Abstract
Since thrombin activation of endothelial cells (ECs) is well-known to increase endothelial permeability by disassembly of adherens junctions (AJs) and actinomyosin contractility mechanism involving myosin light chain (MLC) phosphorylation, we investigated the effects of bone marrow-derived progenitor cells (BMPCs) on the thrombin-induced endothelial permeability response. We observed that addition of BMPCs to endothelial monolayers at a fixed ratio prevented the thrombin-induced decrease in transendothelial electrical resistance, a measure of AJ integrity, and increased mouse pulmonary microvessel filtration coefficient, a measure of transvascular liquid permeability. The barrier protection was coupled to increased vascular endothelial cadherin expression and increased Cdc42 activity in ECs. Using small interfering RNA (siRNA) to deplete Cdc42 in ECs, we demonstrated a key role of Cdc42 in signaling the BMPC-induced endothelial barrier protection. Endothelial integrity induced by BMPCs was also secondary to inhibition of MLC phosphorylation in ECs. Thus BMPCs interacting with ECs prevent thrombin-induced endothelial hyperpermeability by a mechanism involving AJ barrier annealing, inhibition of MLC phosphorylation, and activation of Cdc42.
Keywords: adherens junctions, Cdc42, vascular endothelial cadherin, myosin light chain
bone marrow-derived progenitor cells (BMPCs) positive for antigen markers CD34 and Flk-1 contribute to angiogenesis at sites of neovascularization (2, 11, 24). Recruitment and engraftment of BMPCs may be required for formation of new blood vessels (2, 11, 18, 21, 24). These published studies suggest that BMPC transplantation is a potential therapeutic strategy of neovascularization for ischemic diseases (10, 22, 23). Several studies have also demonstrated the protective role of mesenchymal stem cells in experimental models of acute lung injury (8, 16). We have recently shown BMPC-mediated endothelial barrier protection in the sepsis model of acute lung injury through sphingosine-1-phosphate signaling (31). To address whether the BMPC effects on the endothelium could be extended to other proinflammatory mediators, here we investigated the effects of BMPCs in mitigating increase in endothelial permeability induced by the archetypal mediator thrombin. Thrombin ligation of protease-activated receptor-1 (PAR-1) increases endothelial permeability secondary to disassembly of adherens junctions (AJs) and phosphorylation of myosin light chain (MLC) (28). Loss of vascular endothelial cadherin (VE-cadherin) homotypic interaction induced by thrombin results in increased endothelial permeability (9, 12, 17).
Using mouse BMPCs positive for CD133 and CD34, we observed that BMPCs interacting with pulmonary microvascular endothelial cells (ECs) prevented the thrombin-induced increase in endothelial permeability. The endothelial barrier protection was secondary to inhibition of MLC phosphorylation and strengthening of the AJ barrier through activation of the RhoGTPase Cdc42.
MATERIALS AND METHODS
Mice.
All mice were bred and maintained in the University of Illinois facility according to National Institutes of Health (NIH) guidelines. Approval for animal care and use in these experiments was granted by the Animal Care and Use Committee.
Mouse BMPCs.
Mouse BMPCs were isolated using modification of methods in Refs. 29–31. Briefly, the femur and tibia were stripped from muscle and connective tissue, bone was cut at both ends, and bone marrow was flushed with HBSS using syringe with a 25-gauge needle. The bones were cut into small pieces and incubated with 10 ml of collagenase A solution (1.0 mg/ml in HBSS) in 50-ml tube for 3–4 min at 37°C with gentle shaking. The digested mixtures together with the initial bone marrow HBSS flush were filtered using a 40-μm nylon filter. Mononuclear cells were isolated by density gradient (Ficoll-Paque; Amersham) following centrifugation at 1,600 rpm for 30 min. The cells were resuspended in EBM-2MV endothelial culture basal media using the supplement kit (Lonza) made of 10% FBS, 50 U/ml penicillin and streptomycin, 2 mmol/l l-glutamine (Invitrogen), and additional VEGF (5 ng/ml). The cells were then plated onto fibronectin-collagen-gelatin (1:1:1)-coated tissue culture flasks. The cells were incubated for 48 h at 37°C with 5% CO2, at which time the nonadherent cells, representing 90–95% of the initial culture, were washed away. The adherent cells were then cultured for 21 days. The phenotype of confluent cell population was assessed by determining the expression of protein markers described below using FACS analysis.
BMPC transplantation.
BMPCs (3 × 105 BMPCs in 200 μl of EBM-2MV medium) were injected through external jugular vein.
FACS analysis.
Following 21 days in culture, BMPCs were detached with 1 mmol/l EDTA in PBS and fixed with 100 μl of 4% paraformaldehyde at room temperature for 10 min. The cells were then centrifuged for 5 min, and the supernatant was aspirated. The cells were then incubated with the following antibodies in the dark at room temperature for 30 min: phycoerythrin-labeled anti-Sca-1 (BD Pharmingen), allophycocyanin-labeled anti-CD34 antibodies (BD Pharmingen), goat anti-CD31 (Pharmingen), mouse anti-CD133 (BD Pharmingen), and rabbit anti-VE-cadherin (Santa Cruz Biotechnology). Rabbit anti-mouse or goat anti-rabbit conjugated with FITC (Vector) or Alexa Fluor-labeled anti-goat 594 (Invitrogen) were used as the secondary antibody. The labeled cells were then washed with PBS and resuspended in 0.5 ml of 1 mmol/l EDTA in PBS to prevent aggregation and analyzed with Coulter Elite ESP (Beckman Coulter). The experiment was done in triplicate and repeated twice.
