Abstract
Nodular lymphocyte-predominant Hodgkin’s lymphoma (NLPHL) shows constitutive NF-κB activity in the malignant lymphocyte-predominant (LP) cells. Constitutive NF-κB activity also plays a central pathogenetic role in classical Hodgkin’s lymphoma (cHL), where inactivating mutations in the NFKBIA and TNFAIP3 genes, coding for the negative NF-κB regulators IκBα and A20, respectively, contribute to NF-κB activation. To determine whether mutations in NFKBIA and TNFAIP3 are also involved in the pathogenesis of NLPHL these genes were sequenced from microdissected LP cells of 10 primary NLPHL. We also studied DEV, the only cell line proposedly derived from LP cells, after we had confirmed its derivation from NLPHL by gene expression analysis. A heterozygous somatic missense mutation in the NFKBIA gene was found in one NLPHL, and a heterozygous, possibly subclonal, two base pair insertion in TNFAIP3 in another case. The low mutation frequency and the absence of biallelic destructive mutations propose a minor contribution of NFKBIA and TNFAIP3 mutations to the NF-κB activity of NLPHL, suggesting different mechanisms of NF-κB activation in NLPHL and cHL.
Keywords: mutations, NF-κB regulating factors, Hodgkin’s lymphoma
Introduction
Nodular lymphocyte-predominant Hodgkin’s lymphoma (NLPHL) and classical Hodgkin’s lymphoma (cHL) show marked similarities in their histological appearances.1 The tumor cells of these lymphomas, the Hodgkin and Reed-Sternberg (HRS) cells in cHL and the lymphocyte-predominant (LP) cells, formerly named lymphocytic and histiocytic cells,2 in NLPHL, are embedded in a reactive inflammatory background and often account for only 1% of cells in the tumor tissue. It is well known that HRS cells show strong constitutive NF-κB activity, which is thought to contribute critically to their malignant behavior.3 Recently, it was demonstrated that also LP cells show constitutive NF-κB activity.4 While little is known about the mechanisms leading to the deregulation of the NF-κB pathway in NLPHL, we have some insight into these mechanisms in cHL. Apart from ligand-mediated activation provided by the microenvironment via CD30, CD40 and RANK surface receptors of the TNF receptor family or intrinsic stimuli by the latent membrane protein (LMP) 1 in Epstein-Barr virus (EBV)-positive cases, HRS cells can acquire constant NF-κB activation by genetic lesions, in particular amplification of the REL gene or inactivating mutations of inhibitors of the NF-κB signaling pathway, i.e. the NFKBIA, NFKBIE and TNFAIP3 genes.5–7 The latter genes hence function as tumor suppressor genes in cHL.
NFKBIA codes for IκBα, a protein that prevents the nuclear translocation of NF-κB heterodimers by binding and retaining them in the cytoplasm, thereby inhibiting NF-κB activity. NFKBIA, located on chromosome 14q13 was shown to contain inactivating mutations in Hodgkin’s lymphoma (HL) cell lines and primary cases in about 20% of cases analyzed.8–11 Even more important is the recent finding that 70% of EBV-negative cHL cases contain functionally inactivating mutations of mostly both alleles of the TNFAIP3 gene, located on chromosome 6q23.6 The encoded protein (A20) functions as an inhibitor of NF-κB signaling through the removal of activating Lys63-linked ubiquitins and the Lys48-linked ubiquitination and subsequent proteasomal degradation of receptor-interacting protein (RIP), which is an essential mediator for signal transduction from the TNF-receptor to the IκB kinase (IKK).12 A20 may inhibit also additional factors of the NF-κB pathway, such as TRAF1 and TRAF2.13 Notably, TNFAIP3 mutations are much rarer in EBV-positive cases of cHL, indicating that viral transformation, and in particular NF-κB activation through LMP1, may replace the pathogenetic role of A20 in the EBV-positive cases.6 Given the important role of inactivating mutations in the tumor suppressor genes NFKBIA and TNFAIP3 in cHL, the fact that LP cells are essentially always EBV-negative and the recent recognition of constitutive NF-κB activity also in LP cells of NLPHL, we studied the contribution of inactivating mutations in these two genes to the pathogenesis of NLPHL.
