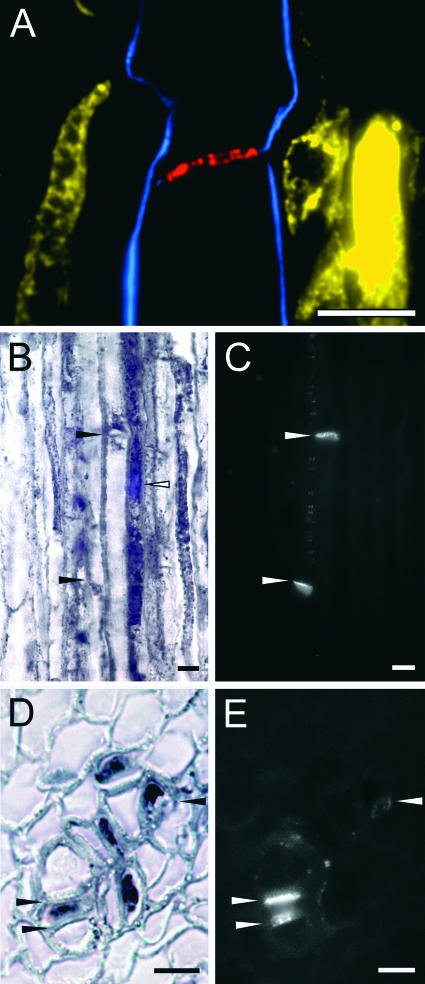
The colocalization of callose was used to positively identity companion cells and sieve elements proximal or adjacent to laticifers as the cell types containing transcripts and enzymes, respectively, involved in alkaloid biosynthesis (Figure 5). Callose, a β-1,3-linked glucan lining the walls of plasmodesmata and characteristically associated with sieve plates, can be detected readily by fluorescence microscopy using a monospecific antibody or after staining with aniline blue (Smith and McCully, 1978). Serial overlays of stem longitudinal sections exposed to COR, MLP, and callose antibodies showed the localization of COR along the peripheral cytoplasm of two adjoining sieve elements separated by a well-defined sieve plate (Figure 5A). The COR-containing sieve elements are adjacent or proximal to laticifers, identified by the presence of MLP (Figure 5A). The staining of callose using aniline blue in root longitudinal sections confirmed the presence of sieve elements adjacent to a typically elongated companion cell to which CYP80B1 transcripts were localized (Figures 5B and 5C). A field of plasmodesmata frequently was found between adjacent sieve elements (Figure 5C). BBE transcripts also were localized specifically to companion cells in root cross-sections (Figure 5D) stained with aniline blue to show the location of a sieve plate and numerous plasmodesmata between DIG-labeled companion cells and adjacent sieve elements (Figure 5E).
Figure 5.
Colocalization of MLP, Biosynthetic Enzymes or Gene Transcripts, and Callose Confirms the Role of Sieve Elements and Companion Cells in Alkaloid Biosynthesis.
(A) Immunofluorescence localization of COR (blue), MLP (yellow), and callose (red) in a serial overlay of LR White–embedded, longitudinal root sections (0.3 μm thick) of opium poppy. Callose was localized using a β-1,3-linked glucan monoclonal antibody.
(B) and (C) In situ hybridization using a DIG-labeled antisense probe for CYP80B1 (B) and localization of callose using aniline blue (C) in a root longitudinal section. Closed arrowheads point to two sieve plates, and the open arrowhead shows a DIG-labeled companion cell.
(D) and (E) In situ hybridization using a DIG-labeled antisense probe for BBE (D) and localization of callose using aniline blue (E) in a root cross-section. Arrowheads show the locations of a sieve plate and pit fields.
Bars = 15 μm.
Immunoreactive Proteins Are Associated with the Parietal Region of Sieve Elements
The localization of CYP80B1, BBE, and COR to the cytoplasm of sieve elements was confirmed by counterstaining with calcofluor white, which binds specifically to cellulose and thus demarcates cell walls. All three enzymes were localized to the parietal region of the sieve element cytoplasm, whereas MLP was found dispersed throughout the cytoplasm of laticifers (Figure 6). Immuno- fluorescence labeling clearly was not associated with cell walls.
Figure 6.

Immunofluorescence Localization of Alkaloid Biosynthetic Enzymes to the Parietal Layer of Sieve Elements.
Root cross-sections were counterstained with calcofluor white.
(A) CYP80B1 (red) and MLP (yellow).
(B) BBE (green) and MLP (yellow).
