Summary
Alcohol addiction is a common affliction with a strong genetic component [1]. Although mammalian studies have provided significant insight into the molecular mechanisms underlying ethanol consumption [2], other organisms such as Drosophila melanogaster are better suited for unbiased, forward genetic approaches to identify novel genes. Behavioral responses to ethanol, such as hyperactivity, sedation, and tolerance, are conserved between flies and mammals [3, 4], as are the underlying molecular pathways [5–9]. However, few studies have investigated ethanol self-administration in flies [10]. Here we characterize ethanol consumption and preference in Drosophila. Flies prefer to consume ethanol-containing food over regular food, and this preference increases over time. Flies are attracted to the smell of ethanol, which partially mediates ethanol preference, but are averse to its taste. Preference for consuming ethanol is not entirely explained by attraction to either its sensory or caloric properties. We demonstrate that flies can exhibit features of alcohol addiction. First, flies self-administer ethanol to pharmacologically relevant concentrations. Second, flies will overcome an aversive stimulus in order to consume ethanol. Third, flies rapidly return to high levels of ethanol consumption after a period of imposed abstinence. Thus, Drosophila ethanol preference provides a new model for studying aspects of addiction.
Results
Flies Prefer to Consume Food Containing Ethanol
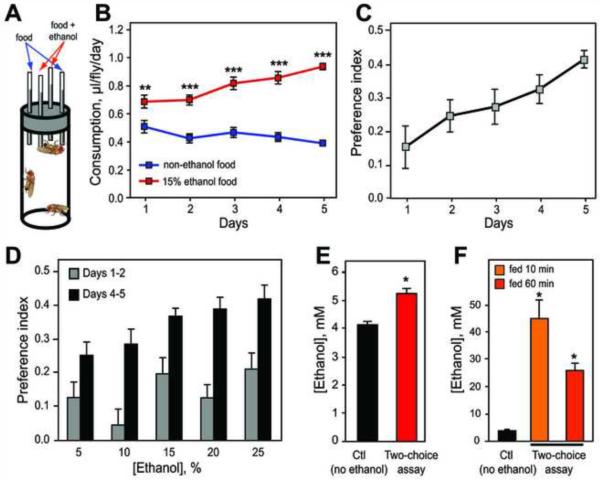
We first characterized the basic parameters of ethanol preference in flies. We used a modified version of the two-choice Capillary Feeder (CAFE) assay [10] to measure ethanol consumption and preference. Our assay is generally analogous to the two-bottle choice assay used in rodent studies of ethanol consumption. In our assay, flies consume liquid food from four capillaries placed vertically through the top of their vials, and consumption is assayed by measuring the descent of each meniscus (Figure 1A). Flies can choose to feed from two capillaries containing non-ethanol food (5% sucrose/5% yeast extract) or two with ethanol-containing food (15% ethanol in 5% sucrose/5% yeast extract). Capillaries are replaced daily. Ethanol preference was quantified by calculating a preference index (PI) defined as (ethanol consumption - non-ethanol consumption) / total consumption. PI can vary between −1 and +1, with positive values indicating preference and negative values indicating repulsion.
Figure 1. Ethanol Preference in Drosophila.
(A) Schematic of the ethanol preference assay (not to scale). Flies choose between liquid food containing 0% or 15% ethanol. Each food type is presented in 2 capillaries to increase the food supply and decrease variability.
(B) Flies consumed a greater amount of 15% ethanol food than non-ethanol food in the preference assay (**p<.01, ***p<.001, two-way repeated measures ANOVA with Bonferroni post-tests, n=16).
(C) PI calculated from consumption values (see text for formula). PI increased over time (p<.01, one-way repeated measures ANOVA, n=16).
(D) The concentration of the ethanol-containing food was varied between 5% and 25% ethanol, and PI values on days 1 and 2 and days 4 and 5 were averaged to compare preference at the beginning and end of the assay. PI increased with increasing ethanol concentration at the end (p<.05) but not the beginning (p>.05) of the assay (one-way ANOVAs, n=16).
