Abstract
• Background and Aims Polyploidy is viewed as an important mechanism of sympatric speciation, but only a few studies have documented patterns of distribution and ecology of different cytotypes in their contact zone. Aster amellus agg. (Asteraceae) is one of the species with documented multiple ploidy levels. The aim of this study was to determine spatial distribution and ecology of two cytotypes, diploid (2n = 18) and hexaploid (2n = 54), of Aster amellus agg. at their contact zone in the Czech Republic.
• Methods Root-tip squashes and flow cytometry were used to determine the ploidy of 2175 individuals from 87 populations. To test whether some differences in ecology between the two ploidy levels exist, in each locality relevés were recorded and abiotic conditions of the sites were studied by estimating potential direct solar radiation, Ellenberg indicator values and above-ground biomass.
• Key Results Together with diploid and hexaploids, minorite cytotypes (triploid, pentaploid and nonaploid) were found. No significant ecological differences between diploid and hexaploid cytotypes were found. In spite of this, no population consisting of both of the two basic cytotypes was found.
• Conclusions The results of this study show that the contact zone of diploid and hexaploid cytotypes in the Czech Republic is much more diffuse than indicated in previous records. Although populations of both cytotypes occur in close proximity (the closest populations of different cytotypes were 500 m apart), each individual population consists of only one basic ploidy level. This was unexpected since there are no clear differences in abiotic conditions between populations. Taken together with the absence of an intermediate tetraploid cytotype and with reference to published world distributional patterns of different ploidy levels, this suggests a secondary contact zone. Detailed genetic study is, however, necessary to confirm this.
Keywords: Aster amellus agg., Asteraceae, contact zone, cytotype, distribution, dry grassland, flow cytometry, polyploidy, productivity, relevés, potential direct solar radiation, Ellenberg indicator values
INTRODUCTION
Polyploidization has long been recognized as an important process in plant evolution (Stebbins, 1950). It is a common phenomenon in plants; it is estimated to occur in 47–70 % of angiosperm species (Ramsey and Schemske, 1998).
Polyploid species often form aggregates of several different cytotypes (Leitch and Bennett, 1997). Existence of different cytotypes requires not only a mechanism enabling the origin of the cytotype, but also a mechanism that would allow its survival and spread. Such evolutionary and ecological processes can be studied especially well in contact zones where plants of different ploidy levels coexist. Here, the interactions between the two entities can be directly observed and it is possible to explore the processes leading to their separation.
According to adaptive explanations for the coexistence of different ploidy levels, some kind of environmental heterogenity is underlying the cytotype distribution patterns. It is assumed that polyploids differ from diploids in their response to spatial environmental variation (‘ecogeographic preferences’, Lewis, 1980). Polyploids may be better adapted to harsh environments (cold, drought, etc.; reviewed by Lewis, 1980). Examples of the coexistence of sympatric cytotypes as a result of niche differentiation are: the diploid (found under tree cover) and tetraploid (in open areas) cytotypes of Dactylis glomerata in north-eastern Spain (Lumaret et al., 1987); the diploid (in bare, unprotected areas) and tetraploid (in more sheltered microsites) cytotypes of Lotus corniculatus in the French Alps (Jay et al., 1991); the spatial variation in abundance of diploid and tetraploid Festuca apennina in the Swiss and Italian Alps (Tyler et al., 1978); and the occupation of a wide range of habitats by different cytotypes in the Antennaria rosea complex (Bayer and Stebbins, 1982).
Cytotype distribution may also reflect environmental heterogenity in the past. Widespread cytotypes may have been superior colonizers of areas that became available after the amelioration of the climate at the end of the Pleistocene or due to human activities such as deforestation and agricultural practices (Manton, 1934; Stebbins, 1950, 1985; Mitchell, 1992; Gornall and Wentworth, 1993).
The type of habitats occupied by the different ploidy levels is not the only difference in distribution between different cytotypes. Higher ploidy levels also sometimes occupy a wider range of habitats. This range can be either completely outside the range of the diploid, or the diploid can occupy a subset of habitats occupied by polyploids. Examples of such distribution include Anthoxanthum alpinum (Felber, 1988), Deschampsia caespitosa (Rothera and Davy, 1986), Solidago nemoralis (Brammal and Semple, 1990) and Plantago media (Van Dijk and Bakx-Schotman, 1997).
Different ecological requirements of cytotypes usually indicate that they have different distributions. In this case, contact zones are maintained by selection against parental types in alien environments and hybrids in parental environments (Barton and Hewitt, 1985; Hewitt, 1988). The converse applies, so when there are no ecological differences between cytotypes, mixed populations can be expected. In such a case it is usually assumed that the different cytotypes interact continuously and cannot be considered independent species. In other words, two basic types of patterns are usually found: (1) ploidy levels with separate habitat requirements and thus separate populations (e.g. Tyler et al., 1978; Lumaret et al., 1987; Jay et al., 1991), or (2) mixed ploidy levels without any ecological differences (e.g. McArthur and Sanderson, 1999; Suda, 2002; Suda et al., 2004).
Cases of cytotypes coexisting in proximity without clear habitat differentiation but also without clear mixing are much more rarely recorded (but see Felber, 1986; Van Dijk et al., 1992). Recently it has been suggested that such patterns can arise if the species can hybridize but the hybrids are non-viable or of low fitness, even if there are no ecological differences between ploidal levels (e.g. Barton and Hewitt, 1985; Husband, 2004). Similarly, such contact zones can be developed upon contact of two independent migration routes (Pannell et al., 2004). The pattern may be maintained by a balance between dispersal rates and frequency-dependent selection against hybrids, as has commonly been reported for hybridogenous species groups (Barton and Hewitt, 1985; Bert and Arnold, 1995; Wang et al., 1997; Kruuk et al., 1999; Bronson et al., 2003).
In the literature on polyploid species contact zones, reports on cases where distribution patterns of the polyploid species are maintained by selection against hybrids are still very rare. It is not clear if this pattern is indeed rare in nature, or whether it has just been rarely recognized; only a decade ago, the estimation of ploidy levels was difficult and different ploidy levels were recognized mainly if cytotypes clearly differed in ecology or morphology. Recent developments in flow cytometry, however, have allowed the estimation of ploidy levels for larger numbers of samples. In many cases, these studies have indicated higher variability of ploidy levels than previously thought to be present (Doležel, 1997). This is also the case for Aster amellus agg.
Aster amellus agg. is an example of a species complex with a documented existence of different sexually reproducing ploidy levels. In this study, all existing Aster amellus agg. populations in the Czech Republic, representing a contact zone between diploid and hexaploid cytotypes, were studied in order to describe the pattern of cytotype distribution. Understanding the factors that result in the distributions of the two cytotypes in the region may provide insights into evolutionary processes in this aggregate. Two basic questions were asked. (1) What is the distribution of the two Aster amellus agg. ploidy levels in the Czech Republic at the regional and local scale? (2) Are there any ecological differences between these cytotypes?
MATERIALS AND METHODS
Species and study site
Aster amellus agg. is a widespread polymorphic species. Its area of distribution in Europe ranges from northern France to Lithuania. In the south it reaches northern Italy and Macedonia (Merx and Schreiber, 1976).
Outside Europe it reaches the Black Sea and northern Caucasus (Meusel and Jäger, 1992). Its basic chromosome number is x = 9. According to Meusel and Jäger (1992), three ploidy levels can be found within the whole area of distribution: 2n = 18 (diploid), 2n = 36 (tetraploid) and 2n = 54 (hexaploid). The specific evaluation of these three taxa (diploid Aster amellus L., tetraploid A. ibericus Stev. and hexaploid A. amelloides Bess.) is justified by their morphological differences, in addition to different chromosome numbers, as well as by their distinct expected areas of origin and different evolutionary history (Májovský et al., 1987). Other published records of ploidy levels of this species mention 2n = 66, counted in a plant of unknown origin in a Botanical garden in Freiburg (Huziwara, 1962), and 2n = 66 and 2n = 76 in garden cultivars of this species (Annen, 1945; Chatterji, 1962).