(C) COR (blue) and MLP (yellow). Bar = 25 μm.
DISCUSSION
A Tale of Three Cell Types
We have shown that the biosynthesis and accumulation of alkaloids in opium poppy involves three cell types of the phloem, two of which have not been implicated previously in plant secondary metabolism. Three key alkaloid biosynthetic genes are expressed in companion cells of the phloem, as shown by the accumulation of the gene transcripts in this cell type throughout the plant (Figures 4 and 5). The relative abundance of CYP80B1, BBE, and COR transcript accumulation (Facchini et al., 1996; Unterlinner et al., 1999; Huang and Kutchan, 2000) is consistent with the level of each protein in various organs of opium poppy (Figure 2). The localization of the CYP80B1, BBE, and COR enzymes to sieve elements (Figure 3) implies that the corresponding gene transcripts are translated in companion cells and that the proteins are transported to adjacent sieve elements. Mature angiosperm sieve elements lack a variety of cellular organelles, including a nucleus and ribosomes, and thus are incapable of basic transcriptional and translational processes. As a result, a sieve element depends on its paired companion cell for survival. Companion cell–specific gene expression and translation of mRNAs that encode proteins found in sieve elements, such as lectins, are well established (Bostwick et al., 1992). The movement of fluorescently labeled phloem proteins from companion cells to sieve elements has been demonstrated (Balachandran et al., 1997).
Symplastic connections among adjoining sieve elements create the contiguous sieve tubes that allow solutes to be transported systemically throughout the plant. The bulk flow of phloem sap in these conductive sieve tubes produces considerable shear forces (Fisher, 1990); thus, alkaloid biosynthetic enzymes and other proteins must be anchored to the parietal region of sieve elements to prevent dislodging and translocation. Many sieve element proteins, such as P proteins, are not translocated along the solute stream (Knoblauch and van Bel, 1998), probably because they are anchored to the sieve element reticulum (SER) (Oparka and Turgeon, 1999). Ultrastructural observations suggest that small protein anchors immobilize the parietal SER and other cellular organelles, forming a channel adjacent to the plasma membrane (Ehlers et al., 2000). Sieve element proteins have been suggested to reside in this channel and along the parietal SER. Immunofluorescence labeling of CYP80B1, BBE, and COR along the cellular periphery supports the localization of alkaloid biosynthesis to the parietal layer of sieve elements (Figures 3, 5, and 6).
The localization of CYP80B1, BBE, and COR gene transcripts and enzymes to companion cells and sieve elements, respectively, is consistent with the association of Tyr/dopa decarboxylase (TYDC) to vascular tissues in opium poppy (Facchini and De Luca, 1995; El-Ahmady and Nessler, 2001). Although TYDC is involved in other biochemical processes in addition to catalyzing the first steps in alkaloid formation and thus is not necessarily a direct marker for morphine and sanguinarine biosynthesis, it is notable that TYDC gene expression was not detected in laticifers (Facchini and De Luca, 1995; El-Ahmady and Nessler, 2001). It also is notable that low levels of TYDC mRNAs were found in xylem parenchyma (Facchini and De Luca, 1995), as were CYP80B1, BBE, and COR transcripts. Although CYP80B1, BBE, and COR represent only three of many biosynthetic enzymes, we suggest that alkaloid formation is restricted to sieve elements. This notion is supported by the positions in the alkaloid biosynthetic pathway of CYP80B1 at a common early step, BBE at the branch point in the sanguinarine pathway, and COR at the penultimate stage in morphine biosynthesis. The colocalization of CYP80B1, BBE, and COR to the same sieve elements shows that both morphine and sanguinarine biosynthesis occurs in the same cell type and implies that different alkaloids accumulate in the same laticifers. Because morphine and sanguinarine biosynthesis requires a common pathway intermediate, (S)-reticuline, the localization of both branch pathways to the same cell has regulatory implications with respect to the relative accumulation of each alkaloid in the plant.
The involvement of multiple, adjacent cell types in alkaloid biosynthesis and accumulation in opium poppy raises intriguing questions about the transport of products from sieve elements to laticifers. Recently, a multidrug-resistance-type, ATP binding cassette (ABC) protein from Coptis japonica (CjMDR1) was shown to transport the benzylisoquinoline alkaloid berberine (Shitan et al., 2003). In situ hybridization showed that CjMDR1 transcripts were most abundant in rhizome xylem tissues; thus, the transporter could function in the translocation of berberine from sites of synthesis to the rhizome, a major site of alkaloid accumulation in C. japonica. A membrane-bound ABC transporter also might reside at the interface between sieve elements and laticifers in opium poppy and participate in the transport of alkaloids between these cell types. It is noteworthy that an ABC transport protein transcript was identified in rice phloem (Asano et al., 2002). However, symplastic transport of alkaloids also must be considered, although plasmodesmata connecting the two cell types have not been reported.