(E) Ethanol concentration in flies during the preference assay was significant compared with control flies that never consumed ethanol (*p<.05, Mann-Whitney test, n=3–5 samples).
(F) Ethanol concentrations in flies that were starved and then refed for 10 or 60 min in the preference assay were higher than those of control flies that were also starved/refed but not offered ethanol (*p<.05 compared with control, Mann-Whitney tests, n=3–12 samples).
In this and all other figures, data are represented as mean ± SEM.
Flies displayed a robust preference for consuming 15% ethanol food over non-ethanol food, and this preference increased over 5 days (Figures 1B and 1C). In addition, the variability in preference decreased over the first 3 days (Figure S1A). Filming of the flies revealed that the drinking frequency from ethanol food relative to non-ethanol food increased from day 1 to day 4 of the assay (Figure S2A), paralleling the increase in ethanol consumption. In contrast, the duration of drinking bouts was lower for ethanol food than non-ethanol food on both day 1 and day 4 (Figure S2B). Thus, preferential ethanol consumption occurs via an increase in frequency, but not duration, of drinking bouts associated with ethanol food.
To further analyze the changes in ethanol preference over time, we examined both shorter and longer time courses. First, we asked whether naive flies exhibit immediate ethanol preference and observed that flies displayed a positive, albeit highly variable, ethanol preference during the first 8 hours of the assay (Figure S1B). Second, we asked whether preference continues to increase after 5 days, and found that preference stabilized after 4–5 days (Figure S1C). We also examined the dose-dependence of ethanol preference by varying the concentration of the ethanol-containing food from 5% to 25% ethanol, and observed that preference increased with increasing ethanol concentration. Interestingly, this relationship was observed at the end (days 4–5) but not the beginning (days 1–2) of the preference assay (Figure 1D), indicating that the dose-dependence of preference develops over time.
Next, we investigated whether flies self-administer ethanol food to pharmacologically relevant ethanol concentrations. We first measured the concentration of ethanol present in populations of flies during day 5 of the preference assay. Flies contained an average of 5.2 mM ethanol (Figure 1E), significantly higher than background measurements of flies that did not consume ethanol (4.1 mM, p<.01). However, this value is an underestimate because flies feed sporadically and metabolize ethanol quickly [5], such that most flies were unlikely to contain significant ethanol levels at the time when measurements were conducted. Unfortunately, these measurements require many flies for a single sample, which therefore encompasses a wide range of ethanol concentrations. We hypothesized that measurements of ethanol levels might be much higher and more uniform if the feeding of flies was synchronized. To accomplish this, we starved flies for 20 hours after 4 days of drinking and then returned them to the preference assay for 10 or 60 minutes. These flies contained an average of 45 or 26 mM ethanol, respectively (Figure 1F). These concentrations are sufficiently high to produce behavioral intoxication, such as locomotor hyperactivity (~15 mM [3]) and loss of postural control (~35 mM [5]). Although the starved/refed flies do not reflect the same conditions as the standard continuous access assay, it is important to note that these flies were able to choose whether or not to consume ethanol and were clearly willing to self-administer ethanol to pharmacologically relevant concentrations.
In our preference assay, the ethanol-containing food has nearly four times the number of calories as the non-ethanol food. To determine whether attraction to calories is the primary reason flies consume ethanol, we tested whether varying the caloric ratio between the ethanol and non-ethanol food would influence ethanol preference. We varied the caloric ratio by using different concentrations of food but a fixed concentration of ethanol (Table 1). At lower food concentrations ethanol provides many more calories than the food, yielding a large caloric ratio. Conversely, higher food concentrations provide substantial calories on their own and yield a more modest caloric ratio. If caloric attraction were a significant factor driving ethanol preference, we would expect greater preference at higher caloric ratios. By varying the food concentration between 1% and 8%, the caloric ratio between ethanol and non-ethanol food ranged from 2.2 to 10.9. Despite this large variation, ethanol preference was not affected (Table 1). We therefore conclude that ethanol preference is unlikely to be a byproduct of caloric attraction.
