Abstract
Hypoxia is a common microenvironment in solid tumors and is correlated with tumor progression by regulating cancer cell survival. Recent studies suggest that activation of double-stranded RNA-dependent protein kinase-like endoplasmic reticulum-related kinase (PERK) and phosphorylation of α subunit of eIF2 (eIF2α) confer cell adaptation to hypoxic stress. However, eIF2α is still phosphorylated at a lowered level in PERK knockout cells under hypoxic conditions. The mechanism for eIF2α kinase(s) (eIF2AK)-increased cell survival is not clear. In this report, we provide evidence that another eIF2AK, the amino acid starvation-dependent general control of amino acid biosynthesis kinase (GCN2), is also involved in hypoxia-induced eIF2α phosphorylation. We demonstrate that both GCN2 and PERK mediate the cell adaptation to hypoxic stress. High levels of eIF2α phosphorylation lead to G1 arrest and protect cells from hypoxia-induced apoptosis. Reduced phosphorylation of eIF2α by knocking out either PERK or GCN2 suppresses hypoxia-induced G1 arrest and promotes apoptosis in accompany with activation of p53 signal cascade. However, totally abolishing phosphorylation of eIF2α inhibits G1 arrest without promoting apoptosis. On the basis of our results, we propose that the levels of eIF2α phosphorylation serve as a “switch” in regulation of G1 arrest or apoptosis under hypoxic conditions.
Introduction
Cells respond to external stimuli by rapid changes in their translational capacity. Stress, such as growth factor depletion, heat shock, and virus infection, rapidly inhibits protein synthesis through phosphorylation of the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α) [1,2]. Four kinases, the double-stranded RNA-dependent protein kinase (PKR), the hemin-regulated inhibitor (HRI), the amino acid starvation-dependent general control of amino acid biosynthesis kinase (GCN2), and the PKR-like endoplasmic reticulum-related kinase (PERK), have been identified to phosphorylate eIF2α and reduce translation initiation in response to stress [2]. Recently, PERK-mediated phosphorylation of eIF2α has been shown to suppress protein synthesis and cell growth on hypoxia [3–6]. In vivo studies show that hypoxia-induced activation of PERK inhibits protein synthesis, decreases cell growth, and promotes tumor adaptation [2–6].
Adaptation to hypoxia could also be regulated by hypoxia-inducible factor 1 (HIF-1), which associates with tumor progression and resistance to radiotherapy and chemotherapy [2,7,8]. HIF-1 is a key mediator in hypoxia [9] and regulates the expressions of more than 70 genes [10] that facilitate metabolic adaptation, cell proliferation, cell cycle arrest, apoptosis, angiogenesis, angioinvasion, and metastasis [11,12]. HIF-1 is a heterodimer composed of α and β subunits. The expression of HIF-1β is constitutive, whereas the level of HIF-1α is highly regulated by oxygen levels [9]. Whereas HIF-1α undergoes rapid degradation and is maintained at basal levels in normoxia, it accumulates through protein stabilization and/or increased expression under hypoxia [13,14]. HIF-1α coordinates with p53, murine double minute 2 (Mdm2), and p21WAF1 in the regulation of cell cycle arrest and apoptosis under hypoxic conditions. However, the regulatory mechanism is controversial [15–17]. There are reports indicating that HIF-1α forms a complex with p53 and stabilizes p53 [18], which promotes cell cycle arrest mediated by p21WAF1 and induces apoptosis [19]. Mdm2 negatively regulates p53 by promoting p53 degradation [20]. Other reports suggest that HIF-1α does not directly associate with p53, but with Mdm2 [17,21], which upregulates HIF-1α levels [17,22,23]. The mechanism for hypoxia-mediated cell cycle arrest and apoptosis remains unclear [24–26]. In this report, we provide pieces of evidence that translation initiation plays a critical role in regulation of hypoxia-induced signaling circuit. Under hypoxic conditions, PERK and GCN2 are activated and coordinately phosphorylate eIF2α. Depending on the levels of eIF2α phosphorylation, the expression and activity of HIF-1α, p53, Mdm2, and p21WAF1 are altered under hypoxic conditions. Our findings not only significantly advance the understanding of the mechanism for hypoxia/eIF2α phosphorylation-mediated G1 arrest and apoptosis signaling circuit but also lead to the discovery of a potential target for antitumor therapies.