Laticifers Function in Alkaloid Accumulation, Not Biosynthesis
The absence of CYP80B1, BBE, and COR proteins (Figure 3) or transcripts (Figure 4) in laticifers shows that the latex is incapable of alkaloid biosynthesis and, instead, serves strictly as the site of alkaloid accumulation. The alkaloid-rich latex of opium poppy was the focus of many early efforts to identify the tissue-specific sites of alkaloid biosynthesis (Roberts et al., 1983). Initial studies suggested that the radiolabeled precursors l-Tyr and l-dopa were incorporated into alkaloids when incubated with isolated latex (Fairbairn and Wassel, 1964; Fairbairn et al., 1968). However, a later study reported that the incorporation of radiolabeled precursors was better with, but not restricted to, isolated latex vesicles (Wilson and Coscia, 1975). Recently, almost 100 isolated proteins were partially sequenced after two-dimensional gel electrophoresis of the cytosolic and vesicular fractions of opium poppy latex (Decker et al., 2000). Several enzymes associated with primary metabolism and various proteins implicated in general cellular processes were identified. However, only one enzyme involved in alkaloid biosynthesis, COR, was detected. The localization of CYP80B1, BBE, and COR to sieve elements suggests that previous studies involving isolated latex were tainted by contamination from the phloem sap. Both the latex and phloem sap exhibit similar positive pressure; thus, the crude lancing of plant organs is certain to cause the exudation of unanchored proteins from both cell types. The absence of membrane-bound enzymes of alkaloid biosynthesis in isolated latex is consistent with this suggestion (Gerardy and Zenk, 1993a, 1993b).
Alkaloid Biosynthesis in Plants Involves Diverse Cell Types
Plant secondary metabolism and accumulation are associated with a diverse array of cell types, which are well represented in the biosynthesis of several distinct alkaloids. Particularly surprising are the alkaloid biosynthetic pathways in which individual enzymes reside in different cell types. Early enzymes of monoterpenoid indole alkaloid formation are localized to leaf epidermis in Catharanthus roseus, whereas late enzymes are found several cell layers away in laticifers and idioblasts, in which the products accumulate (St-Pierre et al., 1999). The first committed and last enzymes in tropane alkaloid biosynthesis in Atropa belladonna and Hyoscyamus niger occur in the pericycle, whereas an intermediate enzyme is found in the adjacent endodermis (Hashimoto et al., 1991; Nakajima and Hashimoto, 1999; Suzuki et al., 1999). Homospermidine synthase, the first step of the pyrrolizidine pathway in Senecio vernalis, is restricted to distinct groups of endodermis and neighboring cortical cells located opposite the phloem (Moll et al., 2002). Although their biosynthetic enzymes are restricted to roots, tropane and pyrrolizidine alkaloids are translocated systemically and accumulate in the cells of other organs (Hartmann et al., 1989; Hashimoto et al., 1991). Despite the evolutionary independence of these alkaloid biosynthetic pathways, the emerging paradigm clearly implicates multiple cell types and the intercellular translocation of pathway intermediates or products.
The involvement of multiple cell types and differential sites of product formation and accumulation also are features of benzylisoquinoline alkaloid biosynthesis in opium poppy. However, the localization of alkaloid biosynthetic enzymes to sieve elements and their corresponding gene transcripts to companion cells is unique among plant secondary metabolic pathways. No other biosynthetic pathway has been localized to sieve elements, which have been shown to possess only a limited number of enzymes. Sieve element cytoplasm typically includes only several hundred polypeptides, few of which have been identified (Kehr et al., 1999). Metabolites and enzymes associated with sieve elements include ascorbate and monodehydroascorbate reductases, which help to maintain an antioxidative environment (Walz et al., 2002), and glutathione, glutaredoxin, and glutathione reductase, which suggest the capacity for glutathione-dependent thiol reduction (Alosi et al., 1988; Szederkenyi et al., 1997). The ability of sieve elements to harbor a complex metabolic pathway has not seriously been considered. Nevertheless, the isolation of glutathione reductase (Alosi et al., 1988), mannitol dehydrogenase (Zamski et al., 1996), and other NAD(P)H-dependent enzymes (Walz et al., 2002) supports the catalytic functionality of COR in sieve elements.