Design and Methods
Generation of gene expression profiles and principal component analysis
The gene expression profiles of primary HRS and LP cells were available through an earlier study.4 Gene expression profiles of the HL cell lines were generated with the same protocol. For the 134 preselected probe sets of differentially expressed genes between cHL and NLPHL with filter criteria fold change ≥1.8 or ≤−1.8 and false discovery rate ≤0.1, we performed principal component analysis. The first principal component, accounting for 63.3% of the variance, was used as an expression signature for the given gene list, which was then applied to the five HL cell lines.
Patient samples and laser microdissection of LP cells
Lymph node samples from 10 patients with NLPHL were collected from the Senckenberg Institute of Pathology at University of Frankfurt. The Institutional Review Board at the University of Frankfurt approved the study. For amplification and sequencing aliquots of 20 pooled as well as single LP cells were obtained by microdissection from 5 μm cryosections stained for CD7514 (Abcam, Cambridge, USA) mounted on membrane covered slides using the PALM Robot-MicroBeam laser microdissection system (PALM, Bernried, Germany).
Amplification and sequencing of NFKBIA
Samples were incubated with 0.5 mg/mL proteinase K at 50°C for 2 h followed by 5 min at 95°C. Exon 1 was amplified using 1.5 mM MgCl2, 1 M betaine, and primers IKB1h (5′-CAGCGCCCCAGCGAGGAAGCAG-3′) and IKB1e (5′-AGCGTTCGGGGCGGTGCAGGAG-3′). Exons 2–6 were amplified in a multiplex PCR containing 2 mM MgCl2, 1 M betaine and primers.10 PCR was performed for 35 cycles. Following first-round amplification semi-nested PCR was performed individually for each exon with primers previously described,10 with IKB1b substituted for IKB1h and IKB2b substituted for IKB2b2 (5′-GCCTGCCAGGAACACTCAGCTC-3′). MgCl2 and betaine concentrations equal to first round conditions were used. Forty-five cycles of PCR were performed. Amplificates were sequenced on an automated sequencer (ABI 3130, Applied Biosystems).
Amplification, sequencing and FICTION analysis of TNFAIP3
All coding exons of TNFAIP3 were amplified in a seminested PCR with 35 cycles in the first round and 45 cycles in the second round of amplification, using 3 mM MgCl2 and 1.05 M betaine as previously described.6 Fluorescence immunophenotyping and interphase cytogenetic (FICTION) analysis of the TNFAIP3 gene was performed using a probe spanning the TNFAIP3 locus and a centromere probe for chromosome 6 as previously described,6 with monoclonal anti-CD20 antibody (L26; DakoCytomation, Glostrup, Denmark) used for immunofluorescence staining of LP cells.
Results and Discussion
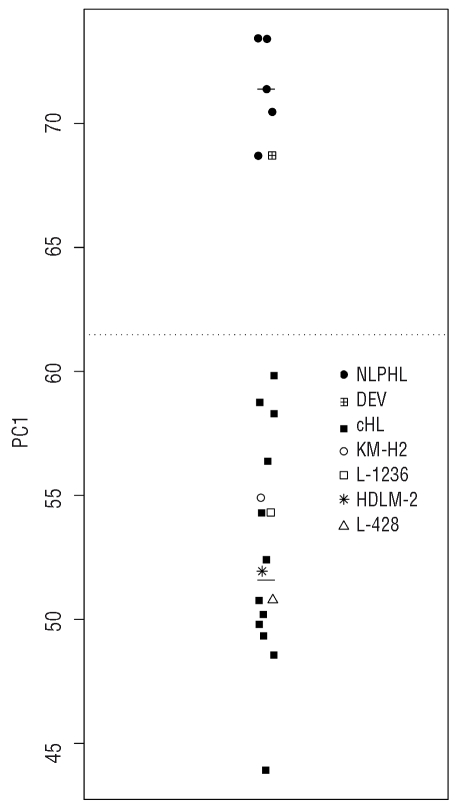
Initially, we wanted to confirm the NLPHL derivation of the DEV cell line, which was originally described to have been established from a cHL patient,15 but which was later reclassified as being derived from NLPHL.16 We used global gene expression profiles from primary microdissected HRS and LP cells,4 and identified a set of 134 genes that best distinguished between them. Gene expression profiles of DEV and four classical HL cell lines were generated and the relatedness of these cell lines to primary HRS and LP cells was assessed by principal component analysis using the above 134 genes. Figure 1 shows that along the first principal component, the four cHL lines co-localize with the population of primary cHL, whereas DEV is located within the population of NLPHL. Thus, regarding genes that are significantly differentially expressed between HRS and LP cells, DEV shows a gene expression profile indistinguishable from primary LP cells, and very different from that of HRS cells. This finding molecularly substantiates the origin of the DEV cell line from NLPHL which is further supported by presence of a BCL6 translocation and CD20 expression, typical features of LP cells, in this cell line.17–19 Having validated the origin of DEV, we analyzed this cell line for mutations in the TNFAIP3 gene, but no mutations were found. The DEV cells have already been shown to lack mutations in the NFKBIA gene.10
Figure 1.