Materials and Methods
Cell Culture and Hypoxic Treatments
Mouse embryonicfibroblast (MEF) wild type(MEFWT), MEFPERK knockout (MEFPERK-/-), MEF GCN2 knockout (MEFGCN2-/-), and MEF S51A mutant (MEFA/A), in which the wild type eIF2α was replaced with a nonphosphorylatable S51A mutated eIF2α, were kindly provided by Dr. RJ Kaufman (University of Michigan Medical School, Ann Arbor, MI). The cells were cultured in Dulbecco's modified Eagle medium (DMEM; Cellgro, Manassas, VA) supplemented with 1% penicillin and streptomycin and 10% fetal bovine serum (Cellgro) at 37°C with 5% CO2. GasPak EZ Anaerobe Pouch System (BD Biosciences, VWR, S. Plainfield, NJ) was used to reduce the oxygen levels to less than 1% (mean, 0.7%) in 90 minutes.
Western Blot Analysis
The cells were washed with cold phosphate-buffered saline (PBS) twice and lysed in buffer with 50 mM Tris-HCl, 150 mM NaCl, 0.05% EDTA, 0.5% IGEPAL CA-630, and a cocktail of protease inhibitors (Roche, Indianapolis, IN). An equal amount of total proteins was separated by SDS-PAGE. Protein was electroblotted onto Immobilon-P membrane (Millipore, Temecula, CA), which was then blocked with 5% milk in PBST (PBS with 0.1% Tween-20). The interested proteins were probed with the corresponding antibodies and visualized by LumiGLO reagent and peroxide (Cell Signaling, Danvers, MA). Anti-eIF2α, anti-phosphorylated eIF2α (Ser 51), and anti-β-actin were purchased from Sigma (St. Louis, MO). Anti-Mdm2 (N-20), anti-p53 (Bp53), anti-HIF-1α, and secondary horseradish peroxidase-linked antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Viability Assay
The cells (1 x 105) were seeded in 12-well plates and were then exposed to normoxia or hypoxia. The viability was measured at different time points by the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, Madison, WI) according to the manufacturer's protocol. After removing the medium, 75 µl of CellTiter-Glo reagent was diluted once with water and added into each well. The cells were incubated with reagent at room temperature on a shaker for 5 minutes and then incubation was continued for 10 minutes. Luminescence of each sample (100 µl) was measured by a Lumat LB9507 luminometer (Berthold Technologies, Oak Ridge, TN).
Cell Cycle Analysis
The cells (2 x 106) were seeded in 100-mm tissue culture plates and exposed to normoxia or hypoxia. The cells were harvested and washed twice with cold PBS. The cells were suspended in 200 µl of PBS and fixed in 4 ml of cold 70% ethanol at -20°C overnight. The cells were centrifuged at 4°C for 10 minutes. The cell pellets were stained by a propidium iodide mixture (100 µg/ml RNase and 50 µg/ml propidium iodide) at 37°C for 30 minutes. The amount of apoptotic cells in a total of 1 x 104 cells was determined by a flow cytometer (Becton Dickinson, San Jose, CA) and analyzed by ModFit LT (Verity Software House, Topsham, ME).
Apoptosis Assay
The cells (2 x 105) were seeded in 12-well plates and exposed to normoxia or hypoxia for 2 days. The cells were analyzed by a Cell Death Detection ELISA kit (Roche Diagnostics), which measures cleaved histone and DNA complex. The assay was performed according to the manufacturer's manual. The absorbance at 405/590 nm was measured by a SPECTRA Max M2 multichannel fluorescence plate reader (Molecular Devices, Sunnyvale, CA).
Luciferase Assay
Cells were seeded in 24-well plates and cotransfected with an inducible luciferase expression vector and a Renilla luciferase expression vector (Panomics, Fremont, CA). At 24 hours after transfection, the cells were exposed to normoxia or hypoxia. The luciferase activities were measured by a Dual-Luciferase Reporter Assay kit (Promega) according to the manufacturer's manual. Luciferase and Renilla luciferase activities were measured by a Lumat LB 9507 luminometer (Berthold Technologies). The Renilla luciferase activity was used to normalize the transfection efficiency.
Glucose Uptake Assay
The cells were washed with serum-free DMEM twice and then cultured under hypoxia with serum-free medium for 12 hours. The cells were washed with Kreb's Ringer phosphate (CRP) buffer (136 mM NaCl, 4.7 mM KCl, 1.25 mM MgSO4, 1.25 mM CaCl2, 8.1 mM Na2HPO4, 1.9 mM NaH2PO4) twice and then incubated with 450 µl of KRP buffer for 30 minutes. Fifty microliters of KRP supplemented with 1 µCi/ml [3H] 2-deoxy-d-glucose and 1 mM glucose was added to each sample and incubated for 30 minutes at 4°C. The buffer was removed, and the cells were washed twice with cold PBS. Cells were lysed with 350 µl of 0.2 M NaOH. Radioactivity was determined by LS 6500 multipurpose scintillation counter (Beckman Coulter, Fullerton, CA).
