Abstract
Objective
Combined hormone replacement therapy (HRT) containing estrogen and progestin (medroxyprogesterone acetate [MPA]) leads to increased risk of breast cancer in postmenopausal women, compared with HRT regimens containing estrogen alone, or placebo. We previously reported that, in animal models, progestins can accelerate the development of mammary tumors by increasing VEGF levels. We furthermore showed that curcumin, an Indian spice derived from the turmeric root, specifically inhibits MPA-induced VEGF secretion from breast cancer cells in vitro. In the present study, we investigated whether curcumin inhibits DMBA-induced, MPA-accelerated tumors in Sprague-Dawley rats.
Design
On Day 0, virgin female Sprague-Dawley rats (55 days old) were given DMBA (20 mg/rat). 60-day timed-release pellets containing 25 mg MPA were implanted into rats on day 30. Curcumin was administered daily at a rate of 200 mg/kg/day from day 26 to day 50 and animals were sacrificed on day 52 (n=15-19 per group).
Results
Treatment with curcumin delayed the first appearance of MPA-accelerated tumors by 7 days, decreased tumor incidence by the end of the experiment, and reduced tumor multiplicity in DMBA-induced MPA accelerated tumors. Curcumin also prevented many of the gross histological changes seen in the MPA-treated mammary gland. Immunohistochemical analyses of mammary tumors showed that curcumin decreased MPA-induced VEGF induction in hyperplastic lesions, although it did not affect the levels of estrogen and progesterone receptors.
Conclusions
We suggest that curcumin be tested as a dietary chemopreventive agent in women already exposed to MPA in an effort to decrease or delay the risk of breast cancer associated with combined HRT.
Keywords: breast cancer, progestins, VEGF, curcumin, DMBA
BACKGROUND
A number of clinical studies have shown that the use of combined estrogen/progestin hormone replacement therapy (HRT) by postmenopausal women increases the incidence of breast cancer and elevates the risk of recurrence of breast tumors compared to treatment with estrogen alone or placebo (1). Due to the time frame within which tumors are detected following estrogen/progestin combination therapy, we and others have suggested that combined HRT may cause existing but undetectable tumors or preneoplastic lesions to progress to frank tumors (2, 3). The molecular basis by which progestin consumption accelerates the growth of mammary tumors is not known. We recently showed that progestins accelerate the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumors as well as human tumor xenografts in nude mice by increasing production of the potent angiogenic molecule vascular endothelial growth factor (VEGF). By promoting angiogenesis in this manner, progestins create a favorable milieu for tumor expansion (3, 4). Consequently, by blocking progestin-induced expression of VEGF or neutralizing VEGF activity by blocking its receptor, VEGFR2, we can potentially reduce the proliferation of breast cancer cells, both in vivo and in vitro (5-7).
Approximately 6 million women in the United States use HRT to treat the symptoms of menopause (8); such extensive exposure to progestin will therefore predispose a large number of post-menopausal women to future development of breast cancer (1, 3, 8). Consumption of an antiangiogenic compound along with combined HRT could prove beneficial by significantly reducing the risk of estrogen and progesterone receptor positive breast cancer associated with HRT regimens that include a progestin component. Curcumin is an Indian spice that has been shown to have antiangiogenic properties in multiple cancer types (9, 10). This phytoestrogen has been reported to have low affinity for both estrogen receptors (ER) and progesterone receptors (PR) (11) and reduces hormone-induced cell proliferation (12). Consideration of curcumin as a chemopreventive agent is gaining popularity (13); however its usefulness as a chemopreventive agent for progestin-dependent breast cancer remains unexplored. We previously reported that curcumin inhibits the progestin-induced elaboration of VEGF from T47-D human breast cancer cells (14) and suggested that curcumin should be considered for use in clinical trials, either as a co-treatment with combined HRT to reduce the risk of breast cancer in postmenopausal women, or perhaps as a chemopreventive additive after HRT, to decrease the development of breast cancer in HRT-exposed women.
Here, we have tested our proposal in a well-established animal model of DMBA-induced breast cancer, in which VEGF is produced and tumors develop rapidly following exposure to progestins (2). We selected MPA as the progestin of choice because it is used worldwide in HRT and because we have previously shown that curcumin acts specifically to inhibit MPA-induced VEGF secretion in breast cancer cells in vitro (14). Prior to undertaking this study we hypothesized that curcumin would delay the formation of MPA-driven DMBA-induced mammary tumors, as well as prevent the elevation of VEGF levels in the mammary gland. Herein we report that curcumin does indeed delay the appearance of MPA-accelerated, DMBA-induced mammary tumors and also blocks the production of VEGF in breast cancer cells. Importantly, curcumin prevents the appearance of gross morphological abnormalities in the mammary glands of rats with DMBA-induced tumors following MPA exposure.
MATERIALS AND METHODS
Animals
Intact virgin female Sprague-Dawley rats (Harlan Breeder, Indianapolis, IN), 40-45 days old, were housed according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care under conditions of 12-hour light/dark cycles and ad libitum access to food and water. All surgical and experimental procedures were in accordance with procedures outlined in the “Guide for Care and Use of Laboratory Animals” (NIH publication 85-23).
Following the protocol previously established by our lab (2), animals were given a single dose of DMBA (20 mg/rat; Sigma Aldrich, St. Louis, MO) in peanut oil via gavage on day 0. From day 26 to day 50, 200 mg/kg curcumin (Acros Organics, Morris Plains, NJ; made daily to a concentration of 200 mg/ml in peanut oil; (15)) was injected daily i.p.; control animals were given peanut oil alone. On day 30, animals were anesthetized and 60-day release pellets containing 25 mg MPA (Innovative Research, Sarasota, FL) or placebo pellets were implanted subcutaneously on the dorsal side (Fig 1A), n=15-19. Animals were palpated 2-3 times weekly throughout the study. The appearance of the first tumor was recorded in each group (MPA group developed tumors more rapidly than other groups); this determined the delay in tumor appearance in all other groups compared with the MPA (2). On day 52 following DMBA administration, the animals were sacrificed and mammary tissues collected.
Figure 1.
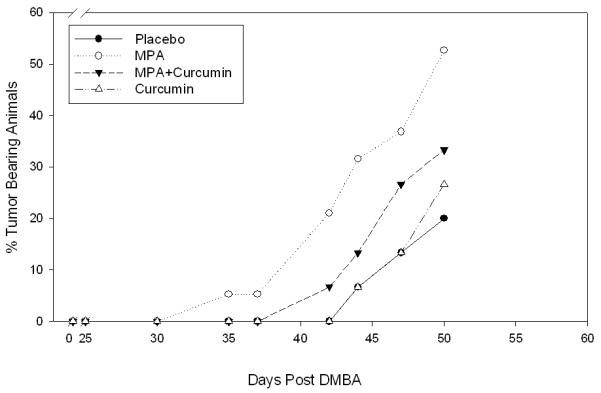
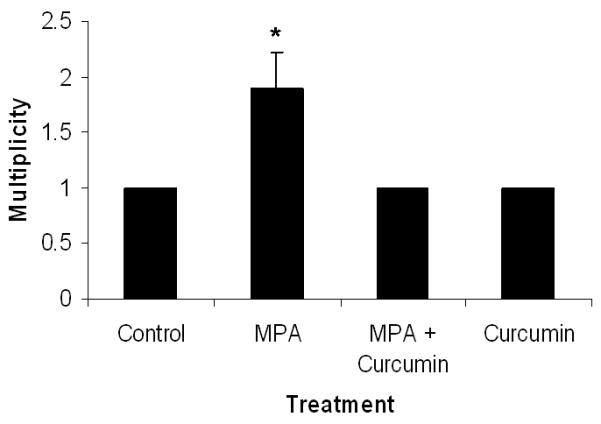
(A) Curcumin delays MPA-accelerated tumorigenesis. Animals were palpated 2-3 times each week and tumors were measured. Latency period differences were compared using a general linear model (PROC GENMOD in SAS) in which the link function was logit and the distribution was binomial. MPA treated DMBA-induced animals were 2.2 times more likely to develop tumors than animals given DMBA alone and 4.4 times more likely than animals given curcumin alone or placebo (n= 15-19/group). (B) Tumor Multiplicity. The average number of tumors per tumor-bearing animal at the conclusion of the study was 1.9 for MPA-treated animals and 1 for Control, MPA + Curcumin and Curcumin alone. *Significantly different from the rest of the groups (ANOVA, p<0.05), Errors bars represent SEM.
Histology and immunohistochemical analysis
The effect of curcumin on mammary tumorigenesis was assessed by measuring levels of VEGF, estrogen receptors (ER-α and ER-β), and progesterone receptors (PR). Both auxiliary and abdominal mammary glands were used for analysis.
Tissues were fixed overnight in 10% neutral buffered formalin for microscopy and 4% paraformaldehyde for immunohistochemistry. Tissues were processed for paraffin infiltration and embedding. Sections (5 μm) were mounted on ProbeOn Plus microscope slides (Fischer Scientific, Inc., Pittsburgh, PA). Light microscopic examination of serial hematoxylin and eosin (H&E)-stained sections representative of a given tissue was performed for classification purposes (16). Prior to immunohistochemistry, sections were dewaxed in xylene, rehydrated through graded concentrations of ethanol, rinsed in distilled water, and stored in PBS at 4°C until use. Sections were heated in 10 mmol/L citrate buffer (pH 6.0) to induce epitope retrieval for PR, ER-α, and VEGF immunolabeling. Slides were treated with 3% H2O2 in absolute methanol (to inactivate endogenous peroxidase activity) before being washed 3 times in PBS and immersed in 10% bovine serum albumin for 20 minutes. Sections were incubated for 60 minutes at room temperature with each of the following polyclonal antibodies: anti-PR antibody (1:50 dilution of a rabbit anti-human PR polyclonal antibody [A0098] that reacts with the DNA binding domain [amino acids 533-547]; DAKO, Carpinteria, CA), anti-ER-α (1:300 dilution of a rabbit anti-ER-α polyclonal antibody [sc-542] raised against an ER-α peptide of mouse origin; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and an anti-VEGF antibody (1:100 dilution of a rabbit anti-VEGF polyclonal antibody [sc-152]; Santa Cruz, Biotechnology). Sections were then washed and sequentially incubated with a secondary antibody (biotinylated swine anti-rabbit IgG [DAKO]) and a streptavidin-linked horseradish peroxidase product (BD PharMingen, San Diego, CA) for 30 minutes at room temperature. Alternatively, some sections were incubated with EnVision+, a horseradish peroxidase—labeled polymer conjugated to anti-rabbit antibodies (DAKO). Bound antibodies were visualized following incubation with 3,3′-diaminobenzidine solution (0.05% with 0.015% H2O2 in PBS; Zymed Corp., San Francisco, CA) for 3-5 minutes. Sections were counterstained with Meyer’s hematoxylin, dehydrated, cleared, and cover-slipped for microscopic examination.
Statistics
Groups were compared with respect to tumor latency and multiplicity at the conclusion of the study. Latency period differences were compared using a general linear model (PROC GENMOD in SAS) in which the link function was logit and the distribution was binomial. Multiplicity data was analyzed using Kruskal-Wallis One Way ANOVA. FoveaoPro 3.0® software analysis was used to determine positive staining by area in immunohistochemical studies. ANOVA was used to statistically compare VEGF staining differences among experimental groups; it was analyzed as a 2×2 factorial in which the difference of means was added. For all statistical comparisons, p < 0.05 was regarded as statistically significant.
RESULTS
Curcumin delays MPA-accelerated tumors in DMBA-treated rats
In our previously established model, we showed that animals treated with MPA 4 weeks after administration of DMBA develop well vascularized mammary tumors earlier than those receiving placebo pellets and concluded that this was due to increased expression of VEGF (2). Here, we used this model to determine whether curcumin inhibits VEGF expression and might therefore prevent the development of MPA-accelerated tumors. Following DMBA administration, MPA pellets were implanted subcutaneously into rats to accelerate the development of mammary tumors (2). Two groups of animals (n=15/group) were given curcumin at a rate of 200 mg/kg/day (15) starting 4 days before implantation of the 25 mg MPA pellet, a dose comparable to that of women prescribed MPA during HRT (2), Treatment was continued and was terminated 50 days after the initial DMBA treatment. Curcumin delayed the appearance of MPA-accelerated tumors (Fig 1A): tumors were first detected in MPA-treated animals on day 35 after DMBA treatment, whereas curcumin delayed the appearance of tumors until day 42. Comparison of latency data from treatments of MPA and MPA + Curcumin did not show a significant difference when analyzed using χ2 test. However, when compared using a general linear model (PROC GENMOD in SAS) in which the link function was logit and the distribution was binomial, according to the calculated odds ratio, the odds of cancer were 2.2 times greater with MPA than with MPA + Curcumin and 3.05 and 4.4 times greater than with curcumin alone or placebo. Curcumin was unable to delay natural tumor development that occurs in response to DMBA (Fig 1A). Thus, curcumin delays DMBA-induced tumors whose development is accelerated by MPA.
Curcumin also reduced the overall incidence of MPA-accelerated tumors (Fig 1A). When the first tumor was detected in the MPA + curcumin group (Day 42; 1/15, 6%), the incidence of tumors had reached 21% (4/19) in the group receiving MPA alone. However, this data also did not reach statistical significance.
Curcumin inhibits multiplicity in MPA-accelerated tumors in DMBA-treated rats
At the conclusion of the study (day 50; Fig 1A) we found that curcumin significantly reduced the number of tumors per tumor-bearing animal in the DMBA-induced MPA-accelerated group from 2 to 1 (Fig 1B).
Curcumin inhibits MPA-induced morphological changes in mammary glands
In order to determine whether curcumin affects MPA-induced changes in mammary gland morphology, non—tumor-bearing mammary tissue was collected at the end of the experiment (day 52). Mammary gland tissue was also analyzed from DMBA-treated rats that were administered curcumin without MPA. Mammary tissue from animals given MPA exhibited extensive proliferation of the mammary epithelium, resulting in a filling in of the ducts and the formation of hyperplastic alveolar nodules (Fig 2). However, hyperplastic ductules and nodule formation were minimal when curcumin was administered prior to MPA, and mammary tissue resembled that of DMBA-treated controls or lesions found in the group treated with DMBA + Curcumin (Fig 2).
Figure 2.
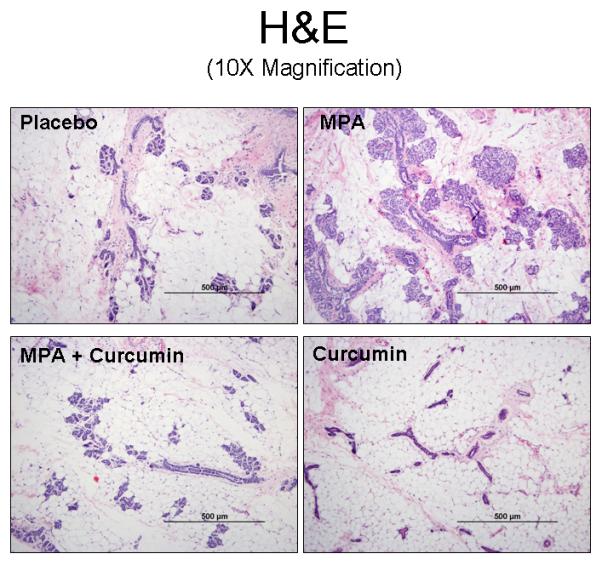
Curcumin prevents MPA-induced morphological changes in the mammary gland. Mammary gland tissue was collected at the conclusion of the study on day 52, sectioned, and stained with H&E. One representative section is shown for each group. (scale bars = 500 μm).
Curcumin blocks MPA-driven VEGF induction in hyperplastic lesions in the mammary gland
Based on our previous in vitro studies (14), we hypothesized that curcumin would reduce levels of MPA-induced VEGF and that this might explain both the delayed onset of tumor formation and the reduction in tumor cell proliferation. Consequently, we measured VEGF expression by immunohistochemistry in sections of mammary gland obtained from each of the experimental groups described in the previous section. Hyperplastic lesions of DMBA-treated, MPA-exposed mammary glands were strongly stained for VEGF (Fig 3A, right panels), whereas curcumin administration reduced the levels of VEGF staining. Although VEGF levels within mammary glands of MPA-treated animals were reduced by 34% when curcumin was co-administered, due to small sample size, there was no statistical significance as analyzed by ANOVA (Fig 3B).
Figure 3.
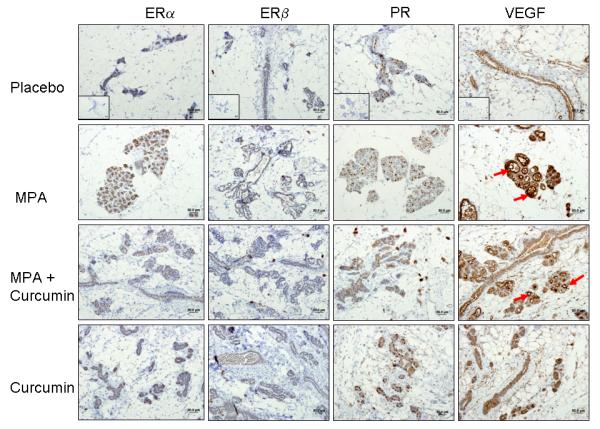
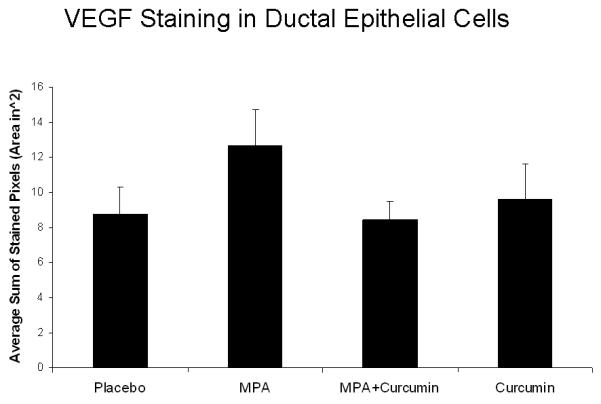
(A) ER and PR expression are unaffected by curcumin and VEGF levels are decreased by curcumin in MPA + curcumin treated DMBA-induced mammary tumors. Mammary gland tissues were collected at the conclusion of the study on day 52, sectioned, and immunostained for ER-α, ER-β, PR and VEGF as described in the Methods section. No significant differences were observed in the intensity of the staining among the treatment groups for ER-α, ER-β, and PR, however, curcumin blocked MPA-driven increases in VEGF levels in hyperplastic lesions (red arrows). Insets represent negative controls with no primary antibody staining for each antibody. (B) Photographs of slides were analyzed using FoveaoPro 3.0 analysis software. Positive VEGF staining was quantified as the number of VEGF-positive pixels in 3 different fields. Though not statistically different, the amount of VEGF was reduced in the group treated with MPA + Curcumin compared to the group treated with MPA alone. No significance was determined when analyzed using ANOVA.
Estrogen and progesterone receptor levels are not affected by curcumin treatment
Signaling through ERs is critical for PR expression (17, 18), and PR activity is essential for VEGF induction (5). Although curcumin does not bind to either ERs or PRs with high affinity (11, 12), if curcumin were to affect the levels of these receptors, it could provide a mechanism for the observed reduction in VEGF in tumor cells and the subsequent delay in the appearance of tumors following MPA exposure. However, immunostaining for ER (ER-α and ER-β) and PR did not differ among the mammary tissues from any of the experimental groups (Fig. 3A), demonstrating that curcumin does not affect the expression of either receptor type.
DISCUSSION
In this report, we provide evidence that the dietary compound curcumin, used as a spice mainly in Asian countries, delays the appearance of progestin-accelerated breast tumors in a DMBA-induced tumor model and reduces the risk of tumor development. This effect is exerted through inhibition of VEGF production by tumor cells (Fig. 3A), a finding that is concordant with the results of our in vitro studies using cultured breast cancer cells (14). Importantly, we also observed that curcumin reduced the multiplicity of tumors generally observed following acceleration of tumor development with progestins and that it preserved the morphology of mammary glands in MPA-treated animals; the abnormal proliferation of intraductal tumor cells leading to hyperplastic lesions in MPA-treated animals was absent in curcumin-treated animals. There were more proliferating lobules in the MPA-treated group than in controls, whereas curcumin-treated animals were histologically similar to the placebo group, suggesting that the turmeric root derivative helps maintain normal morphology. Singletary et al. showed that curcumin, when administered before DMBA delays DMBA-induced mammary tumorigenesis (15); however, in the current study we are the first to show that MPA-accelerated, DMBA-induced mammary tumors can be delayed by curcumin and that the morphology of the mammary gland can be protected. A limitation of our study is the relatively small sample size used for comparative purposes, with tumor incidence, latency and VEGF staining in the hyperplastic regions of the mammary gland consequently not attaining significance. However, the ability of curcumin to protect against increased multiplicity of MPA-induced tumors and to preserve the normal cellular structure of the mammary gland provides a strong rationale for considering its use as a chemopreventive agent against progestin-dependent mammary tumors in women who have already been exposed to combined HRT containing MPA.
The specific mechanism by which curcumin inhibits angiogenesis remains to be elucidated; however, there are studies suggesting that cyclin D (19) and p21 (20) are involved in its anticancer properties. To date, though, no studies have been published that would shed light on how curcumin affects progestin-dependent mammary cancers. A number of studies in other models report that NF-κB is a target for curcumin (10, 21, 22), and that in breast and ovarian cancer cells curcumin inhibits NF-κB activation and thereby decreases VEGF mRNA expression. Curcumin is also reported to reduce the expression of matrix metalloproteinases as a consequence of decreased NF-κB activity and transcriptional downregulation of AP-1, indicating that it may prevent the inflammatory response usually associated with tumor progression (23). It may be that in our studies curcumin inhibits NF-κB activity, though another possible mechanism of action is the specific induction of cancer cell apoptosis and cell cycle arrest at the G2 phase (21), a possibility that remains to be tested. Curcumin has been shown to exhibit anti-genotoxic activity against DMBA-induced mammary tumors (15). In the earlier study, it was demonstrated that at the same doses used in our studies, curcumin inhibited DMBA-induced mammary tumorigenesis, as well as DMBA-DNA adduct formation in rats when administered prior to DMBA (15). However, in our studies, we focus on the inhibitory activity of curcumin on MPA-accelerated tumors using the DMBA model, in which the turmeric root derivative is administered well after DMBA.
ERs are major regulators of PRs in breast cells (17, 18), and ER-β has been associated with inhibition of angiogenesis and growth of human breast cancer xenografts (24). We therefore sought to determine whether curcumin directly suppresses levels of PR, thereby inhibiting the production of MPA-induced VEGF, or blocks ER signaling, which would indirectly suppress PR levels. However, we detected no differences in the levels of either type of ER (ER-α or ER-β) or PRs in the mammary glands of animals receiving DMBA and subsequently treated with curcumin relative to controls, indicating that curcumin exerts its anticancer properties directly on the