Colony-forming units assay.
Clonogenic assays were performed using either bone marrow-derived cells or lung microvascular ECs and mixture of MethodCult RH4100 (Stemcell Technologies) with EBM-2MV medium (1:1) with 5% FBS. Cell-plating densities for colony-forming units (CFU-C) assays were optimized according to manufacturer's suggestion at a density of 103 cells/well in 3 ml of complete EBM-2MV medium containing 5% FBS and 2× the supplement and addition of 50 U/ml penicillin and streptomycin, 2 mmol/l l-glutamine, and additional VEGF (5 ng/ml). Colonies were identified as large, often irregular, multicentric colonies and scored at 18 days following the manufacturer's instructions. After 18-day incubation, the colonies from bone marrow-derived cells were collected and replated into chamber slides and cultured for another 2 days. They were then fixed and immunostained with antibodies against CD133 and CD34 according to the protocol supplied with MethodCult RH4100 kit.
Mouse lung microvascular ECs.
Primary cultures of mouse lung microvascular ECs were established using cells immunoselected from lungs of 4- to 5-wk-old mice as previously described (26). Briefly, ECs were selected using a rat antibody to mouse CD31 (BD Pharmingen) and secondary antibody coupled to immunomagnetic beads (Dynabeads M-450; Dynal). The rosetted cells were then isolated and washed using a magnetic particle concentrator (Dynal MPC-15; Dynal). Purified ECs were plated onto six-well plates coated with 0.2% gelatin (Sigma-Aldrich) in EBM-2MV complete medium (Lonza) with 10% FBS (Invitrogen). These primary ECs were used at passages 3–4 for all experiments.
Transendothelial electrical resistance.
Endothelial junctional barrier function was determined by measuring real-time changes in transendothelial electrical resistance (TER; Ref. 25). ECs were seeded on a gelatin-coated gold electrode (5.0 × 104 cells/cm2) and grown to confluence to allow AJs to form. Changes in TER in response to human α-thrombin (Enzyme Research Laboratories) were monitored for up to 6 h. The small electrode and the larger counterelectrode were connected to a phase-sensitive lock-in amplifier. A constant current of 1 μA was supplied by a 1-V, 4,000-Hz alternating current signal connected serially to a 1-MΩ resistor between the small electrode and larger counterelectrode. The voltage was monitored by a lock-in amplifier, stored, and processed by a personal computer. The same computer controlled the amplifier output and switched the measurement to different electrodes in the course of an experiment. Data are presented as changes in the resistive portion of resistance normalized to its value at time 0.
Pulmonary uptake of BMPCs.
BMPCs were labeled with rhodamine red fluorophore (CMTMR; Molecular Probes). In each case after injection of 3 × 105 labeled cells into the external jugular vein, the mice were killed at day 4. The location of BMPCs within the mouse lung microvasculature counterstained with VE-cadherin was identified by confocal microscopy. In other studies, 3 × 105 BMPCs labeled with 111In-oxine as described for leukocytes (5) were injected into mice to determine their organ-specific uptake using a gamma counter (Packard Instruments).
Immunofluorescence.
Cells and sections of peripheral lung tissue were fixed for 10 min at room temperature with PBS made of 4% formaldehyde, permeabilized for 30 min in Triton X-100 (0.5% in PBS), and incubated with 5% nonfat skim milk in PBS for 90 min. Cells and sections were incubated for 180 min at room temperature with anti-VE-cadherin antibody (Santa Cruz Biotechnology) or anti-Cdc42 (Santa Cruz Biotechnology) antibody and stained with FITC-conjugated second antibody (Chemicon). Stained cells and sections were visualized with Zeiss LSM 510 confocal microscope.
Triton X-100 solubility.
Cell extracts were separated into Triton X-100-soluble and -insoluble fractions according to published protocol (14). Samples were initially homogenized in extraction buffer I made of 1% Triton X-100, 1% Nonidet P-40, 10 mmol/l Tris·HCl, and 150 mmol/l NaCl (TBS) with 2 mmol/l CaCl2, pH 7.5, and protease inhibitors. Extracts were centrifuged at 12,000 g for 5 min at 4°C to separate Triton X-100-soluble from -insoluble fractions. This supernatant was considered the Triton X-100-soluble fraction. After the first extraction, the pellets were gently washed three times in TBS containing protease inhibitors and then resuspended in the same volume of Triton X-100 supernatant and homogenized in extraction buffer II made of 0.5% SDS, 1% Nonidet P-40, and TBS with protease inhibitors. Extracts were centrifuged at 12,000 g for 5 min at 4°C; this supernatant was considered the Triton X-100-insoluble fraction. The Triton X-100-insoluble protein fractions and total protein were used for immunoblotting.
Immunoblotting.