Clonogenic Assay
The cells (5 x 103) were seeded in six-well plates and cultured in 95% air and 5% CO2 at 37°C for 6 days. The cells were then washed with PBS twice and fixed by cold methanol for 10 minutes at -20°C. The fixed cells were stained by 1% crystal violet in 25% methanol for 10 minutes at room temperature. The cells were finally rinsed with distilled water, and the colonies with a size more than 0.5 mm were counted.
Statistical Analysis
Student's t test was used to analyze the significance of data. P < .05 was considered significant.
Results
Hypoxia-Induced Cell Death Is Protected by PERK and GCN2 in an eIF2α Phosphorylation-Independent Manner
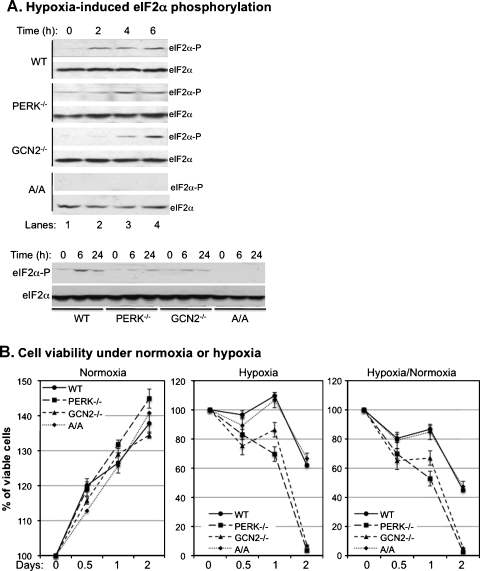
PERK has been suggested to mediate the hypoxia-induced phosphorylation of eIF2α and increase tolerance of tumor cells to hypoxic stress [3,4,26]. However, whereas the thapsigargin-induced eIF2α phosphorylation is totally inhibited in MEFPERK-/- cells, the hypoxia-induced phosphorylation is only partially inhibited in the same cells [26]. The results suggested that other eIF2α kinase (eIF2AK) besides PERK might also be involved in the hypoxia-induced eIF2α phosphorylation. Our recent study demonstrated that GCN2 coordinates with PERK in regulation of UVB-induced phosphorylation of eIF2α [27]. To determine whether GCN2 is also involved in hypoxia-induced phosphorylation of eIF2α, we analyzed time-dependent phosphorylation of eIF2α in MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells after modest hypoxia treatment. As expected, whereas eIF2α was not phosphorylated in MEFA/A cells, an increased phosphorylation of eIF2α was detected at 2 hours after treatment in the other cell lines. Compared with MEFWT cells, the hypoxia-induced phosphorylation of eIF2α was delayed and reduced in MEFPERK-/- cells (Figure 1A), which agreed with previous reports [26,28]. Moreover, our data also showed that the phosphorylation of eIF2α was similarly delayed and reduced in MEFGCN2-/- cells (Figure 1A). These results demonstrate that both PERK and GCN2 are involved in the hypoxia-induced phosphorylation of eIF2α.
Figure 1.
Both PERK and GCN2 mediate hypoxia-induced eIF2α phosphorylation and cell death. MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells were used in the experiments as indicated. (A) The cells were exposed to hypoxia for the indicated time points before immunoblot analysis with phosphorylated eIF2α (Ser 51) and total eIF2α antibodies. (B) The cells were exposed to normoxia or hypoxia for the indicated time points and then viability assays were performed. The bars represent the means of three independent experiments. (C) The cells were exposed to hypoxia for 12 hours with serum-free DMEM and were then fed with glucose supplemented with [3H] 2-deoxy-d-glucose. Total protein was collected, and the radioactivity was measured by liquid scintillation counter. The bars represent the means of three independent experiments. *P < .05 mutant versus wild type. (D) The cells were exposed to normoxia or hypoxia for indicated time points and photographed using microscopy equipped with a Nikon digital camera (Nikon, West Chester, OH).
It has been found that hypoxia-induced PERK activation protects cells from death by increasing eIF2α phosphorylation [3]. To determine whether GCN2 can also protect cells from hypoxia-induced death, we analyzed viabilities of MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells under normoxic or hypoxic conditions. Our data showed that, under normoxic conditions, the growth rates of MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A were within 10% differences (Figure 1B, Normoxia), but under hypoxic conditions, viabilities of MEFGCN2-/- and MEFPERK-/- cells decreased more rapidly than MEFWT and MEFA/A cells (Figure 1B, Hypoxia). After correcting the survival rates in hypoxia with the growth rates in normoxia, our data demonstrated that the survival rates for MEFGCN2-/- and MEFPERK-/- cells were decreased in 48 hours to 2.4 ± 0.1% and 5.1 ± 0.3%, respectively (Figure 1B, Hypoxia/Normoxia). Surprisingly, the survival rate of MEFA/A cells was 47.3 ± 3.2%, which was similar to the 44.9 ± 2.1% survival rate of MEFWT cells (Figure 1B, Hypoxia/Normoxia).