The localization of benzylisoquinoline alkaloid biosynthesis to sieve elements in opium poppy demonstrates the unexpected metabolic competence of this unusual cell type. Our results extend the fundamental physiological role of sieve elements beyond the transport of solutes and information macromolecules. It is interesting to speculate on whether or not benzylisoquinoline alkaloid biosynthesis, in general, is localized to sieve elements or if other secondary metabolic pathways display similar cell type–specific localization. A more general role for these cell types could emerge as additional pathways are localized at the cellular level.
METHODS
Plant Material
Opium poppy (Papaver somniferum cv Marianne) plants were maintained in a growth chamber at 23°C with a photoperiod of 14 h. Plant organs were harvested 2 to 3 d after anthesis except for carpels, which were collected 3 to 5 d after anthesis.
Heterologous Expression and Purification of Proteins
CYP80B1 (Huang and Kutchan, 2000) and BBE1 (Facchini et al., 1996) open reading frames were inserted in frame into pET29 (Novagen, Madison, WI), and the constructs were introduced into Escherichia coli strain BL21(DE3). The COR (Unterlinner et al., 1999) open reading frame was inserted in frame into pRSET, and the constructs were introduced into E. coli strain ER2566 (New England Biolabs, Boston, MA). Heterologous expression was performed according to the pET29 manual. Briefly, 1 L of NZY broth (86 mM NaCl, 20 mM MgSO4, 5 mg/L yeast extract, and 10 mg/L casein hydrolysate) containing 50 mg/L kanamycin (pET29-BBE) or 25 mg/L ampicillin (pRSET-CYP80B1 and pRSET-COR) was inoculated with 5 mL of overnight bacterial culture and incubated at 37°C. At a density of OD600 = 0.5, the cultures were induced for 4 h with 400 μM isopropyl-β-d-thiogalactopyranoside. Cells were pelleted, resuspended in homogenization buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 10 μm phenylmethylsulfonyl fluoride [PMSF], and 5 mM 2-mercaptoethanol), and ruptured using a French press (Spectronic Instruments, Rochester, NY). Cell debris and protein inclusion bodies were recovered by centrifugation. The rinsed pellet was solubilized in homogenization buffer containing 6 M urea, and the solution was passed through a 0.20-μm filter. Recombinant proteins were affinity purified using a Ni2+-charged HiTrap column according to the manufacturer's instructions (Pharmacia Biotech).
Preparation of Antibodies
Antibodies were prepared from purified antigens using repeated subcutaneous injections as described by Harlow and Lane (1988). Antigen proteins were dialyzed against 146 mM NaCl, resuspended at a concentration of 400 μg/mL, emulsified 1:1 with Freund's complete adjuvant, and injected into mice (100 μL) or rabbits (500 μL). Preimmune sera were collected from each animal, and IgG fractions were purified using an Affi-Gel Protein A MAPSII Kit (Bio-Rad). Booster injections were performed every 3 weeks until a sufficient titer was achieved. Antibodies against BBE, CYP80B1, and COR were affinity-purified using purified protein immobilized on nitrocellulose membranes (Smith and Fisher, 1984). Sera were incubated with the immobilized antigen for 3 h, rinsed in TBST (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% [v/v] Tween 20), and eluted with 50 mM Gly buffer, pH 2.3. Purified IgGs were neutralized in 1 M Tris-HCl, pH 8.8, dialyzed against TBS (20 mM Tris-HCl, pH 7.5, and 150 mM NaCl) containing 0.2% (w/v) sodium azide, and concentrated using Centricon YM10 spin columns (Millipore, Bedford, MA).
Immunoblot Analysis
Plant tissues were frozen in liquid nitrogen and ground to a fine powder in the presence of 100 mg/g (fresh weight) polyvinyl polypyrrolidone. Tissues were suspended in extraction buffer (50 mM Tris-HCl, pH 7.5, 5 mM EDTA, 5 μM PMSF, and 5 mM 2-mercaptoethanol) and incubated on ice, and the supernatant was collected by centrifugation. Soluble proteins (25 μg) were fractionated by SDS-PAGE (Laemmli, 1970) and transferred to nitrocellulose membranes. Protein blots were incubated with 10 μg/mL CYP80B1, 5 μg/mL BBE, or 25 μg/mL COR antiserum for 3 h, washed in TBST, and incubated for 2 h with either alkaline phosphatase (AP)–conjugated anti-rabbit or anti-mouse secondary antibodies (Bio-Rad). The membranes were washed in TBST and developed in AP buffer (100 mM Tris-HCl, pH 9.5, 100 mM NaCl, and 5 mM MgCl2) containing 20 μM nitroblue tetrazolium and 20 μM 5-bromo-4-chloro-3-indolyl phosphate as substrates (Sambrook et al., 1989).