Published records from central Bohemia (western part of the Czech Republic) mention only diploid individuals (2n = 18, Holub et al., 1970; Kovanda, 1984; Krahulcová, 1990); hexaploid species were recorded from the south-eastern part of the country (southern Moravia; Löve, 1974; Kovanda, 2002). Within the Czech Republic the group is considered to be taxonomically clear (Kovanda, 2002). Hexaploids are considered to be strongly morphologically differentiated from the diploids from central Bohemia, where Kovanda (2005) separated different cytotypes into the two individual species, Aster amellus L. (2x) and Aster scepusiensis Kit. ex Kanitz (6x). A hexaploid cytotype occuring in southern Moravia is also sometimes identified as the east European species Aster amelloides Bess. (Májovský et al., 1987). Aster amellus agg. has recently been a subject of several ecological studies in the Czech Republic (Münzbergová, 2004; H. Plachá and Z. Münzbergová, unpubl. res.; Z. Münzbergová, unpubl. res.). None of these studies, however, were aimed at understanding the distribution patterns of the two ploidy levels. It should be noted that the Aster amellus agg. is a perenial and self-incompatible plant (Kovanda, 2005), and both cytotypes have similar flowering times (T. Mandáková and Z. Münzbergová, pers. obs.).
Selection of material
All literature records mentioning the occurrence of Aster amellus agg. in the Czech Republic were used for to produce an inventory of the distribution of this taxon in the Czech Republic (Opiz, 1815–1835; Ott, 1851; Weicker, 1854; Čelakovský, 1884–1894; Formánek, 1887; Domin, 1904; Podpěra, 1911; Šimr, 1927; Domin, 1930; Rohlena, 1930; Šindelář, 1941; Novák, 1943; Otruba, 1944; Podpěra, 1949; Durdík, 1951; Pijáček, 1951; Neuhäusl and Neuhäuslová-Novotná, 1968; Reitmayer, 1968; Houda, 1969; Holub et al., 1970; Kubát, 1970; Blažková, 1973; Toman, 1974; Fiedler and Válek, 1975; Rivola, 1975; Deyl, 1976; Suchara, 1978; Šimek, 1980; Hanousek, 1981; Sedláček and Dvořák, 1983; Böswartová, 1984; Kolbek, 1986; Pekárek, 1986; Knížetová et al., 1987; Hrouda and Skalický, 1988; Grulich, 1989; Žídková, 1989; Čekanová, 1990; Fišerová, 1990; Saul, 1990; Višňák, 1992; Kolbek and Petříček, 1994; Pořízek and Pivničková, 1994; Šumberová, 1995; Danihelka and Grulich, 1996; Tichý, 1997; Dudová, 1998; Koblížek et al., 1998; Kubát et al., 1999; Müller, 1999; Blažková, 2000; Kovanda, 2002). At flowering time in July, August and September 2003 and 2004, leaf material was sampled in each population to estimate the distribution of the ploidy levels. The leaves from 25 flowering plants per population were selected with the aim to cover most of the variability within each population. Fresh leaves were transported to the laboratory and the ploidy level was estimated using flow cytometry within one day. The DNA amount in each sample was compared to a reference sample, where the number of chromosomes was counted (plants from the populations No. 4 and 9; see Supplementary Information).
Chromosome counts
Chromosome numbers from root tips were studied. A modified lacto-propionic orcein coloration method of Dyer (1963) was used to prepare slides for chromosome counting. Actively growing roots were pre-treated in paradichlorbenzen for 4 h at room temperature, fixed in 3 : 1 ethanol : acetin acid and stored at 4 °C until used. Root-tips were hydrolysed in 1 : 1 HCl : ethanol for 3 min at room temperature, rinsed in water and the meristematic tissue excised and squashed in a drop of lacto-propionic orcein. Chromosomes were counted using a phase-contrast microscope and an immersion objective of magnification 100×. In total, chromosomes from 20 samples from two localities were counted.
Ploidy level estimation
Ploidy level was estimated with a Partec PA II flow cytometer (Partec GmbH., Münster, Germany) using a two-step procedure as originally described by Otto (1990). Approximately 0·5 cm2 of young, fresh leaf of an analysed plant together with leaf tissue of an internal standard (Aster amellus agg. with known chromosome number) were chopped with a new razor blade in 1 mL of ice-cold Otto I buffer (0·1 m citric acid, 0·5 % Tween 20). The suspension was filtered through a 42-μm nylon mesh, centrifuged at 1200 rpm for 5 min, the supernatant was removed and nuclei were resuspended in 100 μL of fresh ice-cold Otto I buffer. After an incubation period (20 min at room temperature with occasional shaking), the staining solution, containing 1 mL Otto II (0.4 m Na2HPO4·12 H2O), fluorochrome (DAPI or propidium iodide) and β-mercaptoethanol (2 μg mL−1), was added. DAPI at a concentration of 4 μg mL−1 and propidium iodide together with RNAse IIA (both at concentrations of 50 μg mL−1) were employed in the ploidy level estimation. The staining lasted 1–2 min for DAPI and 30 min for propidium iodide protocols, respectively. The two types of staining were used for technical reasons; in both cases comparable results were obtained. Fluorescence was recorded for at least 5000 nuclei. Histograms with a coefficient of variation below 3 % were accepted.
Ecology of cytotypes
To test whether differences in ecology between the two ploidy levels exist, one-to-three relevés (depending on the size of the population) were recorded in each locality (with the exception of localities No. 15, 21 and 87) within stands of Aster amellus agg. The relevé size was 1 × 1 m.
At each locality from the České Středohoří Mountains, five 15 × 15 cm plots were randomly selected, with the condition that they did not include any Aster amellus agg. plants. All the above-ground biomass was harvested within these plots at the beginning of August 2002 (all sampled within 2 d), dried to constant weight and weighted. The biomass was used as a correlate of the successional status of the locality.
In addition, abiotic factors at all localities were analysed. Potential direct solar radiation was calculated from knowledge of the slope and aspect of each locality. To characterize abiotic conditions of the sites, Ellenberg indicator values were used (Ellenberg, 1992), which are considered a valuable and easy-to-obtain source of information on site conditions (Schaffers and Sýkora, 2000). Six environmental characteristics were calculated: understorey light conditions (L), temperature (T), continentality (K), soil moisture (F), soil reaction (R) and soil nutrients (N). For each population, average indicator values (averaged over all relevés within each locality) were then used to characterize the populations.
Data analysis
All ecological characteristics were analysed on two different spatial scales. First, only populations from a small district, České Středohoří Mountains (the place of the most intensive contact of cytotypes), and second, all populations from the Czech Republic.
The species composition was analysed using the program Canoco (ter Braak and Šmilauer, 1998) with the aim of evaluating differences in species composition between the localities of the two ploidy levels. The cover data of all species (recorded as percentages) were square-root transformed before the analysis. Detrended correspondence analysis (DCA) was used to describe variation in the data. To test for differences in species composition between sites of the two ploidy levels, canonical correspondence analysis (CCA) was used. Rare species were downweighted. When more than one relevé per locality was recorded, the average was used for the analysis. The analyses were carried out both with species composition as the predictor and ploidy level as the dependent variable, and the other way around.
The above analyses could detect significant differences in species composition between sites even in cases when the differences between the two ploidy levels would in fact be only in geographical distribution. To remove this effect of spatial position of each locality, partial analysis with geographical co-ordinates as covariates was implemented. To do this, the position of each locality was expressed as position on a grid described by the latitude (x) and longitude (y) of the locality. First, the latitude and longitude (x, y), their second and third power (x2, x3, y2, y3), their interaction (xy) and interaction of their powers (x2y, xy2) were used as predictors in CCA analysis to explain differences in species composition between localities. In this analysis, forward selection was used to detect which of the co-ordinates significantly contributed to differences in species composition between the sites. The significant co-ordinates were then used as covariates in the analysis of the effect of ploidy level on species composition of the localities.
Differences between the two cytotypes in abiotic factors were tested by analysis of variance (ANOVA) using the program NCSS (2001). Similarly to the multivariate analysis, this analysis used the mean value of the environments per sample if multiple values were available.