To further confirm the above results, we analyzed cell viabilities using metabolic and morphologic analyses. Glucose uptake assay was used to measure the metabolic activities of the cells in hypoxia. Our data showed that whereas the metabolic activities of MEFWT and MEFA/A cells were maintained at 75.4 ± 0.3% and 83.5 ± 0.4%, respectively, the activities in MEFGCN2-/- and MEFPERK-/- cells were decreased to 36.0 ± 0.2% and 42.3 ± 0.2%, respectively (Figure 1C). In addition, the morphologic analysis also showed that the MEFGCN2-/- and MEFPERK-/- cells were more sensitive to hypoxia than MEFWT and MEFA/A cells (Figure 1D). These results confirmed that the hypoxia-induced cell death depended on but not linearly related to the levels of eIF2α phosphorylation.
To determine whether PERK or GCN2 influences cell survival in hypoxia independently of eIF2α, we tried to knockout PERK or GCN2 in the MEFA/A cells using a siRNA method. Our results showed that when PERK or GCN2 siRNA reduced the viability of MEFWT cells under hypoxia, they did not affect the viability of MEFA/A cells (Figure W1A). The preliminary result suggests that PERK- and GCN2-mediated hypoxia-induced cell death is eIF2α-dependent. However, our data are not conclusive because the transfection efficiency is less than 20% based on the control FITC-labeled scramble siRNA (Figure W1B).
Hypoxia-Induced Cell Cycle Arrest Is Dependent on PERK and GCN2 by Regulating HIF-1α and p21WAF1
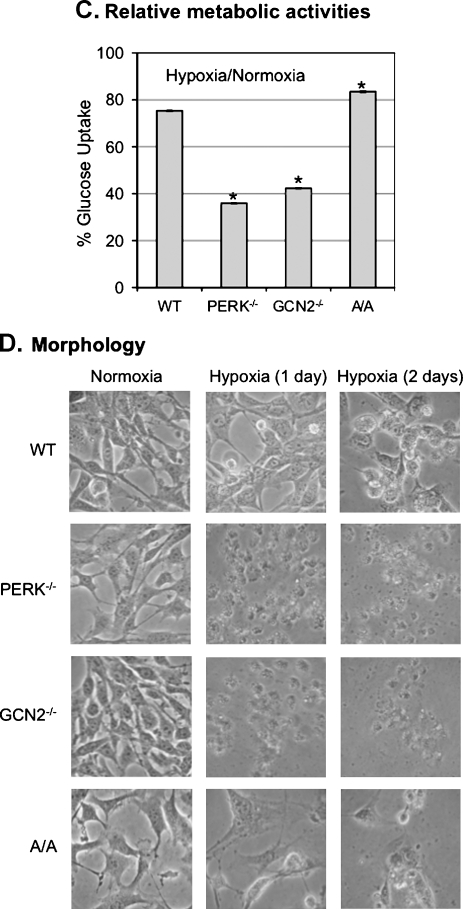
Both PERK and GCN2 contribute to cell cycle arrest on endoplasmic reticulum stress [29]. To determine whether both PERK and GCN2 play a role in the regulation of cell cycle arrest in hypoxia, we performed cell cycle analysis in MEFWT, MEFGCN2-/-, MEFPERK-/-, and MEFA/A cells. Our data showed that hypoxia induced G1 arrest in MEFWT cells but not in MEFPERK-/-, MEFGCN2-/-, or MEFA/A cells (Figure 2A). These data demonstrate that the activation of PERK/GCN2 and the phosphorylation of eIF2α are essential for hypoxia-induced cell cycle arrest.
Figure 2.
Hypoxia induces G1 arrest in MEFWT but not in MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells. (A) The cells were treated with hypoxia for 12 hours before the cell cycle analysis using flow cytometry. The data were analyzed by ModFit software. The bars represent the means of three independent experiments. *P < .05 hypoxia versus normoxia. (B) The cells were treated with hypoxia for the time points as indicated, and the protein levels of HIF-1α and p21WAF1 were analyzed by Western blot.
To assess the molecular mechanism for PERK/GCN2-mediated eIF2α phosphorylation in regulation of cell cycle arrest, we analyzed the expressions of HIF-1α and p21WAF1, the key cell cycle regulators in hypoxia [25,30]. Our data showed that HIF-1α expression was induced in MEFWT (Figure 2B). The hypoxia-inducibility of HIF-1α expression was eliminated or reduced in MEFPERK-/- and MEFGCN2-/- cells (Figure 2B). HIF-1α was not detected in MEFA/A cells in either normoxia or hypoxia (Figure 2B). In contrast to HIF-1α, the maximal inducibility of p21WAF1 in hypoxia was achieved in MEFWT cells (Figure 2B). The inducibility, but not the expression levels of p21WAF1, was correlated to the hypoxia-induced cell cycle arrest. These results suggest that the hypoxia-induced cell cycle arrest is mediated by the inductions of HIF-1α and p21WAF1, which is regulated by PERK/GCN2-mediated eIF2α phosphorylation.