Table 1.
Caloric Ratio Between Ethanol and Non-Ethanol Food Does Not Influence Ethanol Preference
| Sucrose/Yeast Extract Conc., % | Non-Ethanol Food Calories, kcal/L | 10% Ethanol Food Calories, kcal/L | Caloric Ratio Ethanol/Non-Ethanol Food | PI (±SEM) |
|---|---|---|---|---|
| 1 | 56 | 608 | 10.9 | .09 (±.02) |
|
| ||||
| 3 | 167 | 719 | 4.3 | .07 (±.02) |
|
| ||||
| 5 | 279 | 831 | 3.0 | .10 (±.03) |
|
| ||||
| 8 | 446 | 998 | 2.2 | .11 (±.05) |
In different experiments, the concentration of sucrose and yeast extract was varied between 1% and 8%, while the ethanol concentration of ethanol food was always 10%. Consequently, the caloric ratio between ethanol and non-ethanol food ranged from 2.2 to 10.9. This variation did not affect ethanol preference (p>.05, one-way ANOVA, n=24).
Olfactory Attraction and Gustatory Aversion Differentially Influence Ethanol Preference
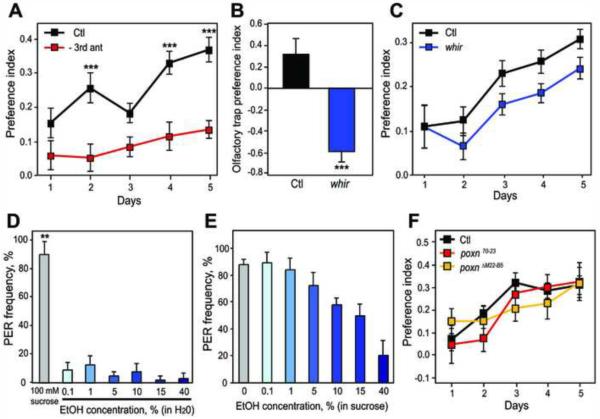
We have shown that ethanol preference is an innate, robust behavior. We next sought to characterize the sensory inputs that influence ethanol preference. To test the role of olfaction, we removed the primary olfactory organs, the third antennal segments [11]. Antennectomized flies fail to startle when ethanol vapor is presented, indicating that they cannot detect its smell [3]. Antennectomized flies exhibited decreased ethanol preference compared with controls (Figure 2A). These flies did not appear sickly and exhibited only an 8% decrease in overall consumption compared with controls (Figure S3), indicating that their decreased preference does not reflect general impairment. To further confirm the role of olfaction, we took advantage of the finding that repeated ethanol vapor exposures can kill the olfactory receptor neurons (ORNs) in the antennae and cause antennae to turn black (French and Heberlein, in press). Flies with black antennae had decreased ethanol preference compared with identically-treated flies with normal antennae (Figure S4). Overall, these data demonstrate that olfaction is an important mediator of ethanol preference.
Figure 2. Olfactory Attraction and Gustatory Aversion Differentially Influence Ethanol Preference.
(A) Flies lacking the third antennal segment had decreased ethanol preference compared with control flies (***p<.001, two-way repeated measures ANOVA with Bonferroni post-tests, n=24).
(B) Wild type flies exhibited positive preference for ethanol in the olfactory trap assay, while whir mutants exhibited olfactory repulsion (***p<.001 for whir vs. control, Student's unpaired t test, n=12).
(C) whir mutants exhibited positive ethanol preference. whir preference displayed a non-significant decrease compared with the control (p=.06, two-way repeated measures ANOVA, n=24).