Protein concentrations were determined using the BCA protein assay (Pierce). Equal amounts of the protein lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated for 180 min at room temperature with the following antibodies: anti-VE-cadherin (1:1,000; Santa Cruz Biotechnology); anti-RhoA (1:100; Santa Cruz Biotechnology); anti-Cdc42 (1:1,000; Santa Cruz Biotechnology); anti-Thr18/Ser19 phosphorylated MLC (MLCp) (1:500; Cell Signaling Technology); and anti-MLC (1:1,000; Cell Signaling Technology). After wash with TBS-Tween, the blots were incubated for 60 min at room temperature with horseradish peroxidase-conjugated antibodies, respectively: anti-goat (1:15,000; Santa Cruz Biotechnology) for VE-cadherin; anti-mouse IgG (1:15,000; Santa Cruz Biotechnology) for RhoA; and anti-rabbit (1:15,000; Sigma-Aldrich) for Cdc42, MLCp, and MLC. Signals from immunoreactive bands were visualized by fluorography using an ECL reagent (Pierce). The intensity of individual bands in immunoblots were quantified using the NIH Image program.
Actin stress fiber formation.
Content of actin stress fibers was determined in confluent ECs grown in a slide chamber (Nalge Nunc International). Cells were washed with PBS, fixed in 4% formaldehyde/PBS for 15 min, and permeabilized for 5 min at room temperature in Triton X-100 (0.05% in PBS). Cells were stained for 90 min with 1 μg/ml tetramethylrhodamine isothiocyanate (TRITC)-phalloidin in PBS. Stained ECs were analyzed by confocal microscopy.
RhoGTPase activity assay.
The GTP-bound active forms of RhoA and Cdc42 were determined by pull-down assays (1, 12). Cells were washed with ice-cold PBS five times and lysed in lysis buffer (50 mmol/l Tris, pH 7.4, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mmol/l NaCl, 10 mmol/l MgCl2, 10 μg/ml each of aprotinin and leupeptin, and 1 mmol/l phenylmethylsulfonyl fluoride). After centrifugation at 18,000 g at 4°C for 2 min, the extracts were incubated at 4°C for 60 min with glutathione-Sepharose beads coupled with glutathione-S-transferase (GST)-rhotekin fusion protein for determination of Rho activity or GST-p21-activated kinase (PAK) for determination of Cdc42 activity. Bound RhoA and Cdc42 proteins were quantified by Western blotting as described above.
Transfection of siRNA.
Small interfering RNA (siRNA) were transduced in ECs by electroporation using the basic EC Nucleofector kit (VPI-1001; Lonza) with the Amaxa Nucleofector Device (Lonza). Briefly, mouse lung microvascular ECs, at 60% confluency, were trypsinized and mixed with 5 μg of siRNA to Cdc42 or control nonsilencing siRNA along with 100 μl of transfection solution. Cells were electroporated by Amaxa Nucleofector Device using the manufacturer's recommended program (S-005). At 24 h after transfection, the culture medium was changed.
Pulmonary microvascular permeability.
Mice (n = 8) were injected intravenously with 0.3 × 106 BMPCs or ECs (in 200 μl of EBM-2 medium) through the external jugular vein. Mice were killed 4 days after BMPC or EC injection to determine the lung capillary filtration coefficient (Kfc) and final lung wet-to-dry weight ratio measurements. Kfc was measured to determine pulmonary microvascular permeability (28). The lung preparations were ventilated and perfused with RPMI 1640 (Invitrogen) at constant flow. The rate of weight gain for a given increase in capillary pressure was normalized by dry lung weight to calculate Kfc value. Kfc was measured from the rate of lung wet weight after a step increase in venous pressure (+6 cmH2O). The amount of fluid filtered in a 5-min period was determined by logarithmic extrapolation of the slower component to time 0. Kfc was computed in units of milliliters per minute per centimeter of water per dry gram. Lungs excised at the end of the experiment were dried in an oven at 60°C for determination of the dry lung weight. In a separate study, lungs were directly excised and weighed for determination of final wet lung weight. The lungs were then stored in an oven at 60°C. The lungs were weighed until the dry weight was stable for >5 days to allow determination of wet-to-dry weight ratio.
Statistical analysis.
Statistical analysis was performed using ANOVA with Fisher protected least significant differences post hoc test if appropriate. A value of P < 0.05 was considered significant.
RESULTS
Characterization of BMPCs.
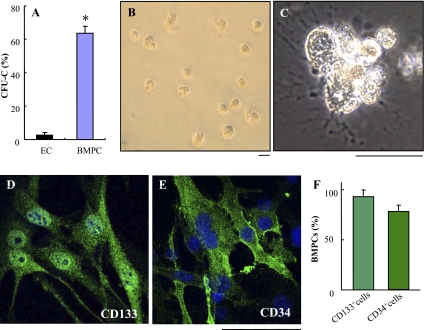
Following 21 days in culture, the bone marrow-derived cells were collected for FACS analysis. These bone marrow-derived cells expressed stem/progenitor cell markers CD133 (92 ± 5%), Sca-1 (83 ± 6%), and CD34 (78 ± 5%) (Supplemental Fig. S1, available in the data supplement online at the AJP-Lung Cellular and Molecular Physiology web site). However, only a few bone marrow-derived cells expressed mature endothelial markers VE-cadherin and CD31 (Supplemental Fig. S1). To further determine whether these bone marrow-derived cells are progenitor cells, we performed CFU-C assay. As shown in Fig. 1, there was a marked difference in the CFU-C production by the bone marrow-derived cells compared with lung microvascular ECs. Bone marrow-derived cells (65 ± 8%) formed colonies at 18 days (Fig. l, A–C), whereas very few colonies were formed by lung microvascular ECs (4 ± 2%). Immunocytochemistry also shows the majority of the cells from the colonies of bone marrow-derived cells expressed the stem/progenitor cell markers CD133 (Fig. 1, D and F) and CD34 (Fig. 1, E and F). These data demonstrated that the bone marrow-derived cells exhibit the characteristics of progenitor cells and thereby are designated as BMPCs.