RESULTS
Cytotype distribution
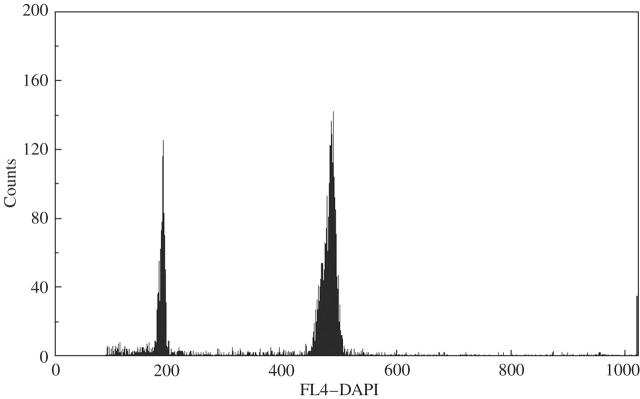
In the Czech Republic, a ploidy level for a total of 87 populations of Aster amellus was estimated (Supplementary Information). Two basic ploidy levels were determined: diploid (2n = 18) and hexaploid (2n = 54). The analysis of DNA content of nuclei isolated from leaf tissue showed that most of the nuclei were in G0/G1 phase of the cell cycle and thus formed a dominant peak in histograms of DNA content. For channels of peak localization and coefficients of variation (CV) see Fig. 1. Cell nuclei of a diploid Aster amellus agg. individual with known chromosome number were used as an internal standard.
Fig. 1.
Ploidy analysis in Aster amellus agg.. Histograms of relative nuclear DNA content of particular Aster amellus agg. cytotypes. Cell nuclei of the diploid Aster amellus agg. individual with known chromosome number were used as an internal standard. The x-axis constitutes relative DNA content, the y-axis number of nuclei. The G0/G1 peak of the diploid A. amellus agg. was on channel 187 (CV = 2·4), that of hexaploid A. amellus agg. on channel 484 (CV = 2·33); i.e. the peak ratio is 2·59.
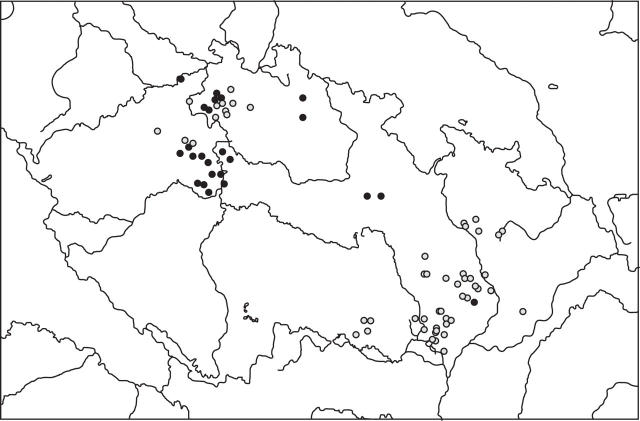
The hexaploid taxon was represented by 57 populations, the diploid taxon by 30 populations. The distribution of particular cytotypes is shown in Fig. 2 (see also Supplementary Information: Aster amellus agg. localities in the Czech Republic with GPS co-ordinates and ploidy levels). In Moravia (eastern part of the Czech Republic), all populations except for one were hexaploid. In Bohemia (western part of the Czech Republic) both cytotypes co-occur. No population consisting of both of the two basic cytotypes was found.
Fig. 2.
Distribution of Aster amellus agg. in the Czech Republic. Solid circles represent localities of the diploid, open circles localities of the hexaploid plants.
Aster amellus agg. populations were, however, not cytologically uniform. In three cases, triploid individual in diploid populations were found. In hexaploid populations, one pentaploid individual and three nonaploid individuals were determined (Fig. 3; see also Supplementary Information).
Fig. 3.
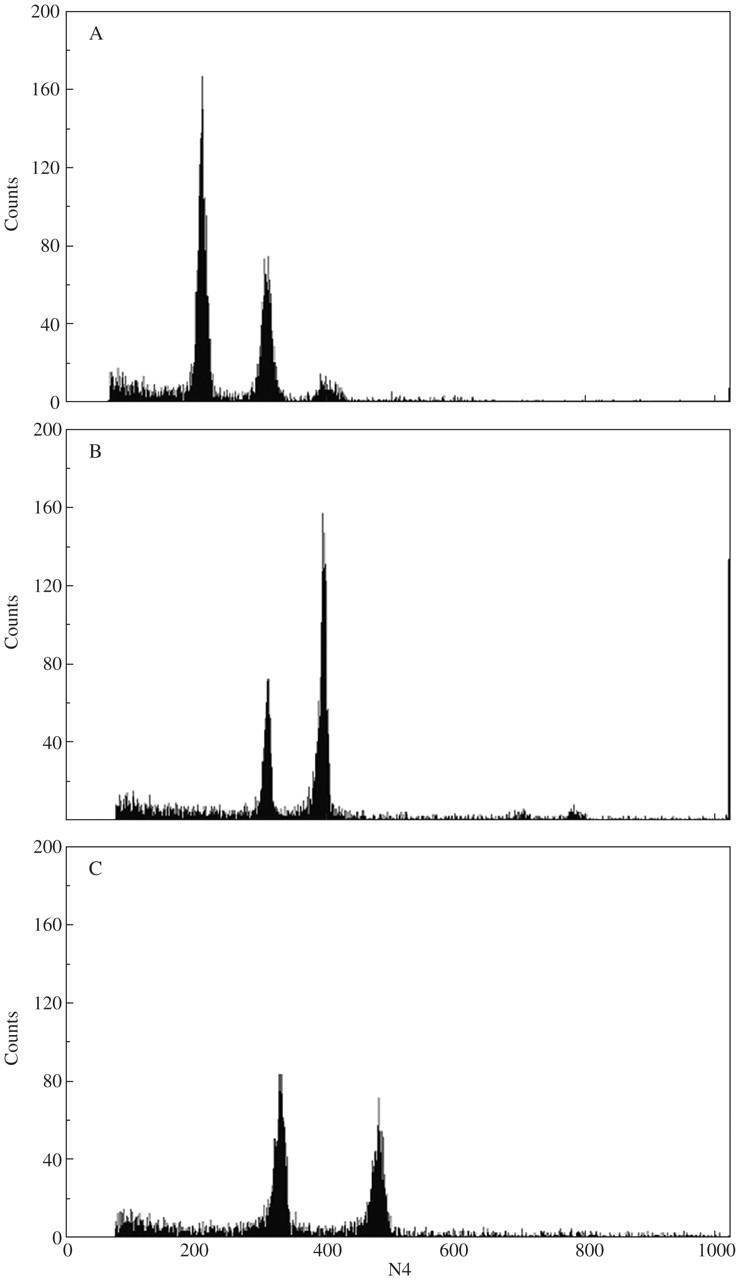
Ploidy analysis in Aster amellus agg.. Histograms of relative nuclear DNA content of particular Aster amellus agg. cytotypes. (A) Cell nuclei of a diploid Aster amellus agg. individual with known chromosome number were used as an internal standard. (B, C) Cell nuclei of a hexaploid Aster amellus agg. individual with known chromosome number were used as an internal standard. The x-axis constitutes relative DNA content, the y-axis the number of nuclei. In (A) the G0/G1 peak of the diploid A. amellus agg. was on channel 208 (CV = 2·78), that of the triploid A. amellus agg. was on channel 308 (CV = 2·79); i.e. the peak ratio is 1.48. In (B) the G0/G1 peak of the pentaploid Aster amellus agg. was on channel 309 (CV = 1·46), that of the hexaploid Aster amellus agg. was on channel 397 (CV = 1·38); i.e. the peak ratio is 1·28. In (C) the G0/G1 peak of the hexaploid Aster amellus agg. was on channel 329 (CV = 2·3), that of the nonaploid Aster amellus agg. was on channel 481 (CV = 1·93); i.e. the peak ratio is 1·46.