(D) Ethanol diluted in water did not elicit significant PER (p>.05 for all concentrations). 100 mM sucrose was used as a positive control and elicited significant PER (**p<.01, one sample t tests, n=3 experiments).
(E) When added to 100 mM sucrose, ethanol caused a dose-dependent decrease in PER frequency (p<.001, one-way repeated measures ANOVA, n=3 experiments).
(F) poxnΔM22-B5 and poxn70–23 mutants exhibited ethanol preference similar to the control (p>.05, two-way repeated measures ANOVA, n=16).
To directly show that flies are attracted to the smell of ethanol, we conducted an olfactory trap assay [12] using ethanol. In this assay, flies choose between an ethanol-containing trap and a control trap on the basis of olfaction alone, since they cannot physically contact the ethanol prior to making a choice. Wild-type flies preferred olfactory traps containing 15% ethanol over control traps (Figure 2B), confirming that ethanol has attractive olfactory properties. However, the fact that both antennectomized flies and flies with ablated antennal ORNs still showed a positive preference for consuming ethanol (Figures 2A and S4) suggests that olfactory attraction is not essential for ethanol preference. To test this hypothesis, we utilized white rabbit (whir) mutant flies [13], which are strongly repulsed by the smell of ethanol (Figure 2B). Interestingly, whir flies still displayed a significant preference for consuming ethanol food (Figure 2C). whir exhibited a trend toward decreased preference compared with the control (p=.06). Although whir flies also have an ethanol sensitivity phenotype [13], they demonstrate that ethanol preference can be dissociated from olfactory attraction to ethanol and can even exist in the presence of strong olfactory repulsion.
Next, we tested the role of gustation in ethanol preference. Most ethanol-naive mammals, including rodents [14] and humans [15], perceive the taste of ethanol as predominantly bitter and generally aversive. To determine whether ethanol represents an attractive or repulsive gustatory stimulus in flies, we tested whether it elicits the proboscis extension reflex (PER), an appetitive response that precedes feeding [16]. Palatable liquids elicit PER when applied to gustatory neurons on the legs or labellum [16]. In contrast, unpalatable compounds fail to elicit PER on their own, and can be distinguished from tasteless compounds because they decrease the PER elicited by a palatable substance when added to the same solution [16, 17]. We tested ethanol concentrations ranging from 0.1% to 40% and found that all concentrations failed to elicit significant PER (Figure 2D). To determine whether the lack of PER indicates that ethanol is tasteless or taste-aversive, we added the same concentrations of ethanol to 100 mM sucrose, which elicits reliable PER. When added to sucrose, ethanol caused a dose-dependent decrease in PER frequency (Figure 2E), indicating an aversive taste response. Flies therefore appear to be attracted to the smell of ethanol but averse to its taste, providing an interesting example of a single stimulus eliciting conflicting sensory responses.
Gustatory aversion to ethanol might provide an inhibitory input that actively suppresses ethanol consumption. If this were the case, taste-defective flies would have increased ethanol preference due to the absence of this gustatory repulsion. We tested this hypothesis by using pox neuro (poxn) mutant flies, in which taste bristles are transformed into mechanosensory bristles lacking gustatory receptors [18]. Surprisingly, poxn70–23 and poxnΔM22-B5 null mutants displayed ethanol preference similar to control flies (Figure 2F). These results indicate that gustatory inputs do not play a major inhibitory role in ethanol preference, thus highlighting the difference between an initial sensory response and a long-term preference assay.
Ethanol Preference in Flies Exhibits Features of Addiction
Our results indicate that preference for consuming ethanol cannot be explained solely by an attraction to its caloric or sensory properties, suggesting the potential importance of its pharmacological effects. This hypothesis is supported by the fact that flies voluntarily self-administer ethanol to high internal concentrations that can alter behavior (Figure 1F). We therefore investigated whether ethanol preference in flies shares characteristics of addiction that have been modeled in rodents.