Fig. 1.
Bone marrow-derived progenitor cells (BMPCs) exhibit the characteristics of progenitor cells. A–C: colony-forming units (CFU-C) assays demonstrating the progenitor potential of BMPCs. BMPCs or mouse lung endothelial cells (ECs) were plated at a density of 103 cells/well with MethodCult RH4100 mixed with EBM-2MV medium (1:1) containing 5% FBS. Following 18 days in culture, the colonies formed in collagen gels were counted. Data are expressed as means ± SD (n = 3 experiments). *P < 0.001 vs. EC (A). B and C: representative micrographs showing the colonies formed by BMPCs. Scale bar, 100 μm. D–F: the majority of BMPCs expressed the stem/progenitor cell markers CD133 and CD34. At the end of the 18-day clonogenic assay, the cells were collected and replated on chamber slides. Following 2 days in culture, the cells were then fixed in 4% paraformaldehyde and immunostained with antibodies against CD133 or CD34 (green). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). Representative micrographs of immunostaining of CD133 (D) or CD34 (E) are shown. Scale bar, 100 μm. The percentage of CD133+ or CD34+ BMPCs was quantified (F). Data are expressed as means ± SD (n = 3 experiments).
BMPCs abrogate thrombin-induced decrease of endothelial TER.
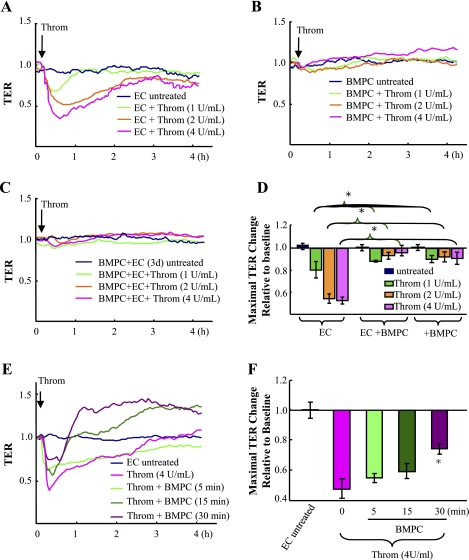
To assess the effects of BMPCs on thrombin-induced endothelial AJ barrier dysfunction, BMPCs were cocultured with ECs (at ratio of 1:3) for 3 days and then challenged with thrombin. Endothelial barrier integrity was determined by real-time measurement of TER with the electrical cell-substrate impedance sensing (ECIS) system in which ECs were grown to confluence directly on the microelectrodes (25). As shown in Fig. 2, thrombin (1, 2, and 4 U/ml) significantly decreased TER of EC monolayers (Fig. 2, A and D), whereas thrombin had no effect on the resistance values when BMPCs alone were cultured to the electrodes (Fig. 2, B and D). Addition of BMPCs into ECs prevented the thrombin-induced decrease in TER (Fig. 2, C and D). In contrast, addition of fibroblast or nonviable BMPC (fixed with paraformaldehyde) into ECs has no protective effects (Supplemental Fig. S2). Intriguingly, transient BMPC pretreatment (as short as 30 min) could also significantly reduce thrombin-induced endothelial barrier dysfunction (Fig. 2, E and F).
Fig. 2.
BMPCs prevent endothelial barrier dysfunction induced by thrombin. A–D: the effects of 3-day coculture of BMPCs with ECs on endothelial barrier function in response to thrombin. Real-time changes in transendothelial electrical resistance (TER) in response to thrombin (1, 2, and 4 U/ml) were monitored in mouse lung EC cultures (A), BMPC cultures (B), or the 3-day (3d) EC-BMPC cocultures (at ratio of 3:1; C). Data are representative of 3 experiments. The maximal changes in TER were quantified (D). Data are expressed as means ± SD (n = 3). *P < 0.05. Throm, thrombin. E and F: the effects of transient BMPC treatment on endothelial barrier function in response to thrombin. BMPCs were added to endothelial monolayers at the indicated times before thrombin challenge (4 U/ml), and real-time changes in TER were then monitored for 4 h (E). Data are representative of 3 experiments. The maximal changes in TER were quantified (F). Data are expressed as means ± SD (n = 3). *P < 0.05 vs. EC treated with thrombin. Thirty-minute pretreatment of BMPCs significantly attenuated thrombin-induced endothelial barrier dysfunction.
BMPCs prevent thrombin-induced increase in vascular permeability in mouse lungs.
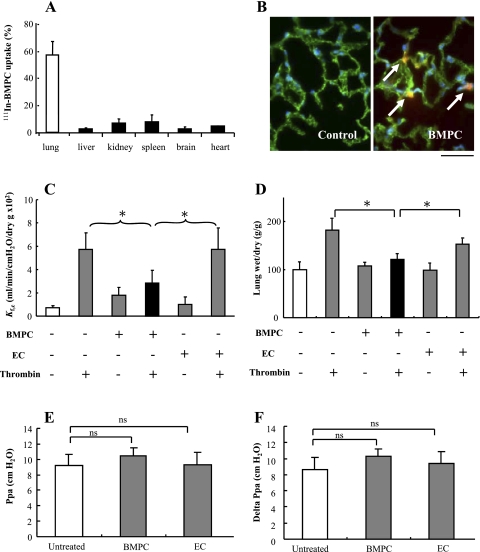
We studied the organ distribution of injected BMPCs and localization of BMPCs in the lung. BMPCs were mainly retained in lungs 4 days postintravenous injection compared with other organs (Fig. 3A). As shown in Fig. 3B, the rhodamine-labeled BMPCs at day 4 after injection were localized within lung microvessels as seen by rhodamine-labeled BMPCs surrounded by lung vascular ECs stained with FITC-conjugated VE-cadherin, an EC marker.