PERK and GCN2 Protect Cells from Hypoxia-Induced Apoptosis
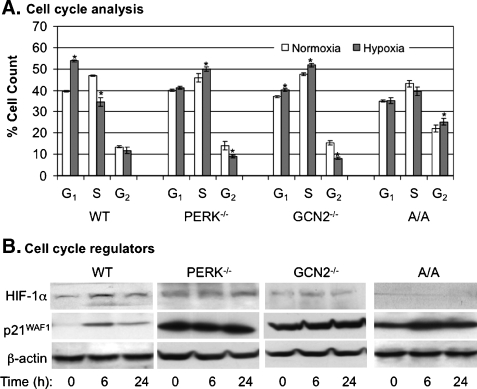
To determine whether GCN2, like PERK, protects cells from hypoxia-induced apoptosis, we assessed the roles of PERK/GCN2-mediated eIF2α phosphorylation in regulation of apoptosis under hypoxia. MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells were exposed to hypoxic stress, and then apoptotic cell death was analyzed by examining the cleavage of histone-DNA complex. Our data showed that higher levels of nucleosome contents were displayed in MEFPERK-/- and MEFGCN2-/- cells than the levels in MEFWT and MEFA/A cells (Figure 3A). The result indicates that both PERK and GCN2 are involved in the regulation of apoptotic cell death in hypoxia.
Figure 3.
Both PERK and GCN2 regulate p53 signaling cascade and apoptosis in hypoxia. MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells were used in the experiments. (A) The cells were exposed to normoxia or hypoxia for 36 hours before apoptotic assays by detecting cleaved histone/DNA complex. The bars represent the means of three independent experiments. *P < .05 mutant versus wild type under normoxia or hypoxia. (B) The cells were treated with hypoxia for the time points as indicated and the protein level of Mdm2 was analyzed by Western blot. (C and D) The cells were cotransfected with p53 luciferase reporter plasmid or Bax luciferase reporter plasmid. Renilla luciferase reporter plasmid was used to normalize the transfection efficiency. Twenty-four hours after transfection, the cells were exposed to normoxia or hypoxia for 24 hours, and the activities of p53 and Bax were analyzed by luciferase assay. The bars represent the means of three independent experiments. *P < .05 mutant versus wild type.
Because p53 plays a central role in regulation of apoptosis during hypoxia [31–34], we determined whether PERK and GCN2 would protect cells from hypoxia-induced apoptosis via regulating p53 signaling pathway. Our data demonstrated that p53 was increased in the four cell lines on hypoxic stress (Figure 3B). However, the maximal inducibility of p53 was achieved in MEFPERK-/- and MEFGCN2-/- cells (Figure 3B). At the same time, the expression of a p53 negative regulator, Mdm2, was decreased in all cell lines independent of eIF2α phosphorylation levels (Figure 3B). These results suggest that activation of PERK and GCN2 inhibits hypoxia-induced activation of p53 signaling pathway independent of Mdm2. To confirm the results that the maximal inducibility of p53 was achieved in MEFPERK-/- and MEFGCN2-/- cells, we used p53 transcriptional activity-regulated luciferase expression system to determine p53 DNA binding ability. Our data showed that p53 transcriptional activity was up to 4.0 ± 0.5- and 4.4 ± 0.8-folds in MEFPERK-/- and MEFGCN2-/- cells, whereas the activity was only up to 1.4 ± 0.1- and 1.7 ± 0.1-folds in MEFWT and MEFA/A cells, respectively, in hypoxia (Figure 3C). The promoter activity of a p53 downstream gene, Bax, was also upregulated more in MEFGCN2-/- and MEFPERK-/- cells than in MEFWT and MEFA/A cells (Figure 3D). These results suggest that the lowered viability in MEFPERK-/- and MEFGCN2-/- cells was due to the upregulated apoptosis, which was mediated by p53 signaling pathways.
