Abstract
Two genes (DGD1 and DGD2) are involved in the synthesis of the chloroplast lipid digalactosyldiacylglycerol (DGDG). The role of DGD2 for galactolipid synthesis was studied by isolating Arabidopsis T-DNA insertional mutant alleles (dgd2-1 and dgd2-2) and generating the double mutant line dgd1 dgd2. Whereas the growth and lipid composition of dgd2 were not affected, only trace amounts of DGDG were found in dgd1 dgd2. The growth and photosynthesis of dgd1 dgd2 were affected more severely compared with those of dgd1, indicating that the residual amount of DGDG in dgd1 is crucial for normal plant development. DGDG synthesis was increased after phosphate deprivation in the wild type, dgd1, and dgd2 but not in dgd1 dgd2. Therefore, DGD1 and DGD2 are involved in DGDG synthesis during phosphate deprivation. DGD2 was localized to the outer side of chloroplast envelope membranes. Like DGD2, heterologously expressed DGD1 uses UDP-galactose for galactosylation. Galactolipid synthesis activity for monogalactosyldiacylglycerol (MGDG), DGDG, and the unusual oligogalactolipids tri- and tetragalactosyldiacylglycerol was detected in isolated chloroplasts of all mutant lines, including dgd1 dgd2. Because dgd1 and dgd2 carry null mutations, an additional, processive galactolipid synthesis activity independent from DGD1 and DGD2 exists in Arabidopsis. This third activity, which is related to the Arabidopsis galactolipid:galactolipid galactosyltransferase, is localized to chloroplast envelope membranes and is capable of synthesizing DGDG from MGDG in the absence of UDP-galactose in vitro, but it does not contribute to net galactolipid synthesis in planta.
INTRODUCTION
Biological membranes are essential for all living organisms because they are the structural basis for the boundary between the environment and the interior of the cell. Furthermore, membranes are critical for subcellular compartmentation in eukaryotic cells (i.e., the establishment of organellar structures such as the nucleus, mitochondria, and chloroplasts). In contrast to animals and yeast, in which phospholipids are very abundant, plants contain high amounts of glycolipids, which carry a sugar moiety in the head group (Browse and Somerville, 1994; Vijayan et al., 1998). The sulfolipid sulfoquinovosyldiacylglycerol (SQDG) and the two galactolipids monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) are present in all higher plants and are particularly enriched in chloroplasts (Joyard et al., 1998; Dörmann and Benning, 2002). Recent studies indicate that the amounts of the lipids SQDG and DGDG increase strongly during phosphate deprivation (Essigmann et al., 1998; Härtel et al., 2000). When phosphate is limiting, phospholipids in plant membranes are reduced and at least in part replaced by glycolipids (i.e., SQDG and DGDG). This change in lipid composition is not restricted to plastids, because during phosphate deprivation, DGDG was found to accumulate in extraplastidic membranes (Härtel et al., 2000). In oat roots deprived of phosphate, the amount of DGDG can increase to levels as high as 70% of total lipids in plasma membranes (Andersson et al., 2003).
In addition to serving as a surrogate lipid for phospholipids, galactolipids were found to be critical for the stabilization of photosynthetic complexes in the thylakoids (for review, see Dörmann and Benning, 2002). An Arabidopsis mutant, dgd1, which is strongly decreased in DGDG, was found to be affected in growth and photosynthetic efficiency (Dörmann et al., 1995). Another mutant, mgd1, which carries a mutation in one of the three MGDG synthase genes, is reduced severely in the amount of chlorophyll and contains chloroplasts with altered thylakoid ultrastructure (Jarvis et al., 2000). Two genes (DGD1 and DGD2) that encode functional DGDG synthases have been described in Arabidopsis (Dörmann et al., 1999; Kelly and Dörmann, 2002). Although DGD1 is involved in the synthesis of the predominant fraction of DGDG for chloroplast membranes, the in vivo role of DGD2 has remained unclear.
Previously, DGDG synthesis in plants was attributed to an enzymatic activity termed galactolipid:galactolipid galactosyltransferase (GGGT), which catalyzes the transfer of one galactose from one molecule of MGDG to another, thereby generating DGDG and diacylglycerol (van Besouw and Wintermans, 1978; Heinz and Roughan, 1983; Heemskerk et al., 1990). In in vitro assays with chloroplast membranes, this enzyme was found to synthesize DGDG and the unusual oligogalactolipids trigalactosyldiacylglycerol (TriGDG) and tetragalactosyldiacylglycerol (TetraGDG) (Dorne et al., 1982; Cline and Keegstra, 1983; Heemskerk et al., 1986). The unusual reaction mechanism of the GGGT activity is in contrast to that of DGD2, which was shown to use UDP-galactose instead of MGDG as the galactose donor for galactosylation (Kelly and Dörmann, 2002). At present, the relationship of GGGT to the other enzymes of galactolipid synthesis is not clear.
The regulation of galactolipid synthesis, including DGDG production for chloroplast membranes and for extraplastidic membranes as well as the synthesis of DGDG during phosphate deficiency, requires a complex network of interacting proteins. To elucidate the roles of the two DGDG synthases (DGD1 and DGD2) and to identify additional factors involved in galactolipid synthesis, mutant alleles deficient in the DGDG synthase DGD2 were isolated, taking advantage of the availability of the recently established T-DNA mutant collections of Arabidopsis.
RESULTS
Isolation of Arabidopsis Mutant Alleles Carrying T-DNA Insertions in DGD2
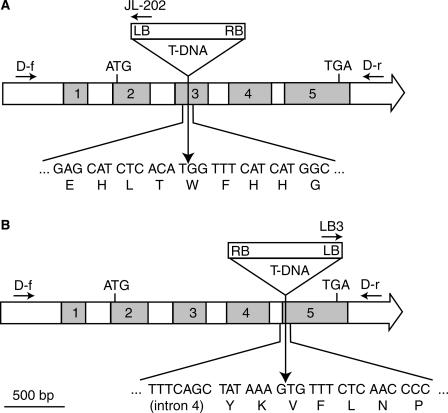
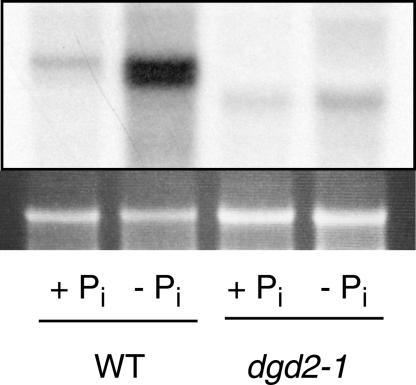
To study the role of the DGDG synthase DGD2 for galactolipid synthesis in Arabidopsis, a reverse genetics approach was chosen. A plant carrying a T-DNA insertion into the DGD2 gene on chromosome 4 (dgd2-1) was identified by PCR screening of the T-DNA insertional mutant population of the University of Wisconsin (Krysan et al., 1999; Sussman et al., 2000). A plant homozygous for the mutation was obtained from a segregating population by PCR analysis. Sequencing of a PCR fragment encompassing the T-DNA/genomic DNA junction revealed that dgd2-1 carries an insertion into the coding sequence (third exon) of DGD2 (Figure 1A). DGD2 mRNA abundance in the homozygous dgd2-1 mutant was reduced strongly. In the wild type, a DGD2 mRNA band of 1.6 kb was observed, but this band was absent from dgd2-1 (Figure 2). In dgd2-1, a weak signal of lower molecular mass (∼1.2 kb) was detected, possibly derived from a truncated mRNA fragment. It has been shown that DGD2 expression is induced during growth under phosphate-limiting conditions (Kelly and Dörmann, 2002). The induction of mRNA expression was estimated to be at least 30-fold by comparing the signal intensities of DGD2 expression in wild-type seedlings grown with 1000 μM or no phosphate (calculated from data of Kelly and Dörmann, 2002). However, even during phosphate deprivation, no mRNA band at the correct molecular mass was observed for DGD2 in dgd2-1 (Figure 2).
Figure 1.
The Two Mutant Alleles dgd2-1 and dgd2-2 Carry T-DNA Insertions in DGD2 on Chromosome 4 of Arabidopsis.