Tissue Fixation and Embedding for Immunocytochemical Localization
Tissue fixation and immunocytochemical localization were performed as described previously (Voznesenskaya et al., 1999). Briefly, tissues were immersed in fixation buffer (50 mM Pipes, pH 7.0, 1.25% [v/v] glutaraldehyde, 2% [v/v] paraformaldehyde, and 5 μM PMSF), cut with a razor blade into 1.5- to 2-mm sections, fixed for 2 h, and rinsed in 50 mM Pipes, pH 7.0, containing 5 μM PMSF. The tissues were dehydrated using a 30 to 100% (v/v) ethanol series with a 2-h incubation in each solution. After dehydration, LR White resin (London Resin Company, London, UK) was introduced into the ethanol series at an initial ratio of 1:4 (v/v) and gradually increased to 1:3, 1:2, 1:1, 2:1, and 3:1 (v/v). Finally, tissues were immersed in pure resin, cast into 1-mL gelatin capsules, and incubated at 60°C for 16 h. Sections were cut 1.0 μm thick using a Reichert-Jung Ultracut E microtome (Leica Microsystems, Wetzlar, Germany).
Tissue Fixation and Embedding for In Situ Hybridization
Organs were immersed in FAA (50% [v/v] ethanol, 5% [v/v] acetic acid, and 3.7% [v/v] formaldehyde), cut with a razor blade into 2- to 5-mm segments, and fixed overnight at 4°C. Tissues were dehydrated using an ethanol/tertiary butanol (t-butanol) series (4:1:5, 5:2:3, 5:3.5:1.5, 4.5:5.5:0, 2.5:7.5:0, and 0:1:0 ethanol:t-butanol:water) with a 2-h incubation in each solution except for the final step, which was overnight. Paraplast Plus (Oxford Labware, St. Louis, MO) was added to a paraffin infiltration series (1:1, 6.7:3.3, and 1:0 wax:t-butanol) with overnight incubations for each step. Embedded tissues were cut into 10-μm sections using an American Optical 620 microtome (Buffalo, NY). Sections were placed onto aminopropyltriethoxysilane-coated slides and incubated overnight at 37°C to promote the firm adhesion of sections to the slides.
Immunocytochemical Localization
Affinity-purified anti-BBE, anti-CYP80B1, and anti-COR IgGs were used at concentrations of 20 μg/mL, 45 μg/mL, and 35 μg/mL, respectively. The anti-MLP (Griffing and Nessler, 1989) IgG fraction was purified using the Affi-Gel Protein A MAPSII Kit (Bio-Rad) and used at a concentration of 20 μg/mL. A mouse monoclonal antibody specific to callose (Biosupplies, Parkville, Australia) was used at a concentration of 15 μg/mL. Tissue sections were incubated with primary antibodies for 2 h and rinsed three times in TBS containing 1% (w/v) BSA (BSA Fraction V; Roche Diagnostics, Mannheim, Germany) and twice in TBS for 10 min. Sections were incubated for 1 h with either Alexa 488–conjugated goat anti-mouse IgG or Alexa 594–conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR) and then rinsed in TBS and water. Slides were sealed using Aquaperm (ThermoShandon, Pittsburgh, PA).
In Situ Hybridization
In situ hybridization was performed as described by St-Pierre et al. (1999). Briefly, CYP80B1, BBE, COR, and MLP (Nessler and Vonder Haar, 1990) open reading frames in pBluescript SK− (Stratagene) served as templates for the synthesis of sense and antisense digoxigenin (DIG)-labeled RNA probes using T3 and T7 RNA polymerases. DIG-labeled probes were hydrolyzed at 60°C in 40 mM sodium carbonate buffer, pH 10, to produce fragments 200 to 400 nucleotides in length. The pH was neutralized using 10% (v/v) acetic acid, and the RNA was resuspended in 50 μL of deionized water.