Fig. 3.
Lung uptake of BMPCs and BMPC-mediated prevention of thrombin-induced increase in lung vascular permeability. A: BMPC uptake in lungs. BMPCs (3 × 105) labeled with 111In-oxine were injected into each mouse through the jugular vein. Approximately 60% of the injected BMPCs were retained in lungs 4 days postinjection. Data are expressed as means ± SD (n = 5 mice). B: representative micrographs of fluorescent staining demonstrating BMPC localization within lung microvessels. Four days postinjection of rhodamine-labeled BMPCs (3 × 105 cells per mouse through the external jugular vein), cryosections of lung tissues were used for assessment of BMPCs localization (red). Lung vascular ECs were immunostained with anti-vascular endothelial cadherin (anti-VE-cadherin; green), and nuclei were counterstained with DAPI (blue). Arrows indicate the rhodamine-labeled BMPCs surrounded by lung vascular ECs. Scale bar, 50 μm. C: effects of BMPCs on pulmonary microvascular hyperpermeability response to thrombin. Four days post-BMPC or mouse lung EC injection (via external jugular vein), the mouse lungs were isolated and perfused for 15 min using RPMI 1640 medium with or without 4 U/ml thrombin. Pulmonary capillary filtration coefficient (Kfc) was then measured to determine pulmonary microvascular permeability. Data are expressed as means ± SD (n = 5). *P < 0.05. BMPC transplantation prevented the thrombin-induced increase in Kfc, whereas EC transplantation had no effect. D: effect of BMPCs on thrombin-induced lung edema formation determined by measuring lung water content. Data are expressed as means ± SD (n = 5). *P < 0.05. In the BMPC treatment group following thrombin challenge, lung wet-to-dry weight ratio was decreased compared with control groups injected with or without mouse lung ECs. E and F: measurement of pulmonary artery pressure without cell perfusion (Ppa; E) and delta Ppa measured after cell perfusion (F). The injection of either BMPCs or ECs did not significantly change Ppa and delta Ppa. Data are expressed as means ± SD (n = 5). ns, not significant.
To address the in vivo relevance of endothelial barrier protection induced by BMPCs, we next assessed alterations in pulmonary microvascular permeability and lung edema formation by measuring Kfc and lung wet weight gain in mice. Each mouse was injected intravenously (jugular vein) with 0.3 × 106 BMPCs or mouse lung ECs and killed 4 days after BMPC or EC injection. Mouse lungs were removed and perfused with medium with or without thrombin challenge (4 U/ml) for 15 min. BMPC transplantation prevented the thrombin-induced increase in Kfc seen in control and EC-transplanted groups (Fig. 3C). Lung wet-to-dry weight ratios were increased after challenge with thrombin in both control and EC treatment groups (Fig. 3D), whereas edema formation was significantly reduced in BMPC-transplanted group (Fig. 3D). BMPC accumulation in lungs was not associated with an increase in pulmonary artery pressure (Fig. 3, E and F).
BMPCs prevent VE-cadherin dissociation from plasma membrane.
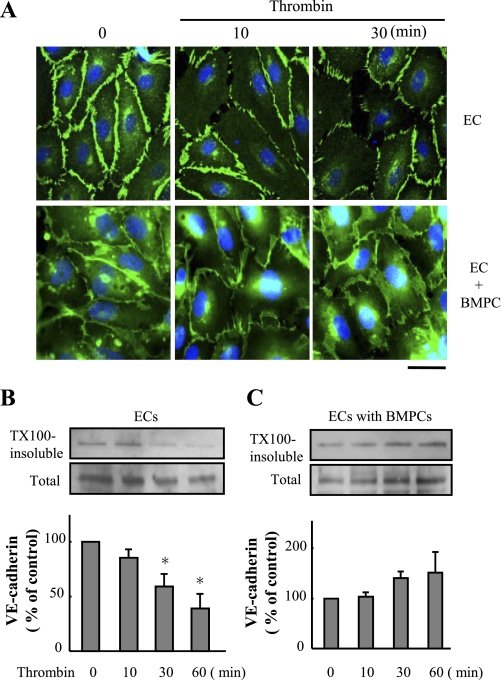
Since VE-cadherin homotypic adhesion is required for endothelial barrier function (9, 12, 14, 17, 20), we determined alterations in VE-cadherin distribution after the addition of rhodamine-labeled BMPCs. We observed that the characteristic time-dependent decrease in VE-cadherin staining after thrombin stimulation of ECs was inhibited by the coculture of BMPCs (Fig. 4A). Western blotting of VE-cadherin showed that BMPCs prevented the decrease in membrane-associated VE-cadherin typically seen after thrombin stimulation of ECs (Fig. 4, B and C).
Fig. 4.