DGD2 is composed of five exons. The locations of the start and stop codons and of the two gene-specific primers (D-f, DGD2-forward; D-r, DGD2-reverse) and the T-DNA border primers (JL-202 and LB3) are shown. Vertical arrows indicate the positions of the T-DNA insertions into the genome as deduced from sequencing of the respective junctions. LB, left border; RB, right border.
(A) The mutant dgd2-1, obtained by PCR screening of a T-DNA mutant collection (University of Wisconsin), carries an insertion into the third exon of DGD2.
(B) A second allele (dgd2-2) was identified in the mutant population from the Torrey Mesa Research Institute. This plant carries a T-DNA in the fifth exon of DGD2.
Figure 2.
DGD2 mRNA Abundance Is Reduced Strongly in dgd2-1.
Total RNA isolated from dgd2-1 leaves was separated and transferred to a nylon membrane before hybridization to a DGD2 cDNA probe. For the wild type (WT), an mRNA band of 1.6 kb was identified, but for dgd2-1, only a weak signal of lower molecular mass was detected. Induction of DGD2 expression by phosphate deprivation (−Pi) was observed for the wild type but not for dgd2-1. The top gel shows the hybridization signal, and the bottom gel shows the ethidium bromide–stained 25S rRNA bands as loading controls.
A second allele (dgd2-2) was identified in the mutant collection of the Torrey Mesa Research Institute/Syngenta (Sessions et al., 2002). A dgd2-2 mutant homozygous for the insertion was obtained in a segregating population by PCR analysis. Sequencing of the PCR fragment revealed that the T-DNA was inserted into exon 5, thereby disrupting the open reading frame of DGD2 (Figure 1B).
The Double Mutant dgd1 dgd2 Is Reduced in Growth and Photosynthetic Efficiency
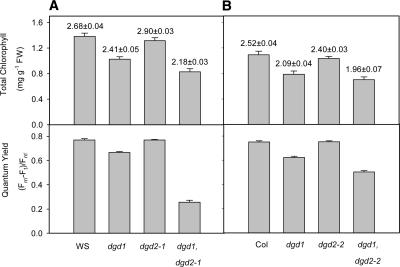
The findings that DGD1 is involved in the synthesis of the predominant fraction of DGDG in Arabidopsis (Dörmann et al., 1995) and that DGD2 expression is very low in the wild type (Kelly and Dörmann, 2002) already suggested that DGD2 is not essential for polar lipid synthesis under optimal growth conditions. Consequently, the growth and development of homozygous dgd2-1 and dgd2-2 plants were not altered compared with those of the wild type (Figure 3). To completely disrupt DGDG synthesis in Arabidopsis, dgd1 was crossed with dgd2-1 or dgd2-2. Arabidopsis dgd1 represents a null mutation of DGD1, because it carries a point mutation resulting in the introduction of a premature stop codon into the open reading frame (Dörmann et al., 1999). Double homozygous plants were strongly reduced in growth, and many of them did not survive on soil for a longer time. The growth of dgd1 dgd2-1 and dgd1 dgd2-2 plants was affected more severely compared with that of the dgd1 single mutant (Figure 3).
Figure 3.
Growth of DGDG-Deficient Arabidopsis Mutants Is Reduced.
Forty-day-old plants raised on soil are shown. Although dgd1 is affected strongly in terms of growth, the growth of dgd2-1 and dgd2-2 plants is similar to that of the wild type (ecotypes Wassilewskija [WS] and Columbia [Col], respectively). The growth of the double mutant alleles dgd1 dgd2-1 and dgd1 dgd2-2 is affected more severely compared with that of the dgd1 single mutant.
The loss of a large fraction of DGDG in dgd1 indicated that DGDG is critical to sustain a normal rate of photosynthesis (Dörmann et al., 1995). Total chlorophyll and quantum yield (as determined from chlorophyll fluorescence) were measured for dgd2-1, dgd2-2, and the double mutant lines dgd1 dgd2-1 and dgd1 dgd2-2 (Figure 4). No differences in chlorophyll content and chlorophyll fluorescence were observed for dgd2-1 and dgd2-2. However, the amount of total chlorophyll and the photosynthetic quantum yield were affected more severely in the double mutant lines compared with dgd1. Therefore, DGDG synthesized via the DGD2 pathway is important to support normal growth and photosynthesis in Arabidopsis.
Figure 4.
Photosynthesis Is Affected in the DGDG-Deficient Mutants dgd1 and dgd1 dgd2-1.
The top graphs show chlorophyll content in leaves of wild-type Wassilewskija (WS), dgd1, dgd2-1, and dgd1 dgd2-1 (A) and wild-type Columbia (Col), dgd1, dgd2-2, and dgd1 dgd2-2 (B). The numbers indicate the chlorophyll a/b ratio. Values represent means and standard errors of three measurements of at least seven plants each. The bottom graphs show photosynthetic quantum yield calculated from the in vivo chlorophyll fluorescence of light-adapted plants (see Methods for units). Values are derived from means and standard errors of at least 15 measurements. FW, fresh weight.
The Two DGDG Synthases, DGD1 and DGD2, Are Critical for DGDG Synthesis during Phosphate Deprivation
To study the impact of the dgd2 mutation on galactolipid synthesis, polar lipids were extracted from leaves of different Arabidopsis lines and lipid classes were quantified (Table 1). The amount of DGDG was very similar in the wild type and dgd2-1, and no differences in the amounts of the other polar lipids, including MGDG, were found. The residual amount of DGDG in dgd1 (∼1.3 mol %; Table 1) (Dörmann et al., 1995) was reduced even further in dgd1 dgd2-1 (i.e., to <0.5 mol %, which is in the range of the detection limit for lipids using this quantification protocol). Similar to dgd1, the relative amounts of the phospholipids and of sulfolipid in dgd1 dgd2-1 were increased in the double mutant. The amount of MGDG was reduced slightly in dgd1 dgd2-1.
Table 1.
Lipid Composition in Leaves of the Wild Type and DGDG Synthase Mutants
| Wild Type
|
dgd1
|
dgd2-1
|
dgd1 dgd2-1
|
|||||
|---|---|---|---|---|---|---|---|---|
| Lipid | + Pi | − Pi | + Pi | − Pi | + Pi | − Pi | + Pi | − Pi |
| MGDG | 48.9 | 45.5 | 45.8 | 44.1 | 44.0 | 47.2 | 38.2 | 39.6 |
| DGDG | 14.5 | 26.3 | 1.3 | 9.9 | 15.9 | 22.4 | – a | – |
| PG | 8.6 | 8.9 | 10.4 | 9.9 | 10.4 | 8.3 | 12.6 | 10.6 |
| SQDG | 1.6 | 4.4 | 1.9 | 3.4 | 2.1 | 2.6 | 4.6 | 6.5 |
| PE | 6.7 | 4.0 | 13.4 | 10.0 | 9.4 | 5.5 | 15.3 | 14.6 |
| PC | 19.7 | 10.8 | 27.2 | 22.7 | 18.2 | 13.9 | 29.3 | 28.7 |
Lipids were quantified by thin layer chromatography/gas chromatography. The values are given in mol % and represent means of three measurements. Standard deviations were all <2 mol %. PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol.
–, not detectable (i.e., <0.5 mol %).
Phosphate deficiency is known to result in an increase in DGDG in Arabidopsis wild-type and dgd1 plants (Essigmann et al., 1998; Härtel et al., 2000; Klaus et al., 2002). This result and the finding that DGD2 expression is induced during phosphate deficiency (Kelly and Dörmann, 2002) suggested that DGD2 is crucial for the synthesis of additional DGDG during phosphate deprivation. Seedlings of the wild type and of the different mutant lines were incubated under phosphate-limiting conditions and used for polar lipid quantification (Table 1). DGDG was increased by 8 to 12 mol % in the wild type and dgd1. A similar increase of DGDG was detected in dgd2-1 but not in dgd1 dgd2-1. This result demonstrated that in addition to DGD2, DGD1 also contributes to DGDG synthesis during phosphate deprivation. Because no increase in DGDG was detected in the double mutant, DGDG synthesis in phosphate-limiting conditions was mediated by only two genes, DGD1 and DGD2.