Sections were deparaffinized and rehydrated using an ethanol series (1:0, 1:0, 9.5:0.5, 7:3, and 1:1 ethanol:water) with a 5-min incubation in each solution. Sections were incubated in prehybridization buffer (100 mM Tris-HCl, pH 8.0, and 50 mM EDTA) containing 5 μg/mL proteinase K (Roche Diagnostics) for 30 min and then blocked in TBS (10 mM Tris-HCl, pH 7.5, and 150 mM NaCl) containing 2 mg/mL Gly. Sections were postfixed in 3.7% (v/v) formaldehyde in PBS (100 mM sodium phosphate buffer, pH 7.2, and 140 mM NaCl), incubated in 100 mM triethanolamine buffer, pH 8.0, containing 0.25% (v/v) acetic anhydride, and finally rinsed in TBS. The slides were inverted onto 100 μL of hybridization buffer (10 mM Tris-HCl, pH 6.8, 10 mM sodium phosphate buffer, pH 6.8, 40% [v/v] deionized formamide, 10% [w/v] dextran sulfate, 300 mM NaCl, 5 mM EDTA, 1 mg/mL yeast tRNA, 500 ng/mL DIG-RNA, and 0.8 unit/mL RNase inhibitor [Invitrogen, Carlsbad, CA]) spread over a cover slip. Slides were sealed in a Petri dish lined with filter paper soaked in 50% (v/v) formamide and incubated overnight at 50°C.
Slides were immersed in 2× SSC (1× SSC = 300 mM NaCl and 30 mM sodium citrate, pH 7.0) at 37°C until the cover slips fell off. Sections were incubated in 50 mg/mL RNase A (Roche Diagnostics) in 500 mM NaCl, 10 mM Tris-HCl, pH 7.5, and 1 mM EDTA for 30 min at 37°C. Slides were washed in 2 L of the following solutions for 1 h: 2× SSC and 1× SSC at room temperature and 0.1× SSC at 60°C. Slides were rinsed in TBST and blocked for 1 h in TBST containing 2% (w/v) BSA. Slides were inverted onto cover slips carrying 100 μL of goat anti-DIG-AP conjugate (Roche Diagnostics) diluted 1:200 in TBST containing 1% (w/v) BSA and incubated for 2 h in sealed Petri dishes lined with filter paper soaked in TBST. After incubation, the slides were rinsed in TBST and AP buffer. Colorimetric development was performed in AP buffer containing 400 μM 5-bromo-4-chloro-3-indolyl phosphate and 428 μM nitroblue tetrazolium for 2 to 24 h.
Aniline Blue, Toluidine Blue O, and Calcofluor White Staining
Deparaffinized and rehydrated tissue sections were stained in 67 mM phosphate buffer, pH 8.5, containing 0.05% (w/v) aniline blue to detect callose. Sections were stained in 0.5% (w/v) calcofluor white to localize cell walls. For general anatomy, LR White sections were stained in benzoate buffer (10 mM sodium benzoate, pH 4.4) containing 0.1% (w/v) toluidine blue O.
Fluorescence and Light Microscopy
Immunofluorescence labeling was viewed using a Leica DM RXA2 microscope (Leica Microsystems, Wetzlar, Germany), and images were acquired with a Retiga EX digital camera (QImaging, Burnaby, British Columbia, Canada). Alexa 488 and Alexa 594 fluorescent labels were detected using Leica L5 and TX2 filters, respectively. Aniline blue and calcofluor white were detected using a Leica A1 filter. Deconvolution and false-color imaging was performed using Open Lab version 2.09 (Improvision, Coventry, UK). Light microscopy images were captured using the Leica microscope and the Retiga camera mounted with a RGB color liquid crystal filter (QImaging).
Upon request, materials integral to the findings of this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact P.J. Facchini, pfacchin@ucalgary.ca.
Acknowledgments
We thank Craig Nessler for the gift of the MLP cDNA and antibodies. P.J.F. is the Canada Research Chair in Plant Metabolic Processes Biotechnology. This work was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to P.J.F.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.015396.