BMPCs retain VE-cadherin in plasma membrane following thrombin challenge. A: representative micrographs of VE-cadherin immunostaining demonstrating BMPCs prevented thrombin-induced decrease of VE-cadherin expression in plasma membrane. Confluent mouse lung EC cultures or EC-BMPC cocultures at 3 days were challenged with thrombin (4 U/ml) for the indicated times. The fixed cells were immunostained with anti-VE-cadherin antibody (green). Nuclei were counterstained with DAPI (blue). Scale bar, 50 μm. B and C: translocation of VE-cadherin in response to thrombin determined by Triton X-100 (TX100) solubility assay. Cells were stimulated with thrombin (4 U/ml) for the times indicated (10, 30, and 60 min) and fractionated with cytoskeleton-stabilizing buffer. Representative immunoblots of anti-VE-cadherin from 3 similar experiments are shown. Bar graphs show quantitative densitometric analysis of each band. Data are expressed as means ± SD (n = 3 experiments). *P < 0.05 vs. control. BMPC treatment inhibited thrombin-induced dissociation of VE-cadherin from plasma membrane.
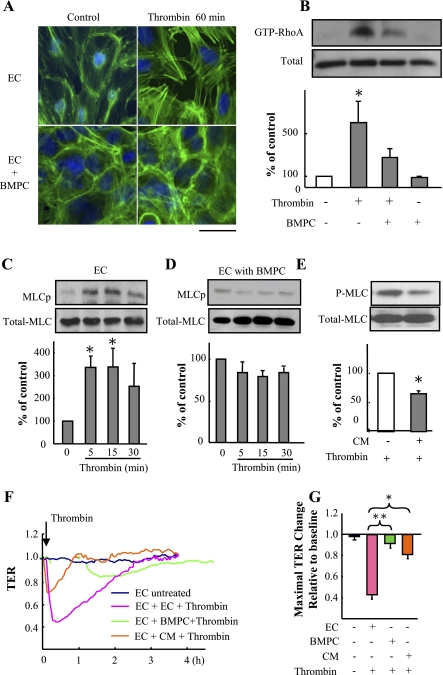
BMPCs suppress thrombin-induced RhoA activation and MLC phosphorylation.
Thrombin induces formation of intercellular AJ gaps and actin stress fibers in ECs (3). As shown in Fig. 5A, coculture of BMPCs with ECs (at ratio of 1:3) significantly reduced actin polymerization and gap formation. We then assessed the amount of active monomeric GTPase RhoA (RhoA-GTP) because of its role in mediating EC actin stress fiber reorganization and interendothelial gap formation (1, 15). BMPCs prevented the increase in thrombin-induced RhoA activity in ECs (Fig. 5B). As RhoA activation induces MLC phosphorylation and increases endothelial permeability (1, 7), we next examined whether BMPC-induced inhibition of RhoA interferes with MLC phosphorylation. In ECs alone, thrombin (4 U/ml for 5, 15, and 30 min) increased MLC phosphorylation (Fig. 5C), whereas BMPCs cocultured with ECs or addition of BMPC-conditioned media prevented the phosphorylation response (Fig. 5, D and E). We also observed that BMPC-conditioned medium significantly reduced the thrombin-induced decrease in TER (Fig. 5, F and G).
Fig. 5.
BMPCs inhibit thrombin-induced RhoA activation and myosin light chain (MLC) phosphorylation. A: actin stress fiber distribution in ECs cultured with or without BMPCs basally or following thrombin challenge (4 U/ml). ECs were cocultured with BMPCs (at ratio of 3:1) for 3 days before thrombin challenge. Fluorescent staining was performed using FITC-conjugated phalloidin (green). Nuclei were counterstained with DAPI (blue). Representative fluorescent images from 3 experiments are shown. Scale bar, 50 μm. B: effect of BMPCs on thrombin-induced RhoA activation. ECs were cocultured with BMPCs (at ratio of 3:1) for 3 days before addition of thrombin (4 U/ml). Pull-down assays were performed to determine GTP-bound RhoA (active form; GTP-RhoA). Representative immunoblots of anti-RhoA antibody from 3 experiments are shown in the top. The intensity of each band was quantified, and data are expressed as means ± SD (n = 3). *P < 0.05 vs. EC-BMPC coculture treated with thrombin. C–E: effect of BMPCs on MLC phosphorylation in response to thrombin. ECs were cocultured with BMPCs (at ratio of 3:1) for 3 days before thrombin (4 U/ml) was added. Five minutes postthrombin challenge, MLC phosphorylation was assessed by immunoblotting using an antibody specific for phosphorylated MLC (MLCp, P-MLC). Representative immunoblots from 3 experiments are shown. Data are expressed as means ± SD (n = 3). *P < 0.05 vs. control EC (C). BMPC treatment suppressed thrombin-induced MLC phosphorylation in mouse lung EC (D). BMPC-conditioned medium (CM) significantly reduced thrombin-induced increase in endothelial MLC phosphorylation (E). *P < 0.05 vs. control. F and G: TER assay demonstrating treatment with BMPC-conditioned medium reduced thrombin-induced increase in endothelial permeability. Representative real-time TER measurements of 3 experiments are shown (F). Peak TER changes were presented by bar graphs (G). Data are expressed as means ± SD (n = 3 experiments). *P < 0.05. **P < 0.01.