The fatty acid composition of MGDG in dgd2-1 and the wild type, as well as that of dgd1 dgd2-1 and dgd1, was very similar (Table 2). In addition, the fatty acid composition of DGDG from dgd2-1 was not changed compared with that in the wild type, reflecting the fact that DGD2 does not contribute significantly to DGDG synthesis under optimal growth conditions. Previously, it was shown that DGDG isolated from dgd1 and the wild type grown under phosphate limitation is particularly enriched in palmitic acid and that most of it is localized to the sn-1 position of the glycerol backbone (Härtel et al., 2000) (Table 3). This is reflected by a high C16:C18 fatty acid ratio of ∼0.50 and a constant C16 content in DGDG of dgd1 and wild-type plants grown under phosphate limitation (Table 2) (Härtel et al., 2000; Klaus et al., 2002). In contrast to that in the wild type, the C16:C18 ratio in DGDG of dgd2-1 did not increase during phosphate limitation (0.23 with Pi [+Pi] and 0.21 without Pi [−Pi]), demonstrating that DGD2 is responsible for the synthesis of DGDG molecular species rich in C16 at sn-1 during phosphate deprivation. Furthermore, the fatty acid composition and positional distribution of DGDG in dgd2-1 shows that, in contrast to DGD2, DGD1 is involved in the synthesis of a DGDG molecular species rich in C18 fatty acids during optimal and phosphate-deficient conditions (Tables 2 and 3).
Table 2.
Fatty Acid Composition of Galactolipids in the Wild Type and DGDG Synthase Mutants
| Wild Type
|
dgd1
|
dgd2-1
|
dgd1
dgd2-1
|
|||||
|---|---|---|---|---|---|---|---|---|
| Lipid | + Pi | − Pi | + Pi | − Pi | + Pi | − Pi | + Pi | − Pi |
| MGDG | ||||||||
| 16:0 | 2.1 | 4.3 | 4.4 | 10.0 | 1.9 | 3.9 | 5.5 | 7.2 |
| 16:1 | 1.4 | 1.6 | 1.7 | 1.9 | 1.7 | 1.6 | 2.1 | 1.5 |
| 16:2 | 0.5 | 0.5 | 0.6 | 0.5 | 0.2 | 0.3 | 0.4 | 0.4 |
| 16:3 | 30.9 | 26.4 | 13.7 | 4.3 | 31.2 | 26.6 | 9.9 | 6.3 |
| 18:0 | 0.7 | 1.1 | 0.3 | 0.4 | 0.2 | 0.5 | 0.4 | 0.4 |
| 18:1 | 0.1 | 0.1 | 0.2 | 0.7 | 0.7 | 0.9 | 0.5 | 0.4 |
| 18:2 | 2.3 | 2.9 | 2.0 | 2.8 | 2.4 | 3.9 | 1.6 | 2.0 |
| 18:3 | 61.7 | 62.8 | 76.9 | 79.0 | 61.5 | 62.1 | 79.4 | 81.5 |
| C16:C18 | 0.54 | 0.49 | 0.26 | 0.20 | 0.54 | 0.48 | 0.22 | 0.18 |
| DGDG | ||||||||
| 16:0 | 16.4 | 26.9 | 26.0 | 29.8 | 14.1 | 13.7 | – a | – |
| 16:1 | 2.1 | 2.9 | 4.2 | 2.3 | 1.6 | 1.4 | – | – |
| 16:2 | 0.9 | 0.4 | 1.6 | 0.3 | 1.0 | 0.5 | – | – |
| 16:3 | 1.8 | 0.9 | 3.4 | 0.9 | 2.0 | 1.6 | – | – |
| 18:0 | 2.3 | 3.3 | 3.7 | 5.3 | 1.5 | 1.3 | – | – |
| 18:1 | 0.1 | 0.1 | 6.4 | 4.9 | 1.1 | 1.0 | – | – |
| 18:2 | 4.1 | 6.7 | 9.2 | 12.5 | 3.5 | 4.2 | – | – |
| 18:3 | 71.7 | 58.4 | 45.2 | 44.0 | 74.7 | 76.1 | – | – |
| C16:C18 | 0.27 | 0.45 | 0.55 | 0.50 | 0.23 | 0.21 | – | – |
Lipids were isolated by thin layer chromatography, and fatty acid content was measured by gas chromatography of methyl esters. Values are given as mol % and represent means of three measurements. Standard deviations were all <2 mol %. C16:C18 indicates the ratio of total C16 fatty acids to total C18 fatty acids.
–, not detectable (i.e., <0.5 mol %).
Table 3.
Fatty Acid Positional Distribution in Galactolipids of dgd2-1
| Wild Type
|
dgd2-1
|
|||
|---|---|---|---|---|
| Lipid | + Pi | − Pi | + Pi | − Pi |
| MGDG | ||||
| C16 | 74.1 ± 1.3 | 57.0 ± 0.8 | 68.0 ± 1.0 | 59.8 ± 0.7 |
| C18 | 25.1 ± 1.6 | 41.8 ± 1.2 | 31.8 ± 1.0 | 39.1 ± 1.0 |
| DGDG | ||||
| C16 | 17.4 ± 2.2 | 16.0 ± 5.1 | 16.7 ± 0.5 | 17.1 ± 0.9 |
| C18 | 80.6 ± 2.9 | 82.3 ± 4.8 | 82.7 ± 0.6 | 82.3 ± 0.9 |
Plants raised in the presence or absence of phosphate were used for lipid isolation by thin layer chromatography. Positional analysis of fatty acids was performed after lipase treatment and separation by thin layer chromatography via gas chromatography of methyl esters. Values represent the total C16 and C18 fatty acid composition of lyso-lipids (sn-2 position) and are given as mol % of the means ± sd of three measurements.
DGD1 Encodes a UDP-Galactose–Dependent DGDG Synthase Specific for α-Glycosidic Bonds and Is Induced by Phosphate Deficiency
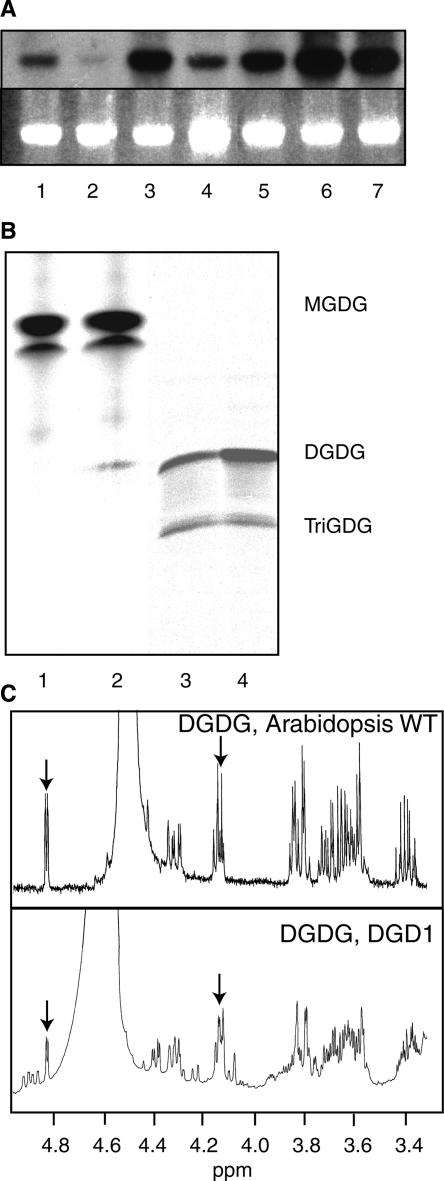
To corroborate the finding that the increase in DGDG synthesis in dgd2-1 during phosphate deficiency was caused by the induction of DGD1 expression, RNA gel blot analysis was performed with total RNA isolated from the wild type, dgd1, and pho1 (Figure 5A). The pho1 mutant is affected in the transport of phosphate from the root to the shoot, and for this reason, the shoot of pho1 is permanently phosphate deficient. In addition, RNA from wild-type plants grown in the presence of different amounts of phosphate was included. The DGD1 band was induced strongly in pho1 and in wild-type plants deprived of phosphate. Induction of DGD1 expression was calculated to be approximately sixfold compared with the signal intensities of wild-type seedlings grown in the presence of 1000 and 0 μM phosphate (Figure 5A). The lower induction value of DGD1 compared with DGD2 (30-fold; see above) (Kelly and Dörmann, 2002) during phosphate deprivation can be explained by the higher basal expression level of DGD1 in normal growth conditions. In conclusion, DGD1 and DGD2 expression as well as DGDG synthesis mediated by the two DGDG synthases are induced during phosphate deprivation.