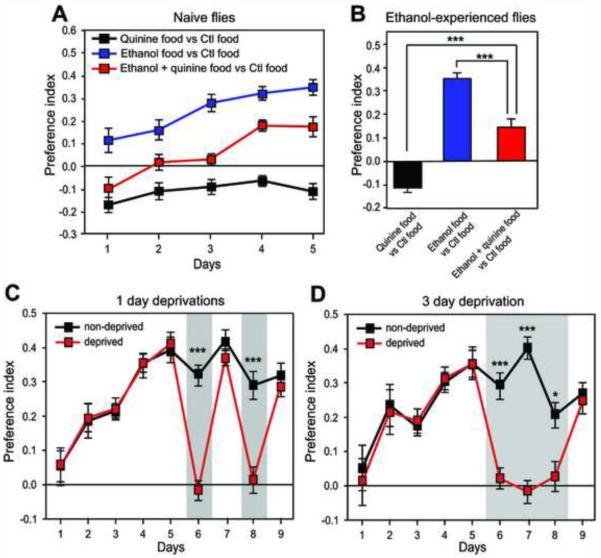
One feature of alcohol addiction is “use of alcohol despite adverse consequences” [19]. Rodent studies have modeled this feature by adding quinine, an aversive compound, to the ethanol solution. Ethanol-experienced rats continue to consume substantial amounts of ethanol even when quinine is added, though intake is usually decreased [20]. First, we tested whether naive flies would exhibit ethanol preference if quinine was added to the ethanol food. These flies did not initially prefer the quinine-laced ethanol food, but developed preference over subsequent days (Figure 3A). This preference does not simply represent habituation to quinine, since flies given a choice between quinine food and normal food exhibited quinine aversion for the entire 5-day period (Figure 3A). Second, we asked whether flies that had been drinking in the standard preference assay for 5 days would maintain ethanol preference when quinine was subsequently added to the ethanol food on day 6. Although preference was decreased compared with ethanol food lacking quinine, these flies displayed a positive preference for quinine-laced ethanol food (Figure 3B). Overall, these results indicate that flies are willing to overcome an aversive stimulus in order to consume ethanol.
Figure 3. Ethanol Preference in Flies Exhibits Features of Addiction.
(A) Over time, naive flies developed ethanol preference when 300 μM quinine was added to the ethanol food throughout the assay. These flies had no preference on days 1–3 (p>.05), but had a positive preference on days 4 (p<.001) and 5 (p<.01). In the absence of ethanol, flies exhibited quinine aversion (p<.05 on all days, one sample t tests, n=16).
(B) Flies that had been drinking in the preference assay for 5 days continued to exhibit ethanol preference when 300 μM quinine was added to the ethanol food on the sixth day (p<.01, one sample t test, n=16), though this preference was decreased compared with controls lacking quinine. All 3 groups are significantly different from each other (***p<.001, one-way ANOVA with Tukey's post-test, n=16).
(C) After 5 days of drinking, flies were divided into two groups, one of which was deprived of ethanol access for two intermittent 1 day intervals (shaded). PI of the deprived group differed from the non-deprived group only during the deprivation periods (day 6 and day 8, ***p<.001). Post-deprivation PI did not differ from pre-deprivation PI (p>.05 for day 7 vs. day 5 and day 9 vs. day 7) or from the non-deprived group (p>.05 for day 7 and day 9).
(D) Same as (C) using a single 3 day deprivation. PI of the deprived group differed from the non-deprived group only during deprivation (days 6–8, *p<.05, ***p<.001). Post-deprivation PI did not differ from pre-deprivation PI or from the non-deprived group (p>.05).
In (C) and (D), one- or two-way repeated measures ANOVAs with Bonferroni's post-tests were used to compare values within the deprived group or between deprived and non-deprived groups, respectively. n=20 in (C) and n=10 in (D).