Ecology of the ploidy levels
In total 156 relevés for Aster amellus agg. were collected at 84 localities. The species data set variability for localities in the České Středohoří Mountains and for all populations in the Czech Republic are shown in Fig. 4. The figure shows the distribution of diploid and hexaploid Aster amellus agg. populations along the floristic composition gradient. In both cases, no clear distributional pattern of diploid and hexaploid populations is visible.
Fig. 4.
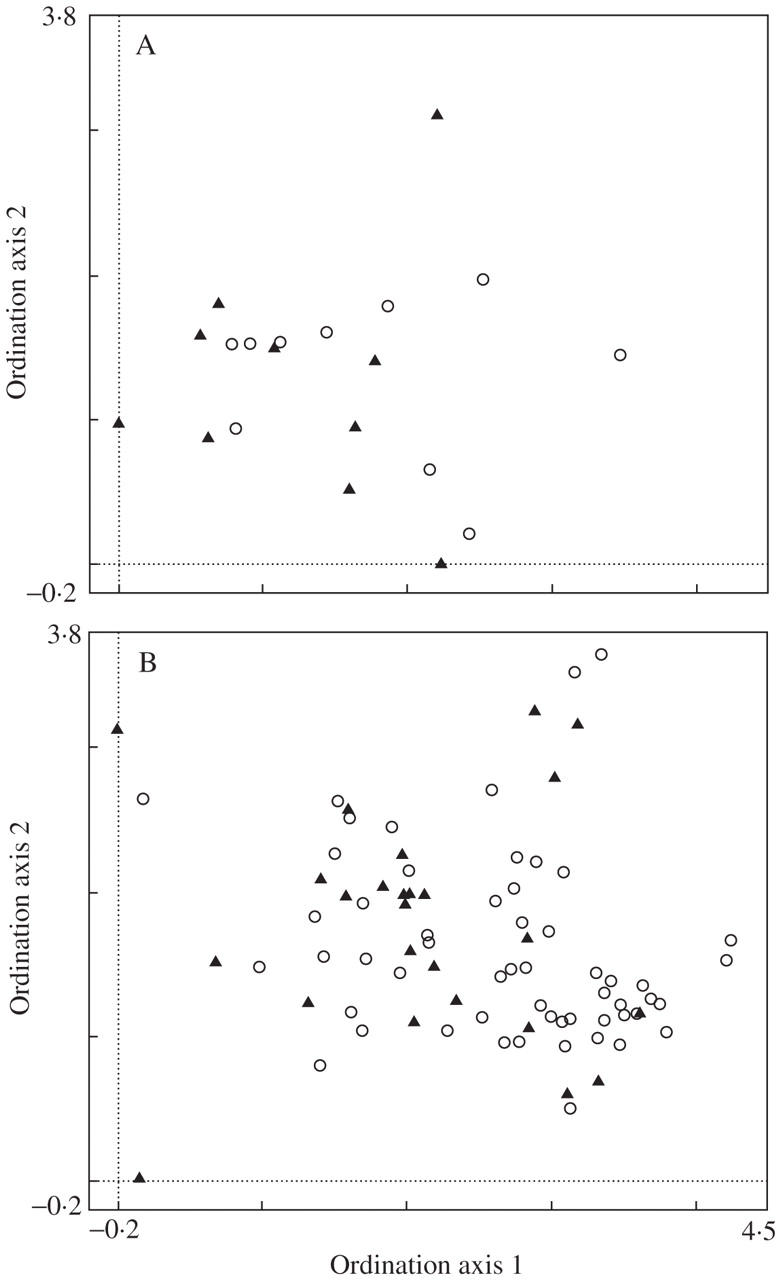
The detrended correspondence analysis of the species data. (A) Shows the distribution of Aster amellus agg. localities along the floristic composition gradient in the České Středohoří Mountains and (B) the distribution in the whole Czech Republic. In (A), the first ordination axis explains 11·9 % of the total variability in the data set, the second ordination axis 9 %. In (B), the first ordination axis explains 5·9 % of the total variability in the data set, the second ordination axis 4·6 %. Triangles represent localities of the diploid, circles localities of the hexaploid plants.
Canonical correspondence analysis testing differences in species composition of localities of the two ploidy levels involving only those within the České Středohoří Mountains was significant (Trace = 0·248, F-ratio = 1·329, P-value = 0·022), as was the dataset for the whole Czech Republic (Trace = 0·141, F-ratio = 2·155, P-value = 0·002). Ploidy level explained 6·9 % of total variation in species composition between localities within the České Středohoří Mountains, and 2·7 % in the whole Czech Republic.
Hexaploid populations more often occur in closed vegetation with species such as Bromus erectus, Salvia verticilata and Galium verum. In contrast, diploid populations more frequently occur in open vegetation dominated by species such as Sesleria varia, Carex humilis and Linum flavum (Fig. 5).
Fig. 5.
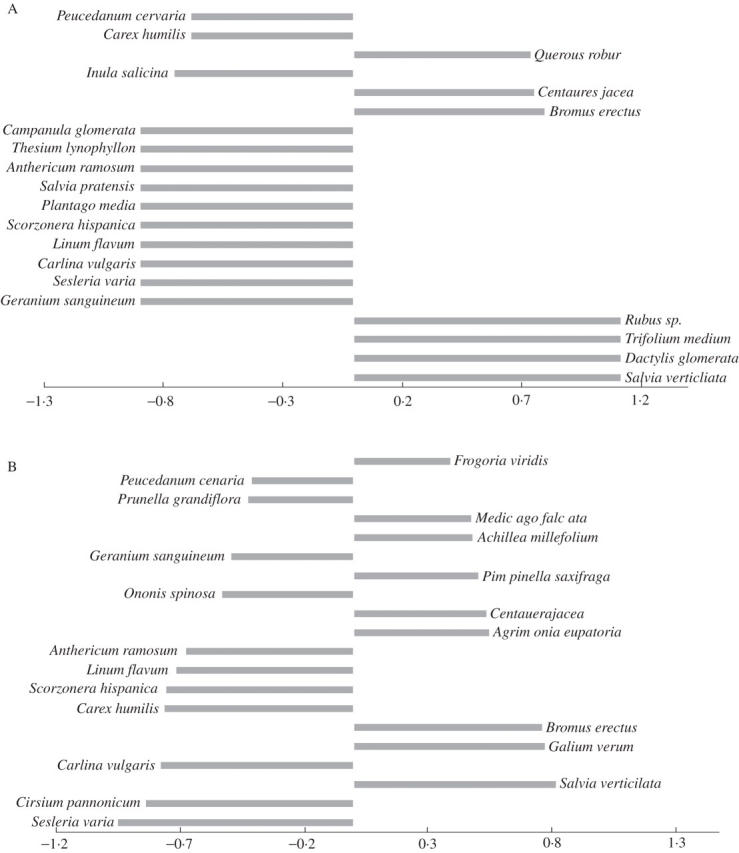
In both parts of the figure, the 20 species with high weight that are most strongly correlated with the first ordination axis are shown. Species correlated with the hexaploid cytotype of Aster amellus agg. are to the right, species correlated with the diploid cytotype are to the left. In (A), the České Středohoří Mountains data set, the first axis explains 6·9 % of the total variation in species composition in (B), the Czech Republic data set, the first axis explains 2·7 %.
In the České Středohoří Mountains data set, geographical co-ordinates did not explain any variation in species composition; in the Czech Republic data set the x and y coordinates were significant, but none of their powers and interactions were. For the Czech Republic data set, the partial analysis with geographical co-ordinates as covariates was not significant (Trace = 0·161, F-ratio = 1·2, P-value = 0·072). The percentage of variability of floristic composition explained by differences of ploidy level declined from 2·7 to 1·7 %. This shows that although differences in vegetative composition between cytotypes can be observed on a small scale, after the differences in geographical position on the large scale are removed the differences in vegetation composition disappear.
There were no significant differences between cytotypes in Ellenberg indicator values and potential direct solar radiation, either in the České Středohoří Mountains or in the Czech Republic as a whole (Table 1). There were also no significant differences in biomass between localities of the two cytotypes, but hexaploids have a greater coefficient of variation (see Table 1). No other environmental variable showed a similar pattern.