Figure 5.
DGD1 Is Induced by Phosphate Deficiency in Arabidopsis; Heterologously Expressed DGD1 Protein Transfers Galactose from UDP-Galactose onto MGDG under Retention on the α-Anomeric Bond.
(A) DGD1 gene expression is induced during phosphate deficiency. The wild type (lane 1), dgd1 (lane 2), and the phosphate transport–deficient mutant pho1 (lane 3) were raised on soil; wild-type seedlings were grown in tissue culture in the presence of different amounts of phosphate (lane 4, 1000 μM; lane 5, 100 μM; lane 6, 10 μM; and lane 7, 0 μM). Total RNA was isolated and used for RNA gel blot hybridization with a DGD1 cDNA probe. Top gel, hybridization signal; bottom gel, 25S rRNA (stained with ethidium bromide).
(B) Heterologously expressed DGD1 encodes a UDP-galactose–dependent DGDG synthase. The C-terminal part of DGD1 (C-DGD1) is not capable of synthesizing DGDG from 14C-MGDG alone (lane 1) but requires the addition of nonradioactive UDP-galactose (lane 2). DGD2 (lane 3) and ND1D2 (lane 4; a chimeric fusion of the N-terminal part of DGD1 and DGD2) both incorporate radioactivity from UDP-14C-galactose into DGDG and TriGDG.
(C) Heterologously expressed DGD1 produces DGDG with an α-glycosidic linkage. 1H-NMR spectra were recorded for DGDG isolated from Arabidopsis wild-type (WT) leaves (top graph) or an E. coli culture expressing cucumber MGD1 and Arabidopsis DGD1 (bottom graph). The arrows indicate peak doublets characteristic of the α-glycosidic (4.8 ppm) and β-glycosidic (4.1 ppm) carbon atoms of the galactose moieties in the head group.
DGD1 was shown to encode a functional DGDG synthase by coexpression in Escherichia coli with cucumber MGDG synthase (Dörmann et al., 1999). However, attempts to measure in vitro DGDG synthesis activity for heterologously expressed DGD1 with radioactive substrates were not successful, presumably because DGD1 accumulation is toxic for E. coli (data not shown). DGD1 contains a long N-terminal extension (∼40 kD) that is required for insertion into the outer envelope and a C-terminal part with sequence similarity to glycosyltransferases (Froehlich et al., 2001). The C-terminal part of DGD1 (C-DGD1) was expressed separately in E. coli, and DGDG synthesis activity was measured with radioactive 14C-MGDG (Figure 5B). However, only after the addition of nonradioactive UDP-galactose was DGDG synthesis detected, indicating that heterologously expressed C-DGD1 encodes a UDP-galactose–dependent DGDG synthase. To elucidate the influence of the N-terminal part of DGD1 (N-DGD1) on galactolipid synthesis, this part of the protein was cloned as an N-terminal fusion with DGD2, resulting in the chimeric construct ND1D2. It was shown previously that DGD2 represents a processive, UDP-galactose–dependent DGDG synthase (Kelly and Dörmann, 2002). No differences in galactolipid synthesis were detected between DGD2 and ND1D2, because the two recombinant enzymes were capable of incorporating radioactivity from UDP-14C-galactose into DGDG and TriGDG (Figure 5B). This result shows that N-DGD1 has no influence on the enzymatic characteristics of galactolipid synthesis in the heterologous E. coli system.
In Arabidopsis wild-type leaf DGDG, the second and first galactose moieties are linked via α- and β-glycosidic bonds, respectively (Carter et al., 1956). To analyze anomeric configuration in DGDG produced by DGD1, DGDG was isolated from E. coli cells coexpressing cucumber MGD1 and Arabidopsis DGD1 (Dörmann et al., 1999) and used for 1H-NMR spectroscopy. As with Arabidopsis leaf DGDG, two characteristic peaks were detected for DGDG produced by DGD1 at 4.8 ppm (α-glycosidic bond) and 4.1 ppm (β-glycosidic bond) (Kojima et al., 1990) (Figure 5C). Thus, the second galactose in DGD1-dependent DGDG is linked in α-configuration. In conclusion, DGD1 and DGD2 both represent UDP-galactose–dependent galactosyltransferases specific for α-glycosidic linkage that are induced during phosphate deprivation (Figure 5) (Kelly and Dörmann, 2002). Therefore, these two DGDG synthases can be distinguished clearly from the previously described GGGT, which was shown to use MGDG as the galactose donor and acceptor and which is independent of UDP-galactose (Heemskerk et al., 1990).
DGD2 Is Localized to the Outer Side of Chloroplast Envelope Membranes
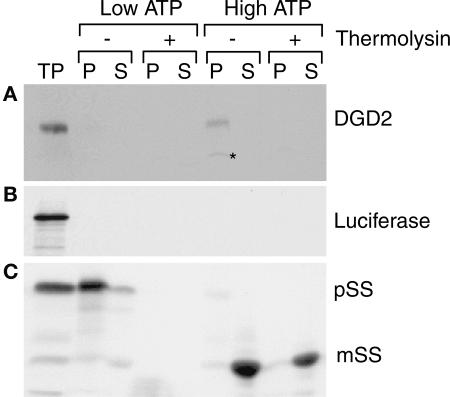
The DGD2 cDNA codes for a polypeptide with a predicted size of 53,906 D (Kelly and Dörmann, 2002). Because this DGDG synthase supposedly is involved in the synthesis of DGDG for extraplastidic membranes, it was important to determine whether the protein localizes to chloroplast membranes. Analysis of the N-terminal sequence did not confirm the prediction of the presence of a chloroplast targeting sequence (data not shown). Thus, import experiments with isolated pea chloroplasts were performed to determine the subcellular localization of DGD2 (Figure 6). In vitro expression of the DGD2 cDNA resulted in the production of a 54-kD protein, which is close to the value calculated from the amino acid sequence. Uptake into pea chloroplasts was dependent on the presence of high amounts of ATP. Furthermore, the protein was found in the membrane pellet fraction and was degraded in the presence of thermolysin, indicating that it localizes to the outer side of chloroplast envelope membranes, most likely to the outer envelope membrane. Interestingly, a lower molecular mass polypeptide (∼45 kD) that was not seen in the translation product appeared consistently after uptake. This finding indicates the presence of a transit peptide, and at least part of DGD2 seems to be processed during uptake into pea chloroplasts. Like DGD1, DGD2 presumably localizes to the outer membrane of chloroplasts (Froehlich et al., 2001). However, in contrast to DGD1, which is inserted into the outer envelope in an ATP-independent manner without processing, DGD2 seems to follow a different route of insertion into the envelope membranes.
Figure 6.
DGD2 Is Localized to Chloroplast Envelope Membranes.
Proteins were radioactively labeled by in vitro expression in the presence of 35S-Met (translation product [TP]). After import experiments with pea chloroplasts in the presence of low ATP (0.1 mM) or high ATP (4 mM), the chloroplasts were treated with (+) or without (−) the protease thermolysin as indicated. Purified intact chloroplasts were recovered, lysed, and separated into pellet (P; membrane proteins) and supernatant (S; stroma proteins) fractions. Fractions were analyzed subsequently by SDS-PAGE. Radioactive proteins were visualized by fluorography.
(A) Import of Arabidopsis DGD2. The star indicates a lower molecular mass polypeptide presumably derived from processing during import.
(B) Luciferase, a control protein not imported into pea chloroplasts.
(C) The precursor protein of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) small subunit (pSS) is shown as a control protein that is imported into pea chloroplasts after processing in an ATP-dependent manner. mSS, mature form of Rubisco small subunit.