A second characteristic of alcohol addiction is relapse, defined as a return to ethanol consumption levels equal to or greater than those observed prior to a period of abstinence [21]. In rodents, relapse can be modeled by the alcohol deprivation effect (ADE), in which animals increase ethanol consumption levels following a period of alcohol deprivation [21]. We tested whether an ADE exists in flies by depriving them of ethanol access for either 1 or 3 days after 5 days in the preference assay. During deprivation, non-ethanol food was substituted for ethanol food and the PI dropped near zero, as expected since all four capillaries contained identical nonethanol food (Figures 3C and 3D). Following 1- or 3-day deprivation, flies rapidly returned to peak values of ethanol preference that were not significantly different from pre-deprivation values or from non-deprived controls (Figures 3C and 3D). A second 1-day deprivation yielded similar results (Figure 3C). After each 1 or 3 day deprivation, PI increased at a much greater rate than was observed with naive flies (Figures S5A and S5B). We did not detect an increase in PI following any deprivation protocol. Nevertheless, the rapid increase in preference after deprivation to peak levels rather than the levels measured early in the assay indicates a strong positive memory for ethanol and meets a criterion for relapse.
krasavietz Exhibits Altered Ethanol Sensitivity, Tolerance, and Preference
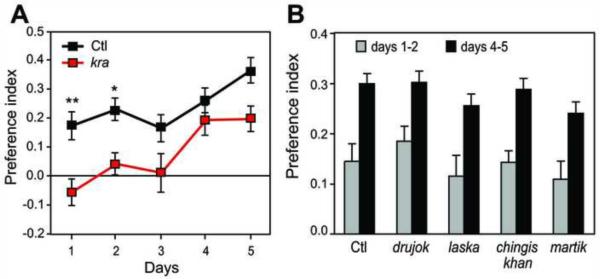
In order to begin identifying genes that influence ethanol preference, we tested whether mutants with known defects in other ethanol-induced behaviors might exhibit altered ethanol preference. We tested 27 mutations affecting ethanol sensitivity or tolerance [4, 9, 22, U.H., unpublished data]. One mutant, krasavietz (kra), exhibited decreased ethanol preference compared with the control (Figure 4A). kra had PI values near zero at the beginning of the assay and did not show ethanol preference until day 4 (Figure 4A). The kra mutation affects a gene also known as exba, which encodes a translation initiation factor. kra was previously found to exhibit decreased sensitivity to ethanol sedation ([22], Figure S6A). Furthermore, kra is the only known mutant with defects in both rapid and chronic tolerance ([22], Figures S6B and S6C), two mechanistically distinct forms of tolerance that differ in their persistence and mode of induction [23].
Figure 4. kra Exhibits Defects in Ethanol Preference.
(A) kra displayed decreased ethanol preference compared with the control (p<.001, two-way repeated measures ANOVA) which was most pronounced at the beginning of the assay (*p<.05, **p<.01, Bonferroni's post-tests, n=25).
(B) The long-term memory mutants drujok, laska, chingis khan, and martik displayed ethanol preference similar to the control (p>.05, two-way repeated measures ANOVA, n=22).
Because kra has been shown to have deficits in long-term memory [24], we asked whether its decreased preference might be due to memory defects. We tested the ethanol preference of 4 other mutants (drujok, laska, chingis khan, and martik) with long-term memory deficits as severe as those of kra [24], but normal ethanol sensitivity and tolerance [22]. All 4 mutants had ethanol preference similar to control flies (Figure 4B), suggesting that long-term memory may not be required for ethanol preference. Thus, the decreased preference of kra is unlikely to be due to a memory defect, and may be related to its altered ethanol sensitivity and/or tolerance.
Discussion
We have characterized ethanol consumption in Drosophila and demonstrated that flies exhibit a robust preference for ethanol-containing food. Furthermore, our assay models several features of mammalian addiction: (1) flies increase ethanol consumption and preference over time; (2) voluntary ethanol consumption leads to pharmacologically relevant ethanol concentrations; (3) caloric or sensory attraction to ethanol does not entirely account for ethanol preference; (4) flies will overcome an aversive stimulus in order to obtain ethanol; and (5) flies exhibit a relapse-like effect after ethanol deprivation. In addition, we have begun to investigate the molecular mechanisms underlying ethanol preference by identifying one mutant, kra, that exhibits deficits in preference.