Relatedness of HL cell lines with cHL and NLPHL by PCA. Probe sets discriminating between cHL and NLPHL (fold change ≥1.8 or ≤−1.8 and false discovery rate ≤0.1, 134 probe sets) were used by principal component analysis to identify the relatedness to DEV and four cHL cell lines (HDLM-2, L-428, L-1236 and KM- H2). The y-axes represent the first principal component score (PC1), the horizontal solid lines show the median score of each cell subset, and the horizontal dotted line indicates the mean of the median scores of the cHL and NLPHL. The first principal component accounts for 63.3% of the total variance. cHL: classical Hodgkin’s lymphoma; NLPHL: nodular lymphocyte-predominant Hodgkin’s lymphoma.
To elucidate a potential pathogenetic role for TNFAIP3 and NFKBIA in primary LP cells, samples of 20 microdissected LP cells from 10 cases of NLPHL were evaluated for mutations in these genes, using a two-rounded, semi-nested PCR followed by direct, bidirectional sequencing. PCR amplicons covered all six exons of NFKBIA and the eight coding exons of TNFAIP3.
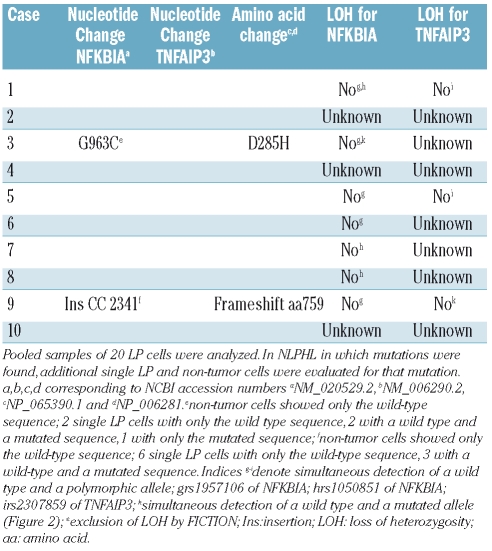
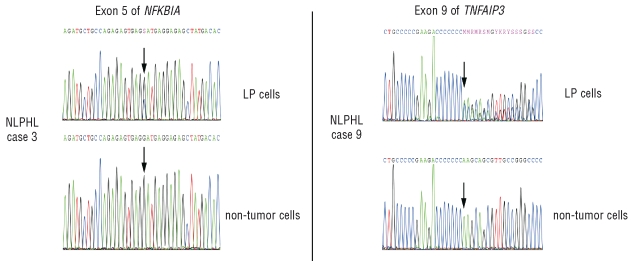
For the NFKBIA gene, a single replacement mutation was found in exon 5 of one NLPHL, leading to an amino acid change at position 285 of the IκBα protein from aspartic acid to histidine (Table 1). The amino acid affected by this mutation is located in the c-terminal PEST domain of IκBα, which is responsible for its proteasomal degradation independent of IKK-mediated phosphorylation.20 A mutant variant with this amino acid change in the PEST domain, which is physiologically masked upon NF-κB binding, might show altered degradation when compared to the wild-type protein. In the sequence with the mutation, a wild-type as well as the mutated allele were recognizable. Sequencing of exon 5 from a microdissected sample of non-tumor cells from this case showed only the wild-type sequence, confirming the somatic origin of this mutation (Figure 2). To establish clonality of this mutation, an additional 6 single LP cells microdissected from different areas of the tumor biopsy were analyzed. Of five evaluable single cell sequences two showed only the wild-type allele, from two cells both the wild-type and the mutated allele were amplified, and one cell sample showed only the mutated sequence. As the PCR efficiency for single molecules from microdissected cells is usually in the range of 30–50%,6,10,21 these results indicate that the LP cells of this case carry a somatic and clonal replacement mutation in exon 5 of one allele of NFKBIA, whereas the second allele is wild-type.