A Third DGDG Synthase Activity Independent from DGD1 and DGD2 Is Present in Arabidopsis Chloroplasts
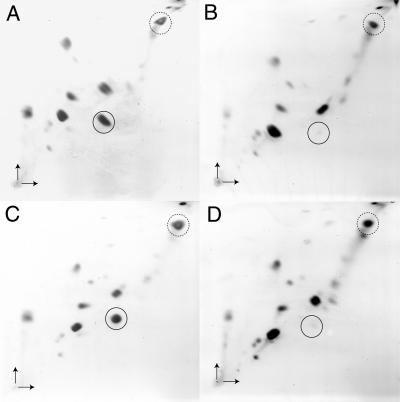
Using one-dimensional thin layer chromatography, it was not possible to unambiguously determine whether the dgd1 dgd2 double mutant is completely devoid of DGDG or whether a minor amount of this lipid still might be present. Therefore, large amounts of polar lipids isolated from wild-type and dgd1 dgd2-1 seedlings grown in the presence and absence of phosphate were separated by two-dimensional thin layer chromatography and stained with iodine. A very faint spot comigrating with DGDG was identified in dgd1 dgd2-1 (Figure 7). However, because the amounts of this lipid were extremely low, it was not possible to perform any further structural analysis. Feeding of radiolabeled acetate to plants is a sensitive method for detecting fatty acids, phospholipids, and galactolipids (Williams and Khan, 1996). Therefore, 2-week-old seedlings of the wild type and of dgd1 dgd2-1 were incubated with 1-14C-acetate in liquid medium overnight. Radioactive lipids were extracted, separated by two-dimensional thin layer chromatography, and visualized by autoradiography. The lipid pattern obtained after feeding radioactive 1-14C-acetate (data not shown) was very similar to that observed by direct iodine staining (Figure 7). A faint radioactive spot comigrating with DGDG was detected in dgd1 dgd2-1, indicating that a very low amount of DGDG was synthesized in this line.
Figure 7.
Polar Lipids in the Wild Type and in the Double Mutant dgd1 dgd2-1.
Lipids isolated from leaf tissue of plants grown under normal (+) or phosphate-deprived (−) conditions were separated by two-dimensional thin layer chromatography and stained with iodine vapor. Arrows indicate the directions of chromatography (vertical arrow, first dimension; horizontal arrow, second dimension). The spots comigrating with MGDG and DGDG standards are indicated by dashed and full circles, respectively.
(A) Wild type + Pi.
(B) dgd1 dgd2-1 + Pi.
(C) Wild type − Pi.
(D) dgd1 dgd2-1 − Pi.
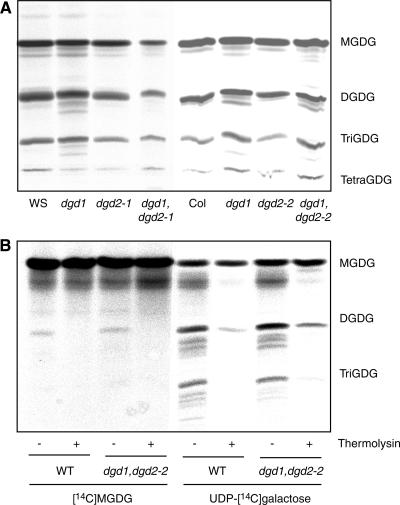
Because the two mutations, dgd1 and dgd2-1, both represent null alleles, there cannot be any residual DGDG synthase activity derived from these two gene loci in the double mutant. Therefore, another enzymatic activity must exist in Arabidopsis that is capable of DGDG synthesis. Chloroplasts were isolated from wild-type and different mutant lines and used for galactolipid synthesis assays with radioactive UDP-14C-galactose. In isolated chloroplasts, not only MGDG and DGDG but also oligogalactolipids carrying three (TriGDG) or four (TetraGDG) galactose moieties in the head group were produced. Figure 8A shows that galactolipid synthesis was detected in dgd1 and dgd2-1, and even chloroplasts isolated from the double mutant dgd1 dgd2-1 were capable of synthesizing MGDG, DGDG, TriGDG, and TetraGDG. The same activity pattern was observed for dgd2-2 and the double mutant dgd1 dgd2-2. Enzyme activities were calculated after scintillation counting of radioactive lipids isolated from thin layer chromatography plates. The activities for the synthesis of the different galactolipids in all lines were very similar (3.9 ± 0.4, 3.3 ± 0.7, 3.0 ± 1.2, and 3.7 ± 1.0 pmol·min−1·mg−1 chlorophyll for the wild type, dgd1, dgd2-1, and dgd1 dgd2-1, respectively). Furthermore, this galactolipid synthesis activity was specific for galactose, because the incorporation of radioactivity into glycolipids was barely detectable with UDP-14C-glucose (data not shown).
Figure 8.
A Galactolipid Synthase Independent from DGD1 and DGD2 Is Present in Chloroplasts of Wild-Type and DGDG-Deficient Mutant Lines.
Chloroplasts were isolated from wild-type and different mutant lines and used for galactolipid synthesis assays with radiolabeled UDP-14C-galactose or 14C-MGDG, as indicated. Lipids were isolated, separated by thin layer chromatography, and visualized by autoradiography.
(A) Chloroplasts isolated from wild-type, dgd1, dgd2, and dgd1 dgd2-2 lines synthesize MGDG, DGDG, and oligogalactolipids from UDP-14C-galactose. Col, Columbia; WS, Wassilewskija.
(B) The DGD1- and DGD2-independent galactolipid-synthesizing activity is localized to the outer side of chloroplast envelopes. Isolated chloroplasts from the wild type (WT) and dgd1 dgd2-2 were treated with the nonpenetrating protease thermolysin as indicated (+ or −). Then, galactolipid synthesis in chloroplasts was measured with 14C-MGDG or UDP-14C-galactose.
In addition to UDP-14C-galactose, 14C-MGDG was used as a substrate for DGDG synthesis in wild-type and dgd1 dgd2-2 chloroplasts (Figure 8B). Therefore, the third galactolipid enzyme present in the double mutant is capable of DGDG synthesis independent of UDP-galactose. Treatment of isolated chloroplasts with the protease thermolysin abolished galactolipid synthesis in the wild type and dgd1 dgd2-2, suggesting that this enzyme is localized to the outer chloroplast envelope (Figure 8B). This finding clearly indicates that a third enzyme of galactolipid synthesis, independent from DGD1 and DGD2, is active in in vitro assays of Arabidopsis chloroplasts. However, this third activity does not contribute to net galactolipid synthesis in planta, because the dgd1 dgd2 lines contained only trace amounts of DGDG.
DISCUSSION
Galactolipid synthesis in Arabidopsis results in the production of different molecular species containing a variety of fatty acids (Browse et al., 1986b). Furthermore, DGDG has been found to accumulate in different subcellular membranes (i.e., plastids and plasma membrane), depending on the phosphate content of the growth medium (Härtel et al., 2000; Andersson et al., 2003). It was anticipated that the two genes that encode functional DGDG synthases in Arabidopsis play different roles in galactolipid synthesis in planta (Dörmann et al., 1999; Kelly and Dörmann, 2002).
Characterization of the dgd1, dgd2, and dgd1 dgd2 alleles demonstrated that under normal growth conditions, DGD1 represents the major DGDG synthase activity in Arabidopsis chloroplasts, whereas DGD2 produces minor amounts of DGDG that can be identified only when the DGD1 pathway is disrupted (i.e., in the dgd1 background). Therefore, the residual amount of DGDG in dgd1 is derived from the DGD2 pathway. The residual amount of DGDG found in dgd1 was suggested to be associated with extraplastidic membranes (Härtel et al., 2000, 2001). The finding that chlorophyll and photosynthetic quantum yield are reduced even further in dgd1 dgd2 compared with dgd1 might be related to a general deregulation of plant metabolism. Furthermore, a fraction of DGDG synthesized via the DGD2 pathway might be associated with thylakoids, thereby stabilizing photosynthetic complexes (Härtel et al., 1997; Reifarth et al., 1997). It is known that DGDG is part of and stabilizes trimeric complexes of the major light-harvesting complex II (Nußberger et al., 1993; Dörmann et al., 1995; Hofmann et al., 1996).