In several respects, flies appear to have a stronger attraction to ethanol that that measured in most rodent assays. First, naive flies exhibit preference for 15% ethanol, while most rodent strains do not display naive preference for ethanol concentrations near 15% [25, 26]. Second, flies exhibit increasing preference with increasing ethanol concentrations up to 25% (the highest concentration tested), while even high-drinking rodent lines typically consume decreasing ethanol volumes as concentration increases within this range [27, 28]. These disparities may be partly explained by a difference in protocols: flies in our assay drink ethanol mixed with food, while rodent studies typically measure consumption of ethanol diluted in water. However, it is also likely that flies have evolved an intrinsically stronger attraction to ethanol than mammals, given that ethanol-containing fermenting plant materials are a major component of their natural diet.
A robust feature of ethanol preference in flies is its change over time. Initially, preference is low and variable, but over several days it becomes high and more consistent. These changes may reflect that flies require time to associate the pharmacological effect of ethanol with the ethanol-containing capillaries, or to reliably discriminate between the two types of capillaries. It is an open question how this discrimination is achieved: flies may utilize the olfactory and gustatory properties intrinsic to the solutions, or they may instead identify the capillaries by their location relative to other subtle cues or even leave their own cues. However, the normal ethanol preference of all 4 long-term memory mutants we tested suggests that long-term memory may not be required for either the display of ethanol preference or the increase in preference over time.
Flies display conflicting responses to the chemosensory properties of ethanol: they are attracted to its smell and averse to its taste. Other studies have reported examples of a single compound possessing both attractive and aversive qualities mediated by distinct sensory systems, such as acetic acid [29] and carbon dioxide [30, 31]. Our finding that gustatory aversion to ethanol does not actively inhibit ethanol preference fits with rodent studies demonstrating a dissociation between naive taste response to ethanol and ethanol consumption [14]. We speculate that olfactory attraction may be the dominating sensory input, or else flies may quickly overcome taste aversion during the preference assay.
No animal model will ever be a perfect model for alcoholism, since it is a human phenomenon comprised of social, cultural, and cognitive factors. However, animal paradigms can model particular facets of addiction, which is what we have established here for Drosophila. Although we do not claim that ethanol preference in flies and mammals are identical phenomena, our paradigm will be useful for identifying molecular mechanisms involved in ethanol consumption, which can then be tested in mammalian models. In addition to its relevance to addiction, because drugs of abuse act through neural pathways for natural rewards (such as food and sex) [32], studying ethanol preference will also contribute to our understanding of general reward pathways in flies.
Experimental Procedures
Details of the ethanol preference assay are described here. All other methods, including statistical analyses, are described in Supplemental Data.
Our CAFE apparatus consisted of a plastic fly vial with small holes for air exchange and an opaque paper cover to eliminate external distractions, capped with a damp cotton plug. Four 5 μl capillaries (VWR) were inserted into each plug via adaptors made of truncated pipette tips. Capillaries were filled by capillary action. A small mineral oil overlay was added to reduce evaporation, and evaporation was minimal (<5% of total consumption per capillary). Capillaries were measured and replaced daily. Preference assays were conducted at 25°C and 70% relative humidity. 8 flies were allocated into each vial by brief CO2 anesthetization. For deprivation and ethanol-experienced quinine experiments, flies were divided into control and experimental groups after 5 days of drinking in order to equalize baseline PIs prior to manipulation.