Table 1.
Significance level (P-values), F-values and coefficients of variance of analyses testing differences in Ellenberg indicator values, potential radiation and biomass between localities of the two cytotypes in the České Střehohoří Mountains and in the Czech Republic as a whole
| České Středohoří Mts. |
Czech Republic |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| P value (DF = 1, DF error = 18) |
F-value |
Coefficient of variance |
P-value (DF = 1, DF error = 78) | F-value | Coefficient of variance |
||||
| 2x | 6x | 2x | 6x | ||||||
| L (light conditions) | 0·71 | 0·14 | 7·11 | 2·17 | 0·24 | 1·37 | 6·91 | 6·10 | |
| Ellenberg indicator value | F (soil moisture) | 0·19 | 1·89 | 2·78 | 5·34 | 0·46 | 0·57 | 8·85 | 4·34 |
| R (soil reaction) | 0·13 | 2·55 | 9·44 | 2·27 | 0·41 | 0·67 | 5·35 | 8·15 | |
| N (soil nutrients) | 0·23 | 0·16 | 8·72 | 0·04 | 0·59 | 0·48 | 9·43 | 0·18 | |
| T (temperature) | 0·23 | 1·53 | 0·02 | 1·57 | 0·13 | 0·30 | 3·47 | 0·06 | |
| K (continentality) | 0·69 | 1·55 | 6·78 | 4·93 | 0·49 | 2·4 | 2·17 | 0·14 | |
| Potential radiation (relative values) | Mean | 0·97 | 0·30 | 0·55 | 1·06 | 0·16 | 2·4 | 1·54 | 0·89 |
| January | 0·95 | 0·30 | 1·27 | 2·72 | 0·38 | 0·78 | 3·35 | 2·13 | |
| June | 0·87 | 0·00 | 1·89 | 5·88 | 0·14 | 2·25 | 0·33 | 0·14 | |
| Biomass (g m−2) | 0·10 | 2·91 | 1·43 | 7·15 | – | – | – | – | |
DISCUSSION
The results from this study are in contrast to previous reports on the cytology of Aster amellus agg. from the Czech Republic. Traditionally, it has been assumed that there are only diploid populations in Bohemia (the western part of the country) and only hexaploid populations in Moravia (the eastern part of the country; see Löve, 1974; Kovanda, 2002, 2005). The results of this study, however, clearly indicate that the hexaploid cytotype occurs also in central Bohemia, where it has never been recorded before. In this region, the hexaploid cytotype is present in the same abundance as the diploid one. On first sight, hexaploid individuals from Bohemia are morphologically similar to the diploid ones and different from the hexaploid species found in Moravia. An analogous situation is found in Moravia, where one diploid population was discovered. This population is morphologically similar to hexaploid plants from nearby populations. This indicates confused taxonomic assessments that demand further study, especially biometric analyses.
Many studies have provided evidence that unreduced gamete formation within diploid populations is the driving force in the formation of polyploids (Bretagnolle and Thompson, 1995; Ramsey and Schemske, 1998). In the case of the current study, minority cytotypes were also detected in the populations: triploids in diploid populations, pentaploid and nonaploids in hexaploid populations. The presence of minorite cytotypes has never been reported for this species before. Because of the absence of the tetraploid cytotype, we expect that triploids originated by fusion of reduced and unreduced gametes of the diploid cytotype, similarly to the origin of nonaploids in hexaploid populations. The origin of pentaploid plants in hexaploid populations is difficult to explain in the absence of a tetraploid parent.
In this study, no intermediate tetraploid cytotype was detected while in other studies of contact zones between different cytotypes, intermediate cytotypes are often detected at low frequencies (Lumaret and Barrientos, 1990; Van Dijk et al., 1992; Bretagnolle and Thompson, 1995; Husband and Schemske, 1998; Burton and Hutband, 1999). It is unlikely that the tetraploid cytotype was missed, as the total number of sampled plants was very high (in total 2175 individuals). The absence of an intermediate cytotype and ploidy-mixed populations indicates limited or no gene-flow between different cytotypes. This could be due the fact that distances between populations of the two ploidy levels (minimum 500 m) are greater than the flying range of pollinators, or due the inviability of hybrids.
When different cytotypes co-exist sympatrically or in contact zones, they often show fine-scale niche differentiation (e.g. Lumaret and Hanotte, 1987; Lumaret et al., 1987; Lumaret and Barrientos, 1990; Felber-Girard et al., 1996; Petit et al., 1997), but no such phenomenon was observed in Aster amellus agg.. No significant ecological differences between the diploid and hexaploid cytotypes were found. Canonical correspondence analysis showed significant differences in the floristic composition of localities of the two basic cytotypes, but after removing the effect of geographical location the differences almost disappeared. There were also no differences in above-ground biomass or in any of the Ellenberg values between localities of the two ploidy levels. There was, however, a much higher variation in biomass between localities of the two ploidy levels, indicating that hexaploid populations occur in a wider range of habitats (from bare, unprotected areas to more sheltered microsites). No other environmental variable, hovewer, showed a similar pattern. A wider ecological amplitude is sometimes documented for polyploids (e.g. Thompson and Lumaret, 1992; Van Dijk et al., 1992; Burton and Husband, 1999).
In spite of the absence of any clear ecological differences between the ploidy levels, no population consisting of both basic cytotypes, diploid and hexaploid, was found. This is an interesting and rare situation because when there are no ecological differences between cytotypes, mixed populations of the cytotypes are expected. A similar case is sometimes seen in allopolyploid complexes (Thompson and Lumaret, 1992). A preliminary allozyme study, however, suggests that the hexaploid cytotype of Aster amellus agg. is of autopolyploid origin (T. Mandáková and Z. Münzbergová, unpubl. res.). The absence of mixed populations without ecological differences between ploidy levels can be explained by founder effects or, more probably, by the fact that the cytotype distribution pattern is a result of secondary contact of cytotypes.
One possibility may be that all individuals in each population originated from a single migration event (founder effect). Alternatively, previously large populations may have expierenced strong reductions in population size (‘bottleneck effect’). In both cases, the ploidy level of the population would be largely a matter of chance. The principal importance of this effect for the formation of population structure has been repeatedly documented for many species (e.g. Agren and Ericson, 1996). Similar patterns could be a result of genetic drift in small populations (Ramsey and Schemske, 1998).
Another explanation for the observed pattern may be secondary contact of the cytotypes. This explanation has been proposed by Thompson and Lumaret (1992). These authors assumed that a similar pattern that was observed by them was a result of postglacial cytotype expansion via different migration routes. In this case, the absence of mixed ploidy-level populations is probably caused by a balance between the ‘minority cytotype exclusion’ (Levin, 1975) and the expansion rate: the cytotypes have different evolutionary histories and should be considered as independent species.
The data presented here give positive evidence that the distribution of diploid and hexaploid Aster amellus agg. is due to a secondary contact after Pleistocene range expansion, and is now maintained by minority cytotype exclusion. (1) We found no clear ecological differences between the 2x and 6x plants, suggesting that environmental selection is not responsible for the different distributions. (2) No mixed populations occur, despite the ecological similarity of the ploidy levels. This indicates strong selection against sympatry between these species. (3) Hybridization between the 2x and 6x plants would yield tetraploids, but none of these were found. This suggests that the hybrids are inviable, due to the divergence of chromosomes since the hexaploids arose. This is supported by published studies showing that many genic and genomic changes can occur within the first few generations after polyploid formation (Song et al., 1995; Galitski et al., 1999; Soltis and Soltis, 1999, 2000; Ozkan et al., 2001; Adams et al., 2003), resulting in functional isolation between the ploidy levels. Thus, whenever the diploid and hexaploid come into contact, production of inviable hybrids means that the minority cytotype is quickly eliminated. Therefore single cytotype areas are maintained.