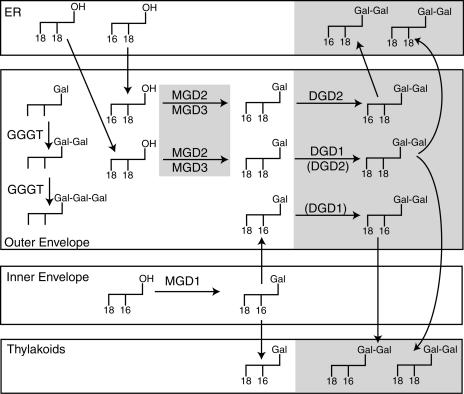
Expression of the genes DGD1 and DGD2 is induced during phosphate deprivation. The DGD2 pathway results in the production of a DGDG molecular species enriched in C16 at the sn-1 position that is derived from the endoplasmic reticulum–localized (eukaryotic) lipid pathway and found predominantly in extraplastidic membranes (Härtel et al., 2000) (Figure 9). Presumably, the large amount of DGDG that accumulates in oat root plasma membrane during phosphate deprivation is derived from DGD2 activity (Andersson et al., 2003). Interestingly, DGD2 was localized to chloroplast envelope membranes (Figure 6), suggesting that under conditions of phosphate limitation, DGDG might be transported from the chloroplast to extraplastidic membranes. DGD1 is involved in the synthesis of DGDG accumulating in chloroplast membranes (Dörmann et al., 1995). The fatty acid composition and positional distribution of DGDG synthesized in dgd2-1 do not change during phosphate deprivation. In dgd2-1, a large fraction of DGDG contains C18 fatty acids in both sn positions of the glycerol backbone (derived from the eukaryotic pathway) (Browse et al., 1986b) and a smaller fraction with C16 at sn-2 that is derived from the chloroplast-localized prokaryotic pathway (Figure 9). This fatty acid distribution is very similar to that of wild-type DGDG, again demonstrating that DGD1 is responsible for DGDG synthesis for chloroplast membranes under normal and phosphate-deficient growth conditions.
Figure 9.
Simplified Scheme of Galactolipid Synthesis in Chloroplast Envelopes.
The different molecular species of galactolipids are derived from the prokaryotic pathway (sn1/sn2, 18/16) or the eukaryotic pathway (18/18 or 16/18) of lipid biosynthesis (Härtel et al., 2000). Genes that are induced (MGD2 and MGD3 [Awai et al., 2001], DGD2 [Kelly and Dörmann, 2002], and DGD1 [this study]) and molecular species that increase during phosphate deprivation are shaded in gray. Enzymes in parentheses indicate minor pathways. ER, endoplasmic reticulum.
Because only trace amounts of DGDG were detected in the double mutant and no increase in DGDG synthesis was observed after phosphate deprivation, it was first assumed that only two genes of DGDG synthesis exist in Arabidopsis. However, galactolipid enzyme assays with chloroplasts isolated from dgd1 dgd2 revealed that a third DGDG synthase independent of DGD1 and DGD2 must be present that results in comparable rates of galactolipid synthesis in all lines. Presumably, this enzyme is responsible for the synthesis of trace amounts of DGDG that are detectable in the double mutant before and after phosphate deprivation (Figure 7). This activity was downregulated strongly in leaves of the wild type and dgd1 dgd2, because no oligogalactolipids were detected in the leaves. However, after chloroplast isolation and organelle rupture, galactolipid synthesis activity was increased strongly. Activation of this enzyme cannot be caused by the induction of mRNA expression; rather, it might result from the removal of an unknown inhibitor or regulatory protein. Alternatively, chloroplast rupture might result in the mixing of different membranes, thereby giving the enzyme access to substrates that it does not have in vivo. Interestingly, Xu et al. (2003) recently isolated an Arabidopsis mutant (tgd1) that accumulates DGDG and oligogalactolipids in planta. This plant carries a mutation in a permease-like gene presumably involved in lipid transport between membranes and a concomitant induction of a third unknown galactosyltransferase activity (processive galactosyltransferase) independent of DGD1 and DGD2 that is capable of DGDG and oligogalactolipid synthesis.
All evidence indicates that the third galactolipid synthase activity described by Xu et al. (2003) and in dgd1 dgd2-1 (this study) is related to the Arabidopsis GGGT (van Besouw and Wintermans, 1978; Heemskerk et al., 1990). This activity has been known for some time, but it could be described only at the biochemical level in different plants, including spinach and pea. The GGGT activity is processive—that is, it produces galactolipids with two (DGDG), three (TriGDG), and four (TetraGDG) galactose moieties in the head group (Figures 8A and 8B). It has been localized to the outer envelope of chloroplast membranes (Dorne et al., 1982; Cline and Keegstra, 1983). Therefore, treatment of isolated chloroplasts with thermolysin is expected to affect GGGT activity (Figure 8B). After chloroplast membrane isolation, galactolipid synthesis catalyzed by GGGT was highly increased; thus, it might mask DGDG synthesis activity catalyzed by DGD1 and DGD2. This explains why DGDG synthesis in isolated chloroplast membranes of dgd1 and dgd2 was very similar to that in the wild type (Dörmann et al., 1995; Klaus et al., 2002; this study).
The GGGT activity was suggested to be responsible for the synthesis of the predominant fraction of DGDG in higher plants (Heemskerk et al., 1990). However, our findings clearly demonstrate that DGD1, and not GGGT, represents the major activity responsible for DGDG synthesis in vivo. GGGT is not involved in the increase of DGDG synthesis during phosphate deprivation. Furthermore, DGD1 is clearly distinct from GGGT, because DGD1 uses UDP-galactose, and not another MGDG molecule, as the galactose donor for galactosylation (Figure 5). By contrast, in vitro assays showed that GGGT is capable of DGDG synthesis without the addition of extra UDP-galactose (Heemskerk et al., 1990) (Figure 7). However, in dgd1 dgd2 chloroplasts, the incorporation of radioactivity from UDP-14C-galactose into DGDG always was much higher compared with the reaction with 14C-MGDG (cf. lanes 3 and 7 in Figure 8B), suggesting that UDP-galactose somehow stimulates DGDG synthesis in the double mutant. Thus, additional studies are required to identify the exact precursors of the GGGT reaction. Until now, the gene that encodes GGGT has remained unknown and its role in galactolipid synthesis has been enigmatic. GGGT might be functional in galactolipid synthesis/trafficking to different membranes. Alternatively, GGGT activity might be associated with a reverse galactosidase reaction and thus could be involved in in vivo galactolipid degradation. Furthermore, GGGT could be crucial for galactolipid synthesis in particular plant species, such as adzuki bean, that were found to be rich in oligogalactolipids (Kojima et al., 1990). However, additional studies are required to elucidate the identity and function of this enzyme of galactolipid metabolism in higher plants.
METHODS
Plant Growth Conditions
Arabidopsis thaliana wild type (ecotypes Columbia [Col] and Wassilewskija) and dgd1 and dgd2 mutants were germinated in Petri dishes containing solidified medium (MS salts [Murashige and Skoog, 1962], 1% [w/v] sucrose, and 1% [w/v] agarose) for 2 weeks before transfer to soil (Dörmann et al., 1995) or to phosphate-deficient medium. For phosphate-deprivation experiments, 2-week-old plants were grown in Petri dishes containing 1 mM or no phosphate for an additional period of 8 days as described (Essigmann et al., 1998). The light regime for all growth conditions was 16 h of light/8 h of dark at a light intensity of 150 μmol·m−2·s−1.
Insertional Mutant Plant Isolation
A mutant carrying a T-DNA insertion in the DGD2 gene of Arabidopsis (dgd2-1) was isolated from a T-DNA–tagged population (ecotype Wassilewskija) by performing several rounds of PCR with pooled genomic DNA as described by Krysan et al. (1999) and Sussman et al. (2000). For screening, gene-specific primers (DGD2-forward [5′-CATGATAGTATTCCCTTATGTGCTCTTCT-3′] and DGD2-reverse [5′-AGGACACGAGTTACTCATAGTTCATATGT-3′]) and a T-DNA border primer (JL-202 [5′-CATTTTATAATAACGCTGCGGACATCTAC-3′]) were used. PCR fragments were separated on agarose gels and, after transfer to nylon membranes (Sambrook et al., 1989), hybridized to a DGD2 cDNA fragment (clone 16) (Kelly and Dörmann, 2002). Mutant plants were selected on medium containing 25 μg/mL kanamycin. The PCR fragment covering the DGD2/T-DNA junction was sequenced directly.