PERK, GCN2, and Phosphorylated eIF2α Promote Recovery of Cells from Hypoxia
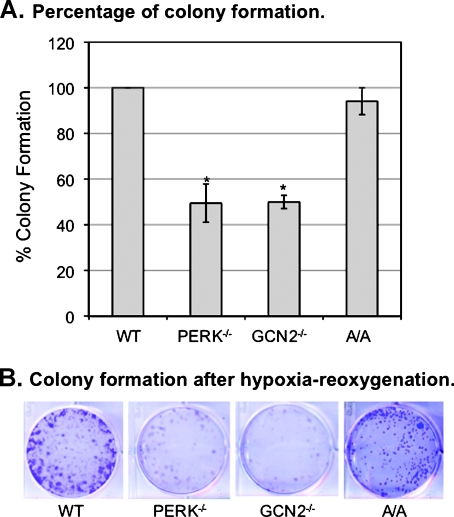
The ability to recover from stress is also an important property to measure the regulation of survival and growth of cells after exposure to a stress. We determined whether PERK/GCN2-mediated eIF2α phosphorylation affects the ability of cells to recover from hypoxia. Clonogenic assay was used to measure survival and recovery of cells from hypoxic stress. The MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells were exposed to hypoxia for 24 hours and then cultured under normal conditions for 6 days. Our data showed that, compared with MEFWT cells, the survival rates after hypoxia were reduced 50.5 ± 8.4% and 50.0 ± 2.9% for MEFPERK-/- and MEFGCN2-/- cells, respectively (Figure 4A). The survival rate of MEFA/A cells was reduced only by 5.9 ± 5.9% (Figure 4A). Noticeably, the sizes of MEFA/A colonies were much smaller than the other three cell lines (Figure 4B), indicating that eIF2α phosphorylation was required for cell growth during recovery but not required for cell survival on hypoxia. These data demonstrate that partial reduction of eIF2α phosphorylation (PERK or GCN2 knockout) decreases cell recovery by lowering survival rate, whereas total elimination of eIF2α phosphorylation (eIF2α S51A mutation) decreases cell recovery by lowering cell growth rate during reoxygenation after hypoxic stress. These results suggest that the hypoxia-induced activation of PERK/GCN2 and eIF2α phosphorylation coordinatively regulate cell growth and apoptosis after hypoxic stress.
Figure 4.
Both PERK and GCN2 mediate recovery of cells from hypoxic stress. MEFWT, MEFPERK-/-, MEFGCN2-/-, and MEFA/A cells were exposed to hypoxia for 24 hours. Cells were collected and stained by Trypan blue. Nonapoptotic cells were counted. Five thousand cells were replated and cultured under normoxia for 6 days. Cells were then fixed by methanol for 10 minutes at -20°C and stained by 1% crystal violet (25% methanol) for 10 minutes, washed by distilled water, and dried. (A) The colonies with a size greater than 0.5 mm were counted. The degree of colony formation was expressed as percentage of MEFWT cells. The bars represent the means of three independent experiments. *P < .05 mutant versus wild type. (B) The plates were photographed using microscopy equipped with a Nikon digital camera.
Discussion
A hypoxic microenvironment in solid tumors often correlates with tumor progression and therapy resistance [35]. HIF-1α is a key regulator in response to hypoxia and has been shown to regulate angiogenesis, metabolic adaptation, proliferation, apoptosis, and invasion [9–12,36–39]. Hypoxia increases cancer cell-induced lymphatic endothelial cell invasion and migration [40], whereas it decreases macromolecular synthesis and slows down cell proliferation [2,41]. Prolonged hypoxia also induces an energy-depleting response that activates mammalian target of rapamycin-signaling network [42]. Recent studies show that hypoxia activates PERK, which phosphorylates eIF2α, reduces protein synthesis, and contributes to hypoxic adaptation [3–6,26,28]. However, in hypoxic cells lacking PERK, the phosphorylation of eIF2α on Ser 51 is still upregulated [26], which indicates that there could be other eIF2AKs activated in hypoxia. In this study, we demonstrate that similar to MEFPERK-/- cells, the phosphorylation of eIF2α in MEFGCN2-/- cells was also delayed in hypoxia (Figure 1A), suggesting that GCN2 is also involved in the phosphorylation of eIF2α in response to hypoxia. Besides PERK and GCN2, HRI and PKR can also phosphorylate eIF2α. Although PKR has been shown not to induce eIF2α phosphorylation under hypoxia [26], we cannot rule out that HRI is also involved in hypoxia-induced eIF2α phosphorylation.