Supplementary Material
Acknowledgments
We thank Peter Cameron for advice on PER experiments, Reza Azanchi for technical assistance, Karla Kaun for advice on statistics, and other members of the Heberlein laboratory for helpful discussions. We are grateful to Robert Messing, Patricia Janak, and members of the Heberlein laboratory for comments on the manuscript. This work was supported by an NSF predoctoral fellowship (A.V.D.) and grants from NIH/NIAAA (U.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Data Supplemental Data include Supplemental Experimental Procedures, 6 figures, and 1 table.
References
- 1.Dick DM, Foroud T. Candidate genes for alcohol dependence: a review of genetic evidence from human studies. Alcohol Clin. Exp. Res. 2003;27:868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- 2.Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict. Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolf FW, Rodan AR, Tsai LT-Y, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J. Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28:261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 5.Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- 6.Maas JW, Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J. Neurosci. 2005;25:118–126. doi: 10.1523/JNEUROSCI.4273-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- 9.Corl AB, Berger KH, Ophir-Shohat G, Gesch J, Simms JA, Bartlett S, Heberlein U. Happyhour, a Ste20 family kinase, implicates EGFR signaling in ethanol-induced behaviors. Cell. 2009;137:949–960. doi: 10.1016/j.cell.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. U.S.A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 12.Woodard C, Huang T, Sun H, Helfand SL, Carlson J. Genetic analysis of olfactory behavior in Drosophila: a new screen yields the ota mutants. Genetics. 1989;123:315–326. doi: 10.1093/genetics/123.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenfluh A, Threlkeld RJ, Bainton RJ, Tsai LT-Y, Lasek AW, Heberlein U. Distinct behavioral responses to ethanol are regulated by alternate RhoGAP18B isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Kiefer SW, Dopp JM. Taste reactivity to alcohol in rats. Behav. Neurosci. 1989;103:1318–1326. doi: 10.1037//0735-7044.103.6.1318. [DOI] [PubMed] [Google Scholar]
- 15.Scinska A, Koros E, Habrat B, Kukwa A, Kostowski W, Bienkowski P. Bitter and sweet components of ethanol taste in humans. Drug Alcohol Depend. 2000;60:199–206. doi: 10.1016/s0376-8716(99)00149-0. [DOI] [PubMed] [Google Scholar]
- 16.Dethier VG. The Hungry Fly. Harvard University Press; Cambridge: 1976. [Google Scholar]
- 17.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Awasaki T, Kimura K.-i. pox-neuro is required for development of chemosensory bristles in Drosophila. J. Neurobiol. 1997;32:707–721. doi: 10.1002/(sici)1097-4695(19970620)32:7<707::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Morse RM, Flavin DK. The definition of alcoholism. JAMA. 1992;268:1012–1014. doi: 10.1001/jama.268.8.1012. [DOI] [PubMed] [Google Scholar]
- 20.Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol. Biochem. Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- 21.Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol. Biochem. Behav. 2004;79:439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin. Exp. Res. 2008;32:895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin. Exp. Res. 2004;28:1469–1480. doi: 10.1097/01.alc.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- 24.Dubnau J, Chiang A-S, Grady L, Barditch J, Gossweiler S, McNeil J, Certa U, Broger C, Tully T. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr. Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 25.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 26.Veale WL, Myers RD. Increased alcohol preference in rats following repeated exposures to alcohol. Psychopharmacologia. 1969;15:361–372. doi: 10.1007/BF00403711. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Lobina C, Agabio R, Diaz G, Fa M, Fadda F, Gessa GL, Reali R, Colombo G. Constant absolute ethanol intake by Sardinian alcohol-preferring rats independent of ethanol concentrations. Alcohol Alcohol. 1997;32:19–22. doi: 10.1093/oxfordjournals.alcalc.a008229. [DOI] [PubMed] [Google Scholar]
- 29.Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 31.Fischler W, Kong P, Marella S, Scott K. The detection of carbonation by the Drosophila gustatory system. Nature. 2007;448:1054–1057. doi: 10.1038/nature06101. [DOI] [PubMed] [Google Scholar]
- 32.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.