A second mutant allele, dgd2-2, was identified by Basic Local Alignment Search Tool (BLAST) comparison of the DGD2 genomic sequence with a database of 100,000 sequences derived from genomic plant DNA/T-DNA junctions of an insertional mutant population (ecotype Col-0) (Sessions et al., 2002). Plants were grown on soil, and mutants carrying the T-DNA insertion were selected by spraying with 0.2% glufosinate (phosphinotricin/BASTA). The PCR fragment containing the junction was amplified with the gene-specific primer DGD2-reverse (see above) and the T-DNA border primer LB3 (5′-TAGCATCTGAATTTCATAACCAATCTCGATACAC-3′) and sequenced.
Homozygous mutant plants for dgd2-1 and dgd2-2 were identified by segregation analysis using the resistance markers kanamycin and glufosinate, respectively. PCR analysis of plants carrying homozygous insertions consistently yielded a single band using the combination of gene-specific and T-DNA border primers, but, as a result of the large insert size, no band was found using the two gene-specific primers. Homozygosity was confirmed by DNA gel blot analysis using a DGD2 cDNA probe (see above). The two mutant alleles each carried a single T-DNA insertion, as demonstrated by PCR, DNA gel blot, and segregation analyses.
Generation of the Double Mutant dgd1 dgd2
The two alleles (dgd2-1 and dgd2-2) were crossed to the galactolipid-deficient dgd1 mutant of Arabidopsis (ethyl methanesulfonate mutant of ecotype Col-2) (Dörmann et al., 1995). After selfing of an F1 plant, F2 plants homozygous for the dgd2 insertion were identified by PCR. Several dgd2 homozygous F2 lines were selfed, and F3 plants were screened for the dgd1 phenotype (reduced amounts of digalactosyldiacylglycerol [DGDG] and reduced amounts of 16:3) (Dörmann et al., 1995) by thin layer chromatography and gas chromatography.
RNA Gel Blot Analysis
Total RNA was isolated from leaves according to Logemann et al. (1987). RNA gel electrophoresis and transfer to nylon membranes were performed according to standard protocols (Sambrook et al., 1989). The membrane was hybridized to an α-32P-dCTP–labeled DGD1 cDNA fragment (Dörmann et al., 1999) or DGD2 cDNA (see above), and hybridization signals were visualized by autoradiography.
Heterologous Expression of DGDG Synthases in Escherichia coli
Subcloning of the C-terminal part of DGD1 (C-DGD1; from Glu-338 to Trp-808) and of DGD2 into the E. coli expression vector pQE31 (Qiagen, Hilden, Germany) were described by Froehlich et al. (2001) and Kelly and Dörmann (2002), respectively. The N-terminal part of DGD1 from Met-1 to Pro-337 (N-DGD1) was amplified by PCR from the full-length DGD1 cDNA clone 22-1 (Dörmann et al., 1999) using the primers D12F (5′-CACGGATCCCATGGTAAAGGAAACTC-3′) and D12R (5′-CACGGATCC- ACAGGCTTCACAAAATC-3′), introducing BamHI sites at the 5′ and 3′ ends. This PCR fragment was ligated into the BamHI site at the 5′ end of the DGD2 cDNA in pQE31 (Kelly and Dörmann, 2002). Chimeric clones containing N-DGD1 in the correct orientation fused N terminally to DGD2 (ND1D2) were identified by restriction analysis. DGDG synthase constructs in pQE31 were transferred into M15(pREP4) cells, and after induction, membrane proteins were used for galactolipid synthase assays.
Lipid and Fatty Acid Analyses
Lipids were extracted from leaves, separated by thin layer chromatography, and stained with iodine vapor (Dörmann et al., 1995). Lipids isolated from thin layer chromatography plates were methylated, and methyl esters were quantified by gas chromatography using pentadecanoic acid as an internal standard, according to Browse et al. (1986a). Two-dimensional thin layer chromatography was performed with chloroform:methanol:water (65:25:4) and chloroform:acetone:methanol:acetic acid:water (50:20:10:10:5) (Benning et al., 1995). Positional analysis of fatty acids was performed as described by Miquel et al. (1998) and Siebertz and Heinz (1977).
Lipids were isolated from wild-type Arabidopsis leaves and E. coli cells expressing cucumber MGD1 and Arabidopsis DGD1 (Dörmann et al., 1999) by thin layer chromatography and dissolved in deuterated solvent (CDCl3/CD3OD, 2:1). 1H-NMR spectra were recorded with a Varian INOVA-600 (600 MHz) NMR spectrometer (Palo Alto, CA) at the Department of Chemistry at Michigan State University (East Lansing).
Chlorophyll and Chlorophyll Fluorescence Measurements
Chlorophyll was determined photometrically in leaf extracts in 80% acetone according to Lichtenthaler (1987). In vivo chlorophyll fluorescence was measured with a pulse amplitude modulation fluorimeter (PAM-2000; Heinz Walz, Effeltrich, Germany). The photosynthetic quantum yield was calculated according to the equation (Fm′ − Ft)/Fm′, where Ft and Fm′ refer to the fluorescence of a light-adapted plant before and after the application of a saturating light pulse, respectively (Schreiber et al., 1986; Krause and Weis, 1991).
In Vitro Protein Expression and Import Experiments with Isolated Pea Chloroplasts
Pea chloroplasts were isolated according to Bruce et al. (1994). Radiolabeled DGD2 protein was obtained by in vitro expression of the DGD2 cDNA (clone 16) (Kelly and Dörmann, 2002) in the presence of 35S-Met, as described by Froehlich et al. (2001). Import experiments with purified chloroplasts were performed in the presence of low (0.1 mM) or high (4.0 mM) ATP, and proteins were recovered after chloroplast isolation from the membrane or stroma fraction as described (Froehlich et al., 2001).
Chloroplast Isolation and Galactolipid Synthesis Assays
Radioactive lipids for enzyme assays were obtained after incubating 14-day-old Arabidopsis wild-type (Col-2) seedlings overnight in 20 mM Mes-KOH, pH 6.0, with 50 μmol of 1-14C-acetate (74 mBq/mmol). Lipids were extracted and separated by thin layer chromatography (see above), and radioactive galactolipids were isolated from the plate. Specific radioactivity of 14C-monogalactosyldiacylglycerol (MGDG) was determined by scintillation counting and fatty acid quantification of methyl esters via gas chromatography.
Leaves were homogenized with a Waring blender (Ultraturrax, IKA Labortechnik, Staufen, Germany), and chloroplasts were purified on a 20% Percoll cushion according to the protocol of Price et al. (1994). Purified chloroplasts were treated with the protease thermolysin as described by Xu et al. (2003). Chloroplasts equivalent to ∼22 μg of total chlorophyll were resuspended in 330 μL of assay buffer (0.3 M sorbitol, 20 mM Tricine-KOH, pH 7.6, 5 mM MgCl2, and 2.5 mM EDTA). The reactions contained 1150 pmol of UDP-U-14C-galactose (10,286 MBq/mmol) or 24 nmol of 14C-MGDG (48.6 MBq/mmol). To increase lipid solubilization, 0.5 mM deoxycholate was added to some of the reactions (Figures 8A and 8B; no deoxycholate was added to the reactions with UDP-14C-galactose in Figure 8B). The reaction was stopped, and lipids were extracted with 1.2 mL of chloroform:methanol (2:1) and 300 μL of 1.0 M KCl and 0.2 M H3PO4. Lipids were separated by thin layer chromatography (see above), and radioactivity was visualized by autoradiography or quantified by scintillation counting of isolated lipid bands.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact Peter Dörmann, doermann@mpimp-golm.mpg.de.
Accession Numbers
The protein entry codes for the Arabidopsis proteins described in the article are as follows: DGD1, At3g11670; DGD2, At4g00550.
Acknowledgments
We thank the University of Wisconsin (Madison) and the Torrey Mesa Research Institute (San Diego, CA), a subsidiary of Syngenta, for providing seed pools and seeds for the isolation of the two mutant alleles, dgd2-1 and dgd2-2, respectively. This project was supported in part by grants from the Deutsche Forschungsgemeinschaft (Do520/2-1; SFB 429, Teilprojekt B6).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.016675.