There is a growing body of literature about contact zones that are maintained by a balance between dispersal rates and frequency-dependent selection against hybrids (Barton and Hewitt, 1985; Bert and Arnold, 1995; Wang et al., 1997; Kruuk et al., 1999; Bronson et al., 2003). This theory has seldom been invoked for ploidal complexes, but is explained in this context in Pannell et al. (2004). The description of the Aster amellus agg. contact zone in the present paper suggests that a similar scenario may also hold for this contact zone.
The existence of a secondary contact zone seems a reasonable explanation for the pattern observed in this study and is supported by world distributional patterns (Meusel and Jäger, 1992) and published chromosome counts. For the west- and south-European populations of Aster amellus agg., only diploids are reported in the literature, whereas in the continental part of Eurasia, only hexaploids have been detected (Tamanšjan, 1959; Meusel and Jäger, 1992). The contact zone of both cytotypes seems to be in the Czech Republic.
The secondary contact zone scheme also fits well with the theory of Májovský et al. (1987) on the origin of populations of Aster amellus agg.. According to Májovský, the diploid Aster amellus is the oldest member of the whole group. During the Tertiary, because of the gradual continentalization of the climate, diploids were pushed to the colder and more humid part of the continent, where they found more appropriate conditions. Some populations found a refuge in the Caucasus, in Anatolia or Bulgaria, and gave rise to tetraploid Aster ibericus. Under the most extreme, i.e. most continental, conditions during the Tertiary a third, hexaploid type arose among these populations, which had already reached the Carpathian basin and the Balkans by the end of the glacial periods. During that time, the contact zone of diploids and hexaploids was established in the area of the Czech Republic. The recent distribution of the diploid and hexaploid populations in the European part of the overall distribution area is in support of this scheme. The hexaploid populations have probably arisen by hybridization of diploids and tetraploids and by the subsequent doubling of chromosomes of the triploid hybrid.
This theory is at present mostly speculative, and data on genetic patterns across the whole distribution range would be needed to confirm this. This paper provides preliminary evidence that the two ploidal levels are not ecologically differentiated, which needs to be confirmed by reciprocal transplant experiments. The results of this paper also suggest that hybridization experiments between the diploid and hexaploid types should be carried out to confirm that the two ploidy levels are indeed functionally isolated.
CONCLUSIONS
The results of this study show that, contrary to previous records, the contact zone of diploid and hexaploid cytotypes in the Czech Republic is rather diffuse. Populations of both cytotypes occur in close proximity; however, each individual population consists of only one ploidy level. This is surprising, since there are no clear differences in abiotic conditions between the populations. This, together with the absence of an intermediate tetraploid cytotype, published world distributional patterns and chromosome counts, suggests a secondary contact zone. Detailed genetic study is, however, necessary to confirm this.
SUPPLEMENTARY INFORMATION
Detailed information on Aster amellus agg. localities in the Czech Republic with GPS co-ordinates and ploidy levels are given in Supplementary Information available online at http://aob.oxfordjournals.org/
Acknowledgments
We would like to thank V. Jarolímová for help with counting the chromosome numbers, and Richard Buggs and Martin Lysák for helpful comments on a previous version of the manuscript. This study was supported by grant GAAV no. B6111303. It was also partly supported by MSMT 0021620828 and AV0Z6005908.
LITERATURE CITED
- Adams KL, Cronn R, Percifield R, Wendel JF. 2003. Genes duplicated by polyploidy show unequal contributions to the transcriptome and organ-specific reciprocal silencing. Proceedings of the National Academy of Sciences of the USA 100: 4649–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agren J, Ericson L. 1996. Population structure and morph-specific fitness differences in tristylous Lythrum salicaria. Evolution 50: 126–139. [DOI] [PubMed] [Google Scholar]
- Annen E. 1945. Die Embryosack und Pollenentwicklung bei einigen polyploiden Garten Astern in Vergleich mit der wildwachsenden Aster amellus L. Bulletin de la Société Botanique Suisse 55: 8–121. [Google Scholar]
- Barton NH, Hewitt GM. 1985. Analysis of hybrid zones. Annual Review of Ecology and Systematics 16: 113–148. [Google Scholar]
- Bayer RJ, Stebbins GL. 1982. A revised classification of Antennaria (Asteraceae: Inuleae) of the eastern United States. Systematic Botany 7: 300–313. [Google Scholar]
- Bert TM, Arnold WS. 1995. An empirical test of predictions of two competing models for the maintenance and fate of hybrid zones — both models are supported in a hard-clam hybrid zone. Evolution 49: 276–289. [DOI] [PubMed] [Google Scholar]
- Blažková J. 1973. Pflanzensociologische Studie űber die Wiesen der sűdbohmischen Becken. Studia ČSAV 1973/10: 1–170. [Google Scholar]
- Blažková D. 2000. Rostlinstvo přírodní rezervace Karlické údolí a okolí. Zpravodaj ochránců přírody okresu Praha-západ 20–21: 23–26. (In Czech). [Google Scholar]
- Böswartová J. 1984. Příspěvek ke květeně středního Povltaví. Bohemia centralis 13: 83–133. (In Czech). [Google Scholar]
- ter Braak CJF, Šmilauer P. 1998. CANOCO Release 4. Reference manual and user's guide to Canoco for Windows. Software for Canonical Community Ordination. New York: Microcomputer Power.
- Brammal RA, Semple JC. 1990. The cytotaxonomy of Solidago nemoralis (Compositae: Asterae). Canadian Journal of Botany 68: 2065–2069. [Google Scholar]
- Bretagnolle F, Thompson JD. 1995. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytologist 129: 1–22. [DOI] [PubMed] [Google Scholar]
- Bronson CL, Grubb TC, Braun MJ. 2003. A test of the endogenous and exogenous selection hypotheses for the maintenance of a narrow avian hybrid zone. Evolution 57: 630–637. [DOI] [PubMed] [Google Scholar]
- Burton TL, Husband BC. 1999. Population cytotype structure in the polyploid Galax urceolata (Diapensiaceae). Heredity 82: 381–390. [DOI] [PubMed] [Google Scholar]
- Čekanová L. 1990. Cytotaxonomická studie druhu Aster amellus L. Master Thesis, Palacký University, Olomouc, Czech Republic. (In Czech).
- Čelakovský L. 1884–1894. Resultate der botanischen Durchforschung Böhmens in den Jahren 1881–1884–1894. Prague.
- Chatterji AK. 1962. Structure and behavior of chromosomes in different varieties of Aster amellus L. and their mode of origin. Caryologia 15: 515–524. [Google Scholar]
- Danihelka J, Grulich V. 1996. Výsledky floristického kursu v Břeclavi (1995). Zprávy České Botanické Společnosti 31, suppl. 1996/1: 1–86. (In Czech). [Google Scholar]
- Deyl Č. 1976. Druhý Příspěvek ke květeně širšího okolí Olomouce. Zprávy Československé Botanické Společnosti 11: 17–26. (In Czech). [Google Scholar]
- Doležel J. 1997. Application of flow cytometry for the study of plant genomes. Journal of Applied Genetics 38: 285–302. [Google Scholar]
- Domin K. 1904. České Středohoří. Praha. (In Czech).
- Domin K. 1930. Schedae ad froram čechoslovenicam exsicatam. Acta Bohemica. 9: 175–259. [Google Scholar]
- Dudová H. 1998. Floristické zpracování Mšeného-lázní. Master Thesis, Charles University, Prague, Czech Republic. (In Czech).
- Durdík M. 1951. Floristické poznámky z Českého Středohoří a okolí. Československé Botanické Listy 3: 134–136. (In Czech). [Google Scholar]
- Dyer AF. 1963. The use of lacto-propionic orcein in rapid aquash methods for chromosome preparations. Stain Technology 38: 85–90. [Google Scholar]
- Ellenberg H. 1992. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18: 1–258. [Google Scholar]
- Felber F. 1986. Distribution des cytodemes d'Anthoxanthum odoratum L. s. lat. en Suisse. Les relations Alpes—Jura. Botanica Helvetica 96: 145–158. [Google Scholar]
- Felber F. 1988. Phenologie de la floraison de populations diploides et tetraploides d'Anthoxanthum alpinum et d'Anthoxanthum odoratum. Canadian Journal of Botany 66: 2258–2264. [Google Scholar]
- Felbert-Girard M, Felber F, Buttler A. 1996. Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum (Poaceae). New Phytologist 133: 531–540. [Google Scholar]
- Fiedler J, Válek B. 1975. Nová lokalita Linum flavum L. v severovýchodních Čechách. Zprávy Československé Botanické Společnosti 10/1: 27–28. [Google Scholar]
- Fišerová D. 1990. Vegetace východní části SPR Karlštejn. Bohemia centralis 19: 41–79. (In Czech). [Google Scholar]
- Formánek E. 1887. Květena Moravy a rakouského Slezska. Brno. (In Czech).