References
- Alosi, M.C., Melroy, D.L., and Park, P.B. (1988). The regulation of gelatin of phloem exudate from Cucurbita fruit by dilution, glutathione, and glutathione reductase. Plant Physiol. 86, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, T., Masumura, T., Kusano, H., Kikuchi, S., Kurita, A., Shimada, H., and Kadowaki, K. (2002). Construction of a specialized cDNA library from plant cells isolated by laser capture microdissection: Toward comprehensive analysis of the genes expressed in the rice phloem. Plant J. 32, 401–408. [DOI] [PubMed] [Google Scholar]
- Balachandran, S., Xiang, Y., Schobert, C., Thompson, G.A., and Lucas, W.J. (1997). Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc. Natl. Acad. Sci. USA 94, 14150–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostwick, D.E., Dannenhoffer, J.M., Skaggs, M.I., Lister, R.M., Larkins, B.A., and Thompson, G.A. (1992). Pumpkin phloem lectin genes are specifically expressed in companion cells. Plant Cell 4, 1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, G., Wanner, G., Zenk, M.H., and Lottspeich, F. (2000). Characterization of proteins in latex of the opium poppy (Papaver somniferum) using two-dimensional gel electrophoresis and microsequencing. Electrophoresis 21, 3500–3516. [DOI] [PubMed] [Google Scholar]
- Ehlers, K., Knoblauch, M., and van Bel, A.J.E. (2000). Ultrastructural features of well-preserved and injured sieve elements: Minute clamps keep the phloem transport conduits free for mass flow. Protoplasma 214, 80–92. [Google Scholar]
- El-Ahmady, S.H., and Nessler, C.L. (2001). Cellular localization of tyrosine decarboxylase expression in transgenic opium poppy and tobacco. Plant Cell Rep. 20, 313–317. [Google Scholar]
- Facchini, P.J. (2001). Alkaloid biosynthesis in plants: Biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 29–66. [DOI] [PubMed] [Google Scholar]
- Facchini, P.J., and De Luca, V. (1995). Phloem-specific expression of tyrosine/dopa decarboxylase genes and the biosynthesis of isoquinoline alkaloids in opium poppy. Plant Cell 7, 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini, P.J., Penzes, C., Johnson, A.G., and Bull, D. (1996). Molecular characterization of berberine bridge enzyme genes from opium poppy. Plant Physiol. 112, 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn, J.W., and Wassel, G. (1964). The alkaloids of Papaver somniferum L.: Biosynthesis in isolated latex. Phytochemistry 3, 583–585. [Google Scholar]
- Fairbairn, J.W., Djote, M., and Paterson, A. (1968). The alkaloids of Papaver somniferum L. VII. Biosynthetic activity of the isolated latex. Phytochemistry 7, 2111–2116. [Google Scholar]
- Fisher, D.B. (1990). Measurement of phloem transport rates by an indicator-dilution technique. Plant Physiol. 94, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardy, R., and Zenk, M.H. (1993. a). Purification and characterization of salutaridine:NADPH 7-oxidoreductase from Papaver somniferum. Phytochemistry 34, 125–132. [Google Scholar]
- Gerardy, R., and Zenk, M.H. (1993. b). Formation of salutaridine from (R)-reticuline by a membrane-bound cytochrome P-450 enzyme from Papaver somniferum. Phytochemistry 32, 79–86. [Google Scholar]
- Griffing, L.R., and Nessler, C.L. (1989). Immunolocalization of the major latex proteins in developing laticifers of opium poppy (Papaver somniferum). J. Plant Physiol. 134, 357–363. [Google Scholar]
- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Hartmann, T., Ehmke, A., Eilert, U., Von Borstel, K., and Theuring, C. (1989). Sites of synthesis, translocation and accumulation of pyrrolizidine alkaloid N-oxides in Senecio vulgaris L. Planta 177, 98–107. [DOI] [PubMed] [Google Scholar]
- Hashimoto, T., Hayashi, A., Amano, Y., Kohno, J., Iwanari, H., Usuda, S., and Yamada, Y. (1991). Hyoscyamine 6β-hydroxylase, an enzyme involved in tropane alkaloid biosynthesis, is localized at the pericycle of the root. J. Biol. Chem. 266, 4648–4653. [PubMed] [Google Scholar]
- Huang, F.-C., and Kutchan, T.M. (2000). Distribution of morphinan and benzo[c]phenanthridine alkaloid gene transcript accumulation in Papaver somniferum. Phytochemistry 53, 555–564. [DOI] [PubMed] [Google Scholar]
- Kehr, J., Haebel, S., Blechschmidt-Schneider, S., Willmitzer, L., Steup, M., and Fisahn, J. (1999). Analysis of phloem protein patterns from different organs of Cucurbita maxima Duch. by matrix-assisted laser desorption/ionization time of flight mass spectroscopy combined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Planta 207, 612–619. [DOI] [PubMed] [Google Scholar]