Table 1.
Mutation analysis for NFKBIA and TNFAIP3 in LP cells of NLPHL.
Figure 2.
NFKBIA and TNFAIP3 sequences of 20 pooled LP and non-tumor cells of primary NLPHL. (Left) Electropherograms showing a, presumably heterozygous, single nucleotide replacement mutation in exon 5 of the NFKBIA gene present in LP but not in non-tumor cells from a primary NLPHL (case 3). Additional sequencing of single LP cells further support a heterozygous mutation (Table 1). (Right). Electropherograms showing a 2 bp insertion in exon 9 of the TNFAIP3 gene present in LP but not in non-tumor cells from a primary NLPHL (case 9). Additional sequencing of single LP cells and immunophenotyping and interphase cytogenetics (FICTION) analysis indicate either a subclonal heterozygous mutation or a heterozygous mutation plus a copy number gain of the wild-type allele only in LP cells (Table 1). Positions affected by mutations in LP cells and their equivalent in non-tumor cells are indicated by arrows.
Analysis of the TNFAIP3 gene from LP cells of the 10 cases of NLPHL revealed one case with a heterozygous 2 bp insertion in exon 9, leading to a frameshift at position 759 of the protein (Table 1). As a result of this mutation, this allele should code for a variant of the protein with a replacement of the 32 amino acids of the most c-terminal zinc finger domain by 57 other amino acids. Notably, a similar mutation was found in the activated B cell-like diffuse large B-cell lymphoma cell line RC-K8 with postulated dependence on constitutive NF-κB signaling,22 where the deletion of a single nucleotide in exon 9 leads to a frameshift abrogating the coding sequence of the two terminal zinc finger domains of the protein. This cell line, harboring an additional inactivating mutation of the second allele of TNFAIP3, undergoes growth arrest and apoptosis upon reconstitution of wild-type A20 thus pointing to a potential functional relevance also of the, albeit monoallelic, mutation in the NLPHL analyzed here. The somatic origin of this mutation was proven by amplification of only the wild-type sequence from microdissected non-tumor cells of the respective biopsy (Figure 2). Single LP cells of this NLPHL were further investigated. Of nine sequences obtained from the single LP cells, six showed only the wild-type allele while three showed the 2 bp insertion coexistent with a wild-type allele. FICTION analysis of this case revealed in 11 of 24 nuclei from LP cells (identified as large CD20-positive cells) 3 or 4 copies of the TNFAIP3 gene locus whereas the bystander cells showed no such copy number gain (data not shown). The shift in ratio of detectable unmutated versus mutated alleles might therefore be due to a copy number gain of the (unmutated) TNFAIP3 gene in a fraction of LP cells or the described mutation being a subclonal event.
Based on the presence of a polymorphic or mutated allele together with a wild-type allele in the electropherogram we were able to exclude a loss of heterozygosity in 7 of 10 cases for NFKBIA and 3 of 10 cases for TNFAIP3, i.e. in all informative cases (Table 1).