- Galitski T, Saldanha AJ, Styles CA, Lander ES, Fink GR. 1999. Ploidy regulation of gene expression. Science 285: 251–254. [DOI] [PubMed] [Google Scholar]
- Gornall JR, Wentworth JE. 1993. Variation in chromosome number of Parnasia palustris L. in British Isles. New Phytologist 123: 383–388. [Google Scholar]
- Grulich V. ed. 1989. Výsledky floristického kursu ČSBS v Uherském Hradišti. Uherské Hradiště: Odbor kultury ONV. (In Czech).
- Hanousek J. 1981. Příspěvek k flóře jihovýchodního okraje Drahanské vrchoviny. Zprávy Československé Botanické Společnosti 16/2: 119–124. (In Czech). [Google Scholar]
- Hewitt GM. 1988. Hybrid zones—natural laboratories for evolutionary studies. Trends in Ecology and Evolution 3: 158–167. [DOI] [PubMed] [Google Scholar]
- Holub J, Měsíček J, Javůrková V. 1970. Annoted chromosome counts of Czechoslovak plants. Folia Geobotanica Phytotaxonomia 5: 339–368. [Google Scholar]
- Houda J. 1969. Džbán. Louny: Kulturní správa ONV. (In Czech).
- Hrouda L, Skalický V. 1988. Floristický materiál ke květeně Příbramska I. Vlastivědný sborník Podbrdska, přírodovědné číslo/živá příroda/ 1984/27: 115–194. (In Czech). [Google Scholar]
- Husband BC. 2004. The role of triploid hybrids in evolutionary dynamics of mixed-ploidy population. Biological Journal of the Linnean Society 82: 537–546. [Google Scholar]
- Husband BC, Schemske DW. 1997. Cytotype distribution at a diploid-tetraploid populationa of Chamerion (Epilobium) angustifolium (Onagraceae). American Journal of Botany 85: 1688–1694. [PubMed] [Google Scholar]
- Huziwara Y. 1962. Karyotype analysis in some genera of Compositae. VIII. Further studies on the chromosomes of Aster. American Journal of Botany 49: 119–119. [Google Scholar]
- Jay M, Reynaud J, Blaise S, Cartier D. 1991. Evolution and differentiation of Lotus corniculatus/Lotus alpinus populations from French south-western Alps. Evolutionary Trends in Plants 5: 157–160. [Google Scholar]
- Knížetová L, Pecina P, Pivničková M. 1987. Prověrka maloplošných chráněných území a jejich návrhů ve Středočeském kraji v letech 1982–85. Bohemia centralis 16: 7–262. (In Czech). [Google Scholar]
- Koblížek J, Sutory K, Řepka R, Unar J, Ondráčková S. 1998. Floristická charakteristika vybraných lokalit širšího okolí energetické soustavy Dukovany-Dalešice. Přírodovědní sborník Západomoravského muzea v Třebíči 37: 1–99. (In Czech). [Google Scholar]
- Kolbek J. 1986. Příspěvek ke květeně Chráněné krajinné oblasti Křivoklátsko. Bohemia centralis 15: 29–52. (In Czech). [Google Scholar]
- Kolbek J, Petříček V. 1994. Příspěvek ke květeně Úštěcké pahorkatiny. Severočeskou Přírodou 28: 65–84. (In Czech). [Google Scholar]
- Kovanda M. 1984. Chromosome numbers in selected Angiosperms (2). Prelia 56: 289–301. (In Czech). [Google Scholar]
- Kovanda M. 2002. Observations on Aster amellus L. Thaisia 12: 83–87. [Google Scholar]
- Kovanda M. 2005. Aster. In: Hejný S, Slavík B (eds). Květena ČR, Volume 7. Praha: Academia. Pp. 130–132. (In Czech).
- Krahulcová A. 1990. Selected chromosome counts of the Czechoslovak flora II. Folia Geobotanica Phytotaxonomia 25: 381–388. [Google Scholar]
- Kruuk LEB, Baird SJE, Gale KS, Barton NH. 1999. A comparison of multilocus clines maintained by environmental adaptation or by selection against hybrids. Genetics 153: 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubát K. 1970. Rozšíření některých druhů rostlin v Českém středohoří. Litoměřice. (In Czech).
- Kubát K, Sládek J, Hamerský R, Roubínková O. eds. 1999. Floristický materiál z floristických kurzů a exkurzí Severočeské pobočky ČBS 1987 – 1993. Severočeskou přírodou 31: 67–81. (In Czech). [Google Scholar]
- Leitch IJ, Bennett MD. 1997. Polyploidy in angiosperms. Trends in Plant Sciences 2: 420–476. [Google Scholar]
- Lewis WH. 1980. Polyploidy in species population. In: Polyploidy, biological relevance. New York: Plenum Press.
- Levin DA. 1975. Minority cytotype exclusion in local plant populations. Taxon 24: 35–43. [Google Scholar]
- Löve Á. 1974. IOPB Chromosome number reports XLVI. Taxon 23: 801–812. [Google Scholar]
- Lumaret R, Barrientos E. 1990. Phylogenic relationships and gene flow between sympatric diploid and tetraploid plants of Dactylis glomerata L. Plant Systematics and Evolution 169: 81–96. [Google Scholar]
- Lumaret R, Hanotte C. 1987. Mise en évidence d'un écotype de dactyle (Dactylis glomerata L.) de pelouses dolomitiques subalpines dans les Grisons (Suisse. origine et échanges géniques avec les dactyles des prairies adjacentes. Oecologia Plantarum 8: 3–20. [Google Scholar]
- Lumaret R, Guillerm JL, Delay J, Loutfi A, Izco J, Jay M. 1987. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain). Oecologia 73: 436–446. [DOI] [PubMed] [Google Scholar]
- Májovský J, Murín A, Feráková V, Hindáková M, Schwarzová T, Uhríková A, Váchová M, Záborský J. 1987. Karyotaxonomický prehlad flóry Slovenska. Bratislava: Veda. (In Slovak).
- Manton I. 1934. Vergleichende Chorologie der Zentraleuropaeische Flora. Jena: Gustav Fischer Verlag.
- McArthur DE, Sanderson SC. 1999. Cytogeography and chromosome evolution of subgenus Tridentatae of Artemisia (Asteraceae). American Journal of Botany 86: 1754–1775. [PubMed] [Google Scholar]
- Merx H, Schreiber A. 1976. Aster. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (eds). Flora Europaea, Volume 4. New York: Cambridge University Press, 115.
- Meusel H, Jäger EJ. 1992. Vergleichende Chorologie der zentraleuropäischen Flora. New York: Gustav Fischer Verlag Stuttgart.
- Mitchell WW. 1992. Cytogeographic races of Arctagrostis latifolia. Canadian Journal of Botany 70: 80–83. [Google Scholar]
- Müller J. 1999. Vzácné rostliny v Moravském krasu a na území města Brna. Zprávy České Botanické Společnosti 34/1: 67–72. (In Czech). [Google Scholar]
- Münzbergová Z. 2004. Effect of spatial scale on factors limiting species distributions in dry grassland fragments. Journal of Ecology 92: 854–867. [Google Scholar]
- Neuhäusl R, Neuhäuslová-Novotná Z. 1968. Floristický materiál ke květene Moravy I. Zprávy Československé Botanické Společnosti 3: 147–160. (In Czech). [Google Scholar]
- Novák FA. 1943. Nová chráněná přírodní oblast. Krása našeho Domova 35: 133–137. (In Czech). [Google Scholar]
- Opiz PM. 1815–1835. Botanische Topographie Böheims. 1–3. Prague. (In Czech).