Our results suggest that reduction of eIF2α phosphorylation by PERK or GCN2 knockout sensitized the cells to hypoxia-induced cell death (Figures 1 and 4), but elimination of eIF2α phosphorylation by eIF2α S51A knock-in had no effects on the cell survival under the same conditions (Figures 1 and 4). These results disagree with the previous in vitro study indicating that abolishing eIF2α phosphorylation reduces cell survival in hypoxia [3]. However, our results agree with the in vivo data in the same report, which shows that the tumor volume from MEFA/A cells is much larger than the tumors from MEFPERK-/- cells but is smaller than the tumors from MEFWT cells. Because tumor hypoxia is transient in vivo and reoxygenation also affects tumor survival and growth, we further confirm our in vitro data by investigating the survival and recovery on hypoxia-reoxygenation in the four cell lines. Our data from the viability and colonogenic assays indicate that partial reduction of eIF2α phosphorylation by knocking out either PERK- or GCN2-promoted cell death but did not affect cell growth on hypoxia-reoxygenation (Figure 4). In contrast, totally abolished eIF2α phosphorylation did not influence cell survival but promoted cell growth on hypoxia-reoxygenation (Figure 4). These results suggest that PERK/GCN2 knockout affects cell growth and death on hypoxia-reoxygenation through differential mechanism as eIF2α S51A mutation does. The controversial observation with the previous in vitro data [3] may be due to the experimental conditions. We used moderate hypoxia (∼1% oxygen), which is close to the physiological condition in solid tumor [43,44]. However, the previous study was performed under extreme hypoxia (<0.02% oxygen) [3].
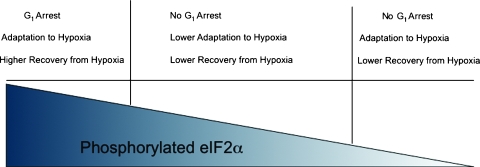
To further analyze the mechanism for eIF2AKs and eIF2α phosphorylation-regulated tumor progression in hypoxia, we analyzed their roles in regulating the expressions of several key hypoxia-induced factors, such as HIF-1α and p53 [25,31–34,39,45]. The correlations among the gene expression patterns, cell cycle arrest, and apoptosis were also studied. Our data showed that the hypoxia-induced eIF2α phosphorylation is critical in the regulation of the inducibility and expression of HIF-1α and p21WAF1 (Figure 2). However, only the inducibilities, but not the levels, of HIF-1α and p21WAF1 expression were correlated to hypoxia-induced cell cycle arrest (Figure 2). Whereas PERK and GCN2 are required for hypoxia-induced HIF-1α/p21WAF1 expression, they suppress hypoxia-induced activation of p53 (Figure 3). Elimination of PERK or GCN2 activity also sensitizes the cells to hypoxia-induced apoptosis (Figure 3). Interestingly, totally abolishing eIF2α phosphorylation has no effects on p53 activation and apoptosis (Figure 3), but affects HIF-1α/p21 WAF1 inducibility and G1 arrest after hypoxic stress (Figure 2). The mechanism could be due to the extremely low expression of HIF-1α in both normoxia and hypoxia (Figure 2B), which plays a critical role in regulation of hypoxia-induced cell cycle arrest [46]. On the basis of these results, we propose that PERK/GCN2-mediated eIF2α phosphorylation serves as a “switch” that determines the fate of cells after hypoxic stress (Figure 5). A lower level of eIF2α phosphorylation “turns on” a death signal by inhibiting G1 arrest and promoting apoptosis through activating p53 signal cascade. In contrast, higher levels of eIF2α phosphorylation “switch” the death signal to an adaptive signal by promoting G1 arrest and reducing apoptosis through activating the HIF-1α/p21WAF1 signaling pathway. Our proposed model has the potential to be used to develop new therapeutics for cancer treatment by targeting PERK/GCN2-meidated eIF2α phosphorylation.
Figure 5.
The model for the roles of eIF2α phosphorylation in cell fate determination.
Supplementary Material
Acknowledgments
The authors thank Andrea Gibson and O. Luke Carpenter for editorial assistance.
Abbreviations
- eIF2α
the α subunit of the eukaryotic initiation factor-2
- EIF2AK
eIF2α kinase
- GCN2
amino acid starvation-dependent general controlof amino acid biosynthesis kinase
- HIF-1α
hypoxia-inducible factor 1 ≠
- HRI
hemin-regulated inhibitor
- Mdm2
murine double minute 2
- MEF
mouse embryonic fibroblast
- PERK
PKR-like endoplasmic reticulum-related kinase
- PKR
double-stranded RNA-dependent protein kinase
Footnotes
This work was partially supported by the National Institutes of Health grants RO1 CA86928 (to S.W.) and R56 CA086928 (to S.W.).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S, Kaufman RJ, Weppler SA, Voncken JW, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koumenis C, Wouters BG. “Translating” tumor hypoxia: unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4:423–436. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 3.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blais JD, Addison CL, Edge R, Falls T, Zhao H, Wary K, Koumenis C, Harding HP, Ron D, Holcik M, et al. PERK-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Mol Cell Biol. 2006;26:9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koritzinsky M, Rouschop KM, van den Beucken T, Magagnin MG, Savelkouls K, Lambin P, Wouters BG. Phosphorylation of eIF2α is required for mRNA translation inhibition and survival during moderate hypoxia. Radiother Oncol. 