NLPHL and cHL show marked similarities in histological appearance, global gene expression, and aberrant activation of several signaling pathways.1,4 In line with a close relationship of these lymphoid malignancies, some common genetic lesions have been identified, for example mutations in the SOCS1 gene as a mechanism contributing to constitutive STAT transcription factor activity.23,24 On the other hand, also distinct genetic lesions have been described in cHL and NLPHL. For example, chromosomal translocations involving the BCL6 locus are frequent in NLPHL but very rare in cHL, and genomic gains of the REL gene have been detected in cHL, but appear to be rare or absent in NLPHL.7 On this background, it was important to clarify whether the mechanisms causing constitutive NF-κB activity in HRS and LP cells are caused by similar genetic lesions, which would further support a close relationship between cHL and NLPHL.
In the present study, out of 10 cases of NLPHL and the NLPHL cell line DEV, only one mutation was found for each of the two genes under investigation and neither one seems to lead to a functional inactivation of the respective gene in the tumor cells. In both instances, the mutation affected only one allele, and in the case of the TNFAIP3 mutation it might even be a subclonal event, that was hence not a primary transforming lesion. This contrasts to cHL, where NFKBIA and TNFAIP3 mutations were found more frequently, were clonal, and often clearly destructive for both alleles, as it is typical for tumor suppressor gene inactivation. Thus, our study revealed that inactivating mutations in the NFKBIA and TNFAIP3 genes do not contribute significantly to the high NF-κB activity in the LP cells of NLPHL, and that the mechanisms of NF-κB activation in NLPHL are distinct from those of cHL. It remains to be determined whether perhaps genetic lesions of other factors of the NF-κB pathway and/or other stimuli cause the high NF-κB activity of LP cells.
Acknowledgments
the authors would like to thank Stefan Gesk and Reina Zühlke-Jenisch for support with the FICTION analysis, Anja Mottok for helpful discussion, and Gwen Lorenz and Ralf Lieberz for excellent technical assistance.
Footnotes
Funding: this work was supported by grants from the Wilhelm Sander-Stiftung (No. 2005.168.2) and the Deutsche Krebshilfe, Mildred Scheel-Stiftung (107736, 107748). E.T. was recipient of a fellowship (F 05/01) granted by the German José Carreras Leukemia Foundation.
Authorship and Disclosures
MS: performed microdissection and sequence analysis of LP cells, analyzed and interpreted the data, wrote the manuscript; RS: designed and supervised the study, wrote the manuscript; ET and VB: performed gene expression analysis; CD: performed principal component analysis; MLH: performed pathological evaluation, contributed the primary biopsies; RSi: molecular cytogenetic analysis, provided funding; RK: designed and supervised the study, wrote the manuscript, provided funding.
RSi received honoraria and reimbursements from Abbott/Vysis for educational activities. The other authors reported no potential conflicts of interest.
References
- 1.Weiss LMWR, Hansmann ML, Chan JKC, Müller-Hermelink HK, Harris NL, Stein H, Jaffe ES. Pathology of Hodgkin Lymphoma. In: Hoppe RTMM, Armitage JO, Diehl V, Weiss LM, editors. Hodgkin Lymphoma. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Poppema S, Delsol G, Pileri SA, Stein H, Swerdlow SH, Warnke RA, et al. Nodular lymphocyte predominant Hodgkin lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., editors. Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. pp. 323–5. [Google Scholar]
- 3.Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100(12):2961–9. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brune V, Tiacci E, Pfeil I, Döring C, Eckerle S, van Noesel CJ, et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J Exp Med. 2008;205(10):2251–68. doi: 10.1084/jem.20080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459(7247):712–6. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz R, Hansmann M-L, Bohle V, Martin-Subero JI, Hartmann S, Mechtersheimer G, et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206(5):981–9. doi: 10.1084/jem.20090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz R, Stanelle J, Hansmann M-L, Küppers R. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol. 2009;4:151–74. doi: 10.1146/annurev.pathol.4.110807.092209. [DOI] [PubMed] [Google Scholar]