References
- Andersson, M.X., Stridh, M.H., Larsson, K.E., Liljenberg, C., and Sandelius, A.S. (2003). Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537, 128–132. [DOI] [PubMed] [Google Scholar]
- Awai, K., Maréchal, E., Block, M.A., Brun, D., Masuda, T., Shimada, H., Takamiya, I.-i., Ohta, H., and Joyard, J. (2001). Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98, 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning, C., Huang, Y.-H., and Gage, D.A. (1995). Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 317, 103–111. [DOI] [PubMed] [Google Scholar]
- Browse, J., McCourt, P.J., and Somerville, C.R. (1986. a). Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal. Biochem. 152, 141–145. [DOI] [PubMed] [Google Scholar]
- Browse, J., and Somerville, C. (1994). Glycerolipids. In Arabidopsis, E. Meyerowitz and C. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 881–912.
- Browse, J., Warwick, N., Somerville, C.R., and Slack, C.R. (1986. b). Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the 16:3 plant Arabidopsis thaliana. Biochem. J. 235, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce, B.D., Perry, S., Froehlich, J., and Keegstra, K. (1994). In vitro import of protein into chloroplasts. In Plant Molecular Biology Manual, S.B. Gelvin and R.A. Schilperoort, eds (Boston, MA: Kluwer Academic Publishers), pp. 1–15.
- Carter, H.E., McCluer, R.H., and Slifer, E.D. (1956). Lipids of wheat flour. I. Characterization of galactosylglycerol components. J. Am. Chem. Soc. 78, 3735–3738. [Google Scholar]
- Cline, K., and Keegstra, K. (1983). Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 71, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörmann, P., Balbo, I., and Benning, C. (1999). Arabidopsis galactolipid biosynthesis and lipid trafficking mediated by DGD1. Science 284, 2181–2184. [DOI] [PubMed] [Google Scholar]
- Dörmann, P., and Benning, C. (2002). Galactolipids rule in seed plants. Trends Plant Sci. 7, 112–118. [DOI] [PubMed] [Google Scholar]
- Dörmann, P., Hoffmann-Benning, S., Balbo, I., and Benning, C. (1995). Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7, 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne, A.-J., Block, M.A., Joyard, J., and Douce, R. (1982). The galactolipid:galactolipid galactosyltransferase is located on the outer membrane of the chloroplast envelope. FEBS Lett. 145, 30–34. [Google Scholar]
- Essigmann, B., Gueler, S., Narang, R.A., Linke, D., and Benning, C. (1998). Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 95, 1950–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich, J., Benning, C., and Dörmann, P. (2001). The digalactosyldiacylglycerol synthase DGD1 is inserted into the outer envelope membrane of chloroplasts in a manner independent of the general import pathway and does not depend on direct interaction with MGDG synthase for DGDG biosynthesis. J. Biol. Chem. 276, 31806–31812. [DOI] [PubMed] [Google Scholar]
- Härtel, H., Dörmann, P., and Benning, C. (2000). DGD1-independent biosynthesis of extraplastidic galactolipids following phosphate deprivation in Arabidopsis. Proc. Natl. Acad. Sci. USA 97, 10649–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtel, H., Dörmann, P., and Benning, C. (2001). A galactolipid pool not associated with the photosynthetic apparatus in phosphate-deprived plants. J. Photochem. Photobiol. B Biol. 61, 46–51. [DOI] [PubMed] [Google Scholar]
- Härtel, H., Lokstein, H., Dörmann, P., Grimm, B., and Benning, C. (1997). Changes in the composition of the photosynthetic apparatus in the galactolipid-deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol. 115, 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk, J.W.M., Storz, T., Schmidt, R.R., and Heinz, E. (1990). Biosynthesis of digalactosyldiacylglycerol in plastids from 16:3 and 18:3 plants. Plant Physiol. 93, 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk, J.W.M., Wintermans, J.F.G.M., Joyard, J., Block, M.A., Dorne, A.-J., and Douce, R. (1986). Localization of galactolipid:galactolipid galactosyltransferase and acyltransferase in outer envelope membrane of spinach chloroplasts. Biochim. Biophys. Acta 877, 281–289. [Google Scholar]
- Heinz, E., and Roughan, P.G. (1983). Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 72, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, E., Wrench, P.M., Sharples, F.P., Hiller, R.G., Wilte, W., and Diederichs, K. (1996). Structural basis of light harvesting by carotenoids: Peridinin-chlorophyll-protein from Aphidinium carterae. Science 272, 1788–1791. [DOI] [PubMed] [Google Scholar]
- Jarvis, P., Dörmann, P., Peto, C.A., Lutes, J., Benning, C., and Chory, J. (2000). Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc. Natl. Acad. Sci. USA 97, 8175–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard, J., Teyssier, E., Miège, C., Berny-Seigneurin, D., Maréchal, E., Block, M.A., Dorne, A.-J., Rolland, N., Ajlani, G., and Douce, R. (1998). The biochemical machinery of plastid envelope membranes. Plant Physiol. 118, 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A.A., and Dörmann, P. (2002). DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J. Biol. Chem. 277, 1166–1173. [DOI] [PubMed] [Google Scholar]
- Klaus, D., Härtel, H., Fitzpatrick, L., Froehlich, J.F., Hubert, J., Benning, C., and Dörmann, P. (2002). Digalactosyldiacylglycerol synthesis in chloroplasts of the Arabidopsis thaliana dgd1 mutant. Plant Physiol. 128, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima, M., Seki, K., Ohnishi, M., Ito, S., and Fujino, Y. (1990). Structure of novel glyceroglycolipids in adzuki bean (Vigna angularis) seeds. Biochem. Cell Biol. 68, 59–64. [PubMed] [Google Scholar]
- Krause, G.H., and Weis, E. (1991). Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349. [Google Scholar]
- Krysan, P.J., Young, J.C., and Sussman, M.R. (1999). T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11, 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382. [Google Scholar]
- Logemann, J., Schell, J., and Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163, 16–20. [DOI] [PubMed] [Google Scholar]
- Miquel, M., Cassagne, C., and Browse, J. (1998). A new class of Arabidopsis mutants with reduced hexadecatrienoic acid fatty acid levels. Plant Physiol. 117, 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nußberger, S., Dörr, K., Wang, N., and Kühlbrandt, W. (1993). Lipid-protein interactions in crystals of plant light-harvesting complex. J. Mol. Biol. 234, 347–356. [DOI] [PubMed] [Google Scholar]
- Price, C.A., Hadjeb, N., Newman, L., and Reardon, E.M. (1994). Isolation of chloroplasts and chloroplast DNA. In Plant Molecular Biology Manual, S.B. Gelvin and R.A. Schilperoort, eds (Dordrecht, The Netherlands: Kluwer Academic Press), section D4, pp. 1–15.
- Reifarth, F., Christen, G., Seeliger, A.G., Dörmann, P., Benning, C., and Renger, G. (1997). Modification of the water oxidizing complex in leaves of the dgd1 mutant of Arabidopsis thaliana deficient in the galactolipid digalactosyldiacylglycerol. Biochemistry 36, 11769–11776. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schreiber, U., Schliwa, U., and Bilger, W. (1986). Continuous recording of photochemical and nonphotochemical quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62. [DOI] [PubMed] [Google Scholar]
- Sessions, A., et al. (2002). A high-throughput Arabidopsis reverse genetics system. Plant Cell 14, 2986–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebertz, H.P., and Heinz, E. (1977). Labeling experiments on the origin of hexa- and octadecatrienoic acids in galactolipids from leaves. Z. Naturforsch. 32, 193–205. [Google Scholar]
- Sussman, M.R., Amasino, R.M., Young, J.C., Krysan, P.J., and Austin-Phillips, S. (2000). The Arabidopsis knockout facility at the University of Wisconsin-Madison. Plant Physiol. 124, 1465–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Besouw, A., and Wintermans, J.F.G.M. (1978). Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim. Biophys. Acta 529, 44–53. [DOI] [PubMed] [Google Scholar]
- Vijayan, P., Routaboul, J.-M., and Browse, J. (1998). A genetic approach to investigating membrane lipid structure and function. In Lipids in Photosynthesis: Structure, Function and Genetics, P.A. Siegenthaler and N. Murata, eds (Dordrecht, The Netherlands: Kluwer Academic Press) pp. 263–285.
- Williams, J.P., and Khan, M.U. (1996). Lipid biosynthesis in Brassica napus leaves. 1. 14C-labelling kinetics of the fatty acids of the major glycerolipids. Plant Physiol. Biochem. 34, 93–100. [Google Scholar]
- Xu, C., Fan, J., Riekhof, W., Froehlich, J.E., and Benning, C. (2003). A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J. 22, 2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]