2007;83:353–361. doi: 10.1016/j.radonc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 8.Vaupel P, Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Clin Biol. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Schmid T, Schnitzer S, Brüne B. Tumor hypoxia and cancer progression. Cancer Lett. 2006;237:10–21. doi: 10.1016/j.canlet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 12.Buchler P, Reber HA, Tomlinson JS, Hankinson O, Kallifatidis G, Friess H, Herr I, Hines OJ. Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer. Neoplasia. 2009;11:196–206. doi: 10.1593/neo.08734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arriola EL, Lopez AR, Chresta CM. Differential regulation of p21waf-1/cip-1 and Mdm2 by etoposide: etoposide inhibits the p53-Mdm2 autoregulatory feedback loop. Oncogene. 1999;18:1081–1091. doi: 10.1038/sj.onc.1202391. [DOI] [PubMed] [Google Scholar]
- 14.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 15.Alarcon R, Koumenis C, Geyer RK, Maki CG, Giaccia AJ. Hypoxia induces p53 accumulation through MDM2 down-regulation and inhibition of E6-mediated degradation. Cancer Res. 1999;59:6046–6051. [PubMed] [Google Scholar]
- 16.Zhang L, Hill RP. Hypoxia enhances metastatic efficiency by up-regulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res. 2004;64:4180–4189. doi: 10.1158/0008-5472.CAN-03-3038. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen AL, Qanungo S, Schneider EA, Jiang BH, Agani FH. Mdm2 and HIF-1α interaction in tumor cells during hypoxia. J Cell Physiol. 2005;204:364–369. doi: 10.1002/jcp.20406. [DOI] [PubMed] [Google Scholar]
- 18.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DE. The p53 tumor suppressor: critical regulator of life & death in cancer. Apoptosis. 2001;6:7–15. doi: 10.1023/a:1009659708549. [DOI] [PubMed] [Google Scholar]
- 20.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 α and Mdm2 modulate p53 function. J Biol Chem. 2003;278:13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 22.Bardos JI, Chau NM, Ashcroft M. Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1α expression. Mol Cell Biol. 2004;24:2905–2914. doi: 10.1128/MCB.24.7.2905-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaRusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD. Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1α and Hdm2. Cancer Res. 2007;67:450–454. doi: 10.1158/0008-5472.CAN-06-2710. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 25.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2α. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu W, Laszlo CF, Miao Z, Chen H, Wu S. The role of nitric oxide synthase in regulation of ultraviolet light-induced phosphorylation of the α-subunit of eukaryotic initiation factor 2. J Biol Chem. 2009;284:24281–24288. doi: 10.1074/jbc.M109.008821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamanaka RB, Bennett BS, Cullinan SB, Diehl JA. PERK and GCN2 contribute to eIF2α phosphorylation and cell cycle arrest after activation of the unfolded protein response pathway. Mol Biol Cell. 2005;16:5493–5501. doi: 10.1091/mbc.E05-03-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1α is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1α as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20:5779–5788. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Biju MP, Wang MH, Haase VH, Dong Z. Cytoprotective effects of hypoxia against cisplatin-induced tubular cell apoptosis: involvement of mitochondrial inhibition and p53 suppression. J Am Soc Nephrol. 2006;17:1875–1885. doi: 10.1681/ASN.2005121371. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Ahmed A, Poon E, Perusinghe N, de Haven Brandon A, Box G, Valenti M, Eccles S, Rouschop K, Wouters B, et al. Small-molecule activation of p53 blocks hypoxia-inducible factor 1α and vascular endothelial growth factor expression in vivo and leads to tumor cell apoptosis in normoxia and hypoxia. Mol Cell Biol. 2009;29:2243–2253. doi: 10.1128/MCB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 36.Green SL, Giaccia AJ. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J Sci Am. 1998;4:218–223. [PubMed] [Google Scholar]
- 37.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000;106:809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 39.Goda N, Dozier SJ, Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal. 2003;5:467–473. doi: 10.1089/152308603768295212. [DOI] [PubMed] [Google Scholar]
- 40.Mikhaylova M, Mori N, Wildes FB, Walczak P, Gimi B, Bhujwalla ZM. Hypoxia increases breast cancer cell-induced lymphatic endothelial cell migration. Neoplasia. 2008;10:380–389. doi: 10.1593/neo.07854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 42.Schneider A, Younis RH, Gutkind JS. Hypoxia-induced energy stress inhibits the mTOR pathway by activating an AMPK/REDD1 signaling axis in head and neck squamous cell carcinoma. Neoplasia. 2008;10:1295–1302. doi: 10.1593/neo.08586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- 44.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 45.Cosse JP, Ronvaux M, Ninane N, Raes MJ, Michiels C. Hypoxia-induced decrease in p53 protein level and increase in c-jun DNA binding activity results in cancer cell resistance to etoposide. Neoplasia. 2009;11:976–986. doi: 10.1593/neo.09632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.