- 8.Cabannes E, Khan G, Aillet F, Jarrett RF, Hay RT. Mutations in the IkBa gene in Hodgkin’s disease suggest a tumour suppressor role for IκBα. Oncogene. 1999;18(20):3063–70. doi: 10.1038/sj.onc.1202893. [DOI] [PubMed] [Google Scholar]
- 9.Emmerich F, Meiser M, Hummel M, Demel G, Foss HD, Jundt F, et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood. 1999;94(9):3129–34. [PubMed] [Google Scholar]
- 10.Jungnickel B, Staratschek-Jox A, Bräuninger A, Spieker T, Wolf J, Diehl V, et al. Clonal deleterious mutations in the IκBα gene in the malignant cells in Hodgkin’s lymphoma. J Exp Med. 2000;191(2):395–402. doi: 10.1084/jem.191.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake A, Shield LA, Cordano P, Chui DT, Osborne J, Crae S, et al. Mutations of NFK-BIA, encoding IkappaBalpha, are a recurrent finding in classical Hodgkin lymphoma but are not a unifying feature of non-EBV-associated cases. Int J Cancer. 2009;125(6):1334–42. doi: 10.1002/ijc.24502. [DOI] [PubMed] [Google Scholar]
- 12.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. Deubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature. 2004;430 (7000):694–9. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 13.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc Natl Acad Sci USA. 1996;93(13):6721–5. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chittal SM, Caveriviere P, Schwarting R, Gerdes J, Al Saati T, Rigal-Huguet F, et al. Monoclonal antibodies in the diagnosis of Hodgkin’s disease. The search for a rational panel. Am J Surg Pathol. 1988;12(1):9–21. doi: 10.1097/00000478-198801000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Poppema S, De Jong B, Atmosoerodjo J, Idenburg V, Visser L, De Ley L. Morphologic, immunologic, enzymehistochemical and chromosomal analysis of a cell line derived from Hodgkin’s disease. Evidence for a B-cell origin of Sternberg-Reed cells. Cancer. 1985;55(4):683–90. doi: 10.1002/1097-0142(19850215)55:4<683::aid-cncr2820550402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.Maggio EM, Van Den Berg A, Visser L, Diepstra A, Kluiver J, Emmens R, et al. Common and differential chemokine expression patterns in rs cells of NLP, EBV positive and negative classical Hodgkin lymphomas. Int J Cancer. 2002;99(5):665–72. doi: 10.1002/ijc.10399. [DOI] [PubMed] [Google Scholar]
- 17.Atayar C, Kok K, Kluiver J, Bosga A, van den Berg E, van der Vlies P, et al. BCL6 alternative breakpoint region break and homozygous deletion of 17q24 in the nodular lymphocyte predominance type of Hodgkin’s lymphoma-derived cell line DEV. Hum Pathol. 2006;37(6):675–83. doi: 10.1016/j.humpath.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Drexler HG. Recent results on the biology of Hodgkin and Reed-Sternberg cells. Leuk Lymphoma. 1993;9(1–2):1–25. doi: 10.3109/10428199309148499. [DOI] [PubMed] [Google Scholar]
- 19.Wlodarska I, Nooyen P, Maes B, Martin-Subero JI, Siebert R, Pauwels P, et al. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101(2):706–10. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- 20.Mathes E, O’Dea EL, Hoffmann A, Ghosh G. NF-κB dictates the degradation pathway of IκBα. EMBO J. 2008;27(9):1357–67. doi: 10.1038/emboj.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson AE, Ling G, Williams C, Backvall H, Ponten J, Ponten F, et al. Analysis of p53 mutations in single cells obtained from histological tissue sections. Anal Biochem. 2000;287(1):25–31. doi: 10.1006/abio.2000.4830. [DOI] [PubMed] [Google Scholar]
- 22.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations in multiple genes caused by deregulation of NF-κB pathway in diffuse large B-cell lymphoma. Nature. 2009;459(7247):717–21. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mottok A, Renne C, Willenbrock K, Hansmann ML, Bräuninger A. Somatic hypermutation of SOCS1 in lymphocyte-predominant Hodgkin lymphoma is accompanied by high JAK2 expression and activation of STAT6. Blood. 2007;110(9):3387–90. doi: 10.1182/blood-2007-03-082511. [DOI] [PubMed] [Google Scholar]
- 24.Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, et al. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25(18):2679–84. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]