- Otruba J. 1944. Světlík tatarský, Euphrasia tatarica Fischer, na stepi u Žerůvek nedaleko Olomouce. Příroda 37: 32. (In Czech). [Google Scholar]
- Ott J. 1851. Fundorte der Flora Böhmens nach weil. Prof. Fr. Ing. Tausch's Herbarium Florae Bohemicae alphabetisch geordnet: 49–60.
- Otto F. 1990. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Crissman HA, Darzynkiewicz Z, eds. Methods in cell biology, Vol. 33. New York: Academic Press, 105–110. [DOI] [PubMed]
- Ozkan H, Levy AA, Feldman M. 2001. Allopolyploidy-induced rapid genome evolution in the wheat (Aegilops–Triticum) group. Plant Cell 13: 1735–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell JR, Obbard DJ, Buggs RJA. 2004. Polyploidy and the sexual system: what can we learn from Mercurialis annua? Biological Journal of the Linnean Society 82: 547–560. [Google Scholar]
- Pekárek P. 1986. Příspěvek ke květeně Ústěcka. Zprávy Československé Botanické Společnosti 21: 215–223. (In Czech). [Google Scholar]
- Petit RJ, Pineau E, Demesure B, Bacilieri R, Ducousso A, Kremer A. 1997. Chloroplast DNA footprints of postglacial recolonization by oaks. Population Biology 94: 9996–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijáček J. 1951. První Příspěvek k průzkumu motýlí fauny na Hlučinsku. Přírodovědecký sborník Ostravského kraje 12: 248–258. (In Czech). [Google Scholar]
- Podpěra J. 1911. Květena Hané. Základy zeměpisného rozšíření rostlinstva na Horním úvalu moravském. Brno. (In Czech).
- Podpěra J. 1949. Jak proniká teplobytná květena do údolí jesenických a beskydských. Přírodovědecký sborník Ostravského kraje 10: 81–95. (In Czech). [Google Scholar]
- Pořízek L, Pivničková M. 1994. Plány péče o zvláště chráněná území a příklady jejich realizace v chráněných územích Kopč a Sprašová rokle u Zeměch v okrese Mělník. Bohemia centralis 23: 155–162. (In Czech). [Google Scholar]
- Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics 29: 467–501. [Google Scholar]
- Reitmayer J. 1968. Příspěvek ke květeně jihomoravské pahorkatiny. Zprávy Československé Botanické Společnosti 3: 17–21. (In Czech). [Google Scholar]
- Rivola M. 1975. Některé nové návrhy chráněných území na Křivoklátsku. Bohemia Centralis 4: 52–63. (In Czech). [Google Scholar]
- Rohlena J. 1930. Přízpěvky k floristickému výzkumu Čech. Časopis národního Musea, sect. 104: 1–16, 69–78. (In Czech). [Google Scholar]
- Rothera SL, Davy AJ. 1986. Polyploidy and habitat differentiation in Deschampsia caespitosa. New Phytologist 102: 449–467. [Google Scholar]
- Saul J. 2000. Významná lokalita Tretorhiza cruciata v severozápadním okolí Brna. Zprávy Československé Botanické Společnosti 35/1: 39–40. [Google Scholar]
- Schaffers AP, Sýkora KV. 2000. Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. Journal of Vegetation Science 11: 225–244. [Google Scholar]
- Sedláček J, Dvořák F. 1983. Vzácné a ohrožené druhy jihovýchodní části Hustopečské pahorkatiny. Zprávy Československé Botanické Společnosti 18: 61–66. (In Czech). [Google Scholar]
- Soltis DE, Soltis PS. 1999. Polyploidy: recurrent formation and genome evolution. Trends in Ecology and Evolution 14: 348–352. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. 2000. The role of genetic and genomic attributes in the success of polyploids. Proceedings of the National Academy of Sciences of the USA 97: 7051–7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Lu P, Tang K, Osborn T. 1995. Rapid genome change in synthetic polyploids of Brassica and its implications for polyploidy evolution. Proceedings of the National Academy of Sciences of the USA 92: 7719–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. 1950. Variation and evolution in plants. New York: Columbia University Press.
- Stebbins GL. 1985. Polyploidy, hybridization and the invasion of new habitats. Annals of the Missouri Botanical Garden 72: 824–832. [Google Scholar]
- Suda J. 2002. New DNA ploidy level in Empetrum (Empetraceae) revealed by flow cytometry. Annales Botanici Fennici 39: 133–141. [Google Scholar]
- Suda J, Malcová R, Abazid D, Banaš M, Procházka F, Šída O, Štech M. 2004. Cytotype distribution in Empetrum (Ericaceae) at various spatial scales in the Czech Republic. Folia Geobotanica 39/2: 161–171. [Google Scholar]
- Suchara I. 1978. Příspěvek ke květeně nejsevernějších opukových strání na Jičínsku. Zprávy Československé Botanické Společnosti 13: 29. (In Czech). [Google Scholar]
- Šimek P. 1980. Chráněné rostliny jižního povodí Želetavky. Zprávy Československé Botanické Společnosti 15: 133–135. (In Czech). [Google Scholar]
- Šimr J. 1927. Vegetace na opuce a její postup na holé půdě. Příspěvek k floristice Českého Středohoří. Věda přírodní 8: 80–81. (In Czech). [Google Scholar]
- Šindelář J. 1941. Studie květeny okolí Kladna. Kladno. (In Czech).
- Šumberová K. 1995. Vybrané lokality xerotermní flóry v jižní části Hustopečské pahorkatiny. Zprávy České Botanické Společnosti 30, suppl. 1995/1: 103–112. (In Czech). [Google Scholar]
- Tamanšjan SG. 1959. Aster L. In: Komarov V. (ed.) Flora URSS. Moskva–Leningrad. 14: 77–110.
- Thompson JD, Lumaret R. 1992. The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends in Ecology and Evolution 7: 302–307. [DOI] [PubMed] [Google Scholar]
- Tichý L. 1997. Lesní vegetace údolí Dyje v okolí Vranovské přehrady a mapa potenciální přirozené vegetace. Zprávy České Botanické Společnosti 32, suppl. 1997/15: 109–130. (In Czech). [Google Scholar]
- Toman M. 1974. Rozšíření druhů Aster amellus L. a Linun flavum L. v Čechách. Zprávy Československé Botanické Společnosti 9: 9–21. (In Czech). [Google Scholar]
- Tyler B, Borrill M, Chorlton K. 1978. Studies in Festuca. 10. Observations on germination and seedling cold tolerance in diploid Festuca pratensis and tetrapoid F. pratensis var. apennina in relation to their altitudinal distribution. Journal of Applied Ecology 15: 219–226. [Google Scholar]
- Van Dijk P, Bakx-Schotman T. 1997. Chloroplast DNA phylogeography and cytotype geography in autopolyploid Plantago media. Molecular Ecology 6: 345–352. [Google Scholar]
- Van Dijk P, Hartog M, Vandelden W. 1992. Single cytotype areas in autopolyploid Plantago media L. Biological Journal of the Linnean Society 46: 315–331. [Google Scholar]
- Višňák R. 1992. Květena města Liberce I. Sborník Severočeského muzea, Přírodní vědy 18: 21–72. [Google Scholar]
- Wang H, McArthur ED, Sanderson SC, Graham JH, Freeman DC. 1997. Narrow hybrid zone between two subspecies of big sagebrush (Artemisia tridentata Asteraceae). 4. Reciprocal transplant experiments. Evolution 51: 95–102. [DOI] [PubMed] [Google Scholar]
- Weicker C. 1854. Aufzählung einiger im nordwestlichen Teile Böhmens gesammelten Pflanzen. Lotos 4: 131–133. [Google Scholar]
- Žídková K. 1989. Inventarizační průzkum květeny Státní přírodní rezervace Bílé stráně a vrchu Satan u Litoměřic. Severočeskou přírodou 23: 55–76. (In Czech). [Google Scholar]