- Knoblauch, M., and van Bel, A.J.E. (1998). Sieve elements in action. Plant Cell 10, 35–50. [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Moll, S., Anke, S., Kahmann, U., Hansch, R., Hartmann, T., and Ober, D. (2002). Cell-specific expression of homospermidine synthase, the entry enzyme of the pyrrolizidine alkaloid pathway in Senecio vernalis, in comparison with its ancestor, deoxyhypusine synthase. Plant Physiol. 130, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima, K., and Hashimoto, T. (1999). Two tropinone reductases, that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 40, 1099–1107. [DOI] [PubMed] [Google Scholar]
- Nessler, C.L., Allen, R.D., and Galewsky, S. (1985). Identification and characterization of latex-specific proteins in opium poppy. Plant Physiol. 79, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler, C.L., and Mahlberg, P.G. (1977). Ontogeny and cytochemistry of alkaloidal vesicles in laticifers of Papaver somniferum L. (Papaveraceae). Am. J. Bot. 64, 541–551. [Google Scholar]
- Nessler, C.L., and Vonder Haar, R.A. (1990). Cloning and expression analysis of DNA sequences for the major latex protein of opium poppy. Planta 180, 487–491. [DOI] [PubMed] [Google Scholar]
- Oparka, K.J., and Turgeon, R. (1999). Sieve elements and companion cells: Traffic control centers of the phloem. Plant Cell 11, 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli, H.H., and Kutchan, T.M. (1998). Molecular cloning and functional heterologous expression of two alleles encoding (S)-N-methylcoclaurine 3′-hydroxylase (CYP80B1), a new methyl jasmo- nate-inducible cytochrome P-450-dependent mono-oxygenase of benzylisoquinoline alkaloid biosynthesis. Plant J. 13, 793–801. [DOI] [PubMed] [Google Scholar]
- Roberts, M.F., McCarthy, D., Kutchan, T.M., and Coscia, C.J. (1983). Localization of enzymes and alkaloidal metabolites in Papaver latex. Arch. Biochem. Biophys. 222, 599–609. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Shitan, N., Bazin, I., Dan, K., Obata, K., Kigawa, K., Ueda, K., Sato, F., Forestier, C., and Yazaki, K. (2003). Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc. Natl. Acad. Sci. USA 100, 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.E., and Fisher, P.A. (1984). Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: Application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J. Cell Biol. 99, 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.M., and McCully, M.E. (1978). A critical evaluation of the specificity of aniline blue-induced fluorescence. Protoplasma 95, 229–254. [Google Scholar]
- St-Pierre, B., Vazquez-Flota, F.A., and De Luca, V. (1999). Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K., Yamada, Y., and Hashimoto, T. (1999). Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 40, 289–297. [DOI] [PubMed] [Google Scholar]
- Szederkenyi, J., Komor, E., and Schobert, C. (1997). Cloning of the cDNA for glutaredoxin, an abundant sieve-tube exudate protein from Ricinus communis L., and characterisation of the glutathione-dependent thiol-reduction system in sieve tubes. Planta 202, 349–356. [DOI] [PubMed] [Google Scholar]
- Thureson-Klein, Å. (1970). Observations on the development and fine structure of the articulated laticifers of Papaver somniferum. Ann. Bot. 34, 751–759. [Google Scholar]
- United Nations (2002a). Narcotic Drugs Estimated World Requirements 2003. Report No. E/F/S.03.XI.2. (Geneva, Switzerland: International Narcotics Control Board, United Nations).
- United Nations (2002b). Global Illicit Drug Trends 2002. Report No. E.02.XI.9. (Geneva, Switzerland: International Narcotics Control Board, United Nations).
- Unterlinner, B., Lenz, R., and Kutchan, T.M. (1999). Molecular cloning and functional expression of codeinone reductase: The penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum. Plant J. 18, 465–475. [DOI] [PubMed] [Google Scholar]
- Voznesenskaya, E.V., Franceschi, V.R., Pyankov, V.I., and Edwards, G.E. (1999). Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae). J. Exp. Bot. 50, 1779–1795. [Google Scholar]
- Walz, C., Juenger, M., Schad, M., and Kehr, J. (2002). Evidence for the presence and activity of a complete antioxidant defence system in mature sieve tubes. Plant J. 31, 189–197. [DOI] [PubMed] [Google Scholar]
- Wilson, M.L., and Coscia, C.J. (1975). Studies on the early stages of Papaver alkaloid biogenesis. J. Am. Chem. Soc. 97, 431–432. [DOI] [PubMed] [Google Scholar]
- Zamski, E., Yamamoto, Y.T., Williamson, J.D., Conkling, M.A., and Pharr, D.M. (1996). Immunolocalization of mannitol dehydrogenase in celery plants and cells. Plant Physiol. 112, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]