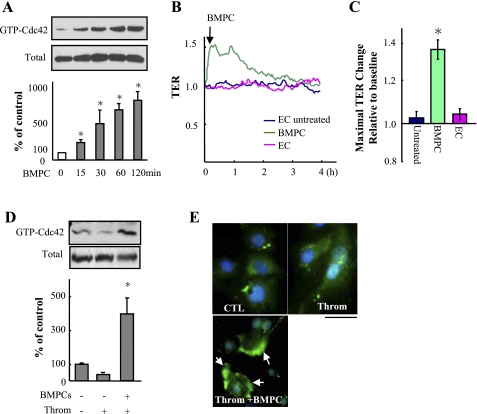
Endothelial barrier protection is mediated by BMPC-induced Cdc42 activation in ECs.
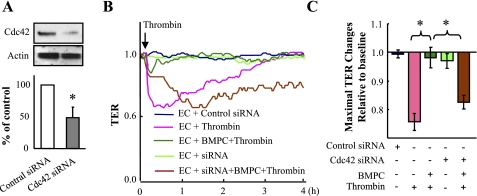
As the RhoGTPase Cdc42 is known to mediate endothelial barrier reannealing (4, 12), we next investigated the role of Cdc42 in signaling the endothelial barrier protective effect of BMPCs. GTP-bound Cdc42 was increased within 15 min after addition of 5 × 105 BMPCs to confluent ECs (Fig. 6A). BMPC addition significantly increased basal TER (Fig. 6, B and C). BMPC addition also induced Cdc42 activation in thrombin-challenged ECs (Fig. 6, D and E). To determine whether Cdc42 activation in ECs was required for BMPC-mediated endothelial barrier protection, ECs were transfected with Cdc42 siRNA to knockdown Cdc42 (Fig. 7A) before addition of either BMPCs or ECs to the Cdc42-depleted ECs (at ratio of 1:3). As shown in Fig. 7, B and C, treatment of ECs with Cdc42 siRNA prevented the recovery of endothelial TER induced by BMPCs, suggesting BMPC-induced activation of Cdc42 in ECs is the critical determinant of BMPC-elicited protective effects on endothelial barrier function.
Fig. 6.
BMPCs induce Cdc42 activation in ECs. A: effect of BMPCs on Cdc42 activity determined by level of Cdc42-GTP. Following BMPCs (50,000 cells per 60-mm dish) addition to confluent mouse lung EC monolayer, the cells were lysed at indicated times. Pull-down assays were performed to determine the GTP-bound active Cdc42 (GTP-Cdc42). Data are expressed as means ± SD from 3 independent experiments. *P < 0.05 vs. control (0 min). B and C: effects of BMPCs on endothelial barrier function basally. TER was measured in pulmonary microvessel endothelial monolayers to which BMPCs or ECs (5,000 cells/per well) were added. Data are representative of 3 experiments. *P < 0.05 vs. controls with/without addition of EC. D: effects of BMPCs on Cdc42 activity in response to thrombin challenge. Five minutes postthrombin challenge (4 U/ml), BMPCs were added to EC monolayer. Cdc42 activity was then determined by the amount of Cdc42-GTP at 15 min after addition of BMPC. Bars are means ± SD of quantitative densitometric analysis from 3 experiments. *P < 0.05 vs. control. E: effects of BMPCs on Cdc42 activity in thrombin-challenged ECs determined by immunofluorescence staining. BMPCs were added to ECs 5 min after thrombin challenge (4 U/ml), and cells were fixed 20 min after thrombin challenge. Cdc42 were stained with anti-Cdc42 antibody (green), and nuclei were counterstained with DAPI (blue). Representative fluorescent images of 3 experiments are shown. Arrows indicate BMPCs-induced membrane expression of Cdc42. Scale bar, 50 μm. CTL, control.
Fig. 7.
Endothelial barrier protection is mediated by BMPC-induced Cdc42 activation in ECs. A: Western blotting demonstrating small interfering RNA (siRNA)-mediated knockdown of Cdc42 in mouse lung ECs. Densitometry from immunoblots showed Cdc42 protein expression was efficiently reduced 72 h after transfection. *P < 0.01 vs. control siRNA-transfected ECs (n = 3 experiments). B–C: TER assay demonstrating BMPC inhibition of thrombin-induced endothelial barrier dysfunction was mediated by Cdc42 activation in ECs. ECs were transfected with either Cdc42 siRNA or scrambled control siRNA. Four hours posttransfection, BMPCs were added to the EC cultures (BMPCs-to-ECs ratio, 1:3). Seventy-two hours posttransfection, thrombin (4 U/ml) were added to the cultures, and TER was monitored to assess the endothelial barrier function (B). Data are representative of 4 independent experiments. Data of maximal TER changes are expressed as means ± SD (C). *P < 0.01.
DISCUSSION
We addressed here the effects of BMPCs in mediating endothelial barrier protection in response to thrombin challenge. We demonstrated that BMPCs added to endothelial monolayers or injected in mice prevented the increase in endothelial permeability induced by thrombin. BMPCs functioned by inhibiting the thrombin-mediated disassembly of VE-cadherin junctions. The barrier protection required BMPC-induced activation of Cdc42 in ECs and attenuation of thrombin-induced RhoA activation and MLC phosphorylation. Thus BMPCs mitigated the increase in endothelial permeability induced by thrombin by interfering directly with the mechanisms mediating the increased permeability response.
The endothelial barrier protective effect of BMPCs was established in studies in which BMPCs were added directly onto ECs. BMPCs prevented the thrombin-mediated decrease in TER and disruption of VE-cadherin junctions. In contrast, addition of ECs, fibroblast, or fixed BMPCs did not have a protective effect. Endothelial barrier protection was also seen in mouse lungs as evident by measuring the Kfc and wet-to-dry weight changes. BMPC injection significantly reduced the thrombin-induced increase in Kfc and lung edema formation compared with control lungs receiving ECs. The protection may be the result of the finding that ∼60% of the injected BMPCs were retained in lungs. Lung BMPC uptake did not increase pulmonary artery pressure, suggesting that BMPC sequestration in lungs did not induce a significant blockage of lung vessels.
Thrombin-induced increases in vascular permeability is known to be mediated by the activation of RhoA and phosphorylation of MLC leading to opening AJs (7, 9). Hence we used thrombin to test the signaling mechanism of BMPC-mediated endothelial protection. BMPCs prevented both RhoA activation and MLC phosphorylation. The protective effects of BMPCs appeared to be due to release of secondary factors since BMPC-conditioned medium elicited a protective response similar to BMPCs. We (31) have recently shown that BMPCs release large amounts of sphingosine-1-phosphate, which is known to prevent the thrombin-induced increase in endothelial permeability (13).
The actin cytoskeletal reorganization and polymerization in response to thrombin are mediated secondary to MLC phosphorylation through activation of the monomeric RhoA-GTP (1, 6). RhoA-mediated MLC phosphorylation results in formation of interendothelial gaps due to EC contraction (1, 7, 15, 27). We observed here that BMPC addition to EC monolayers significantly reduced thrombin-induced actin polymerization in ECs concomitant with reduced RhoA activation and MLC phosphorylation.
We have shown that activated RhoGTPase Cdc42 in contrast to RhoA signals the annealing of endothelial junctions in ECs exposed to thrombin (4, 19). This effect of Cdc42 is secondary to its role in promoting the interaction of α-catenin with β-catenin and the assembly of AJs and AJ interaction with the actin cytoskeleton (4, 12, 17). Therefore, we addressed whether BMPC addition to EC monolayers might signal AJ annealing by a similar mechanism. We observed that BMPCs increased the amount of GTP-bound Cdc42 (the active form) in control EC or thrombin-challenged ECs, whereas depletion of Cdc42 prevented BMPC-mediated endothelial barrier protection. Thus the interaction of BMPCs with ECs activates Cdc42 in ECs and may thereby elicit the integrity of AJs. This finding is consistent with the described role of Cdc42 as an essential determinant of endothelial junction integrity (4, 12).
In summary, our results show that BMPCs prevent thrombin-mediated AJ disassembly by activating Cdc42 and inhibiting MLC phosphorylation secondary to inactivation of RhoA. These results raise the possibility that BMPC transplantation or mobilization of BMPCs can induce endothelial barrier protection by interfering with signaling pathways in ECs mediating the increased permeability in response to proinflammatory mediators.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01-HL-085462 (Y.-Y. Zhao) and R01-HL-090152 (A. B. Malik) and a grant from the Illinois Stem Cell Research Program (A. B. Malik). H. Ohkawara was supported in part by the Banyu Fellowship Program of Banyu Life Science Foundation International.
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Kelly Price for critical reading. We also thank Qiyan Zhou and Nanjia Yu for their expert technical assistance.
REFERENCES
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275: 1308–1311, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Broman MT, Kouklis P, Gao X, Ramchandran R, Neamu RF, Minshall RD, Malik AB. Cdc42 regulates adherens junction stability and endothelial permeability by inducing alpha-catenin interaction with the vascular endothelial cadherin complex. Circ Res 98: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Cooper JA, Lo SK, Malik AB. Fibrin is a determinant of neutrophil sequestration in the lung. Circ Res 63: 735–741, 1988 [DOI] [PubMed] [Google Scholar]
- 6.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 407: 258–264, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 286: L1143–L1153, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103: 634–637, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7: 430–436, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Kouklis P, Konstantoulaki M, Malik AB. VE-cadherin-induced Cdc42 signaling regulates formation of membrane protrusions in endothelial cells. J Biol Chem 278: 16230–16236, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res 94: 159–166, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129: 203–217, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J Biol Chem 278: 33492–33500, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 4: e269, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro P, Ruco L, Dejana E. Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140: 1475–1484, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9: 702–712, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ramchandran R, Mehta D, Vogel SM, Mirza MK, Kouklis P, Malik AB. Critical role of Cdc42 in mediating endothelial barrier protection in vivo. Am J Physiol Lung Cell Mol Physiol 295: L363–L369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca(2+) signalling and PKCalpha activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol 533: 433–445, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111: 2981–2987, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 103: 2776–2779, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation 107: 3093–3100, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Tiruppathi C, Malik AB, Del Vecchio PJ, Keese CR, Giaever I. Electrical method for detection of endothelial cell shape change in real time: assessment of endothelial barrier function. Proc Natl Acad Sci USA 89: 7919–7923, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 272: 25968–25975, 1997 [DOI] [PubMed] [Google Scholar]
- 27.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res 83: 1115–1123, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol Genomics 4: 137–145, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109: 1801–1809, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96: 442–450, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Zhao YD, Ohkawara H, Rehman J, Wary KK, Vogel SM, Minshall RD, Zhao YY, Malik AB. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res 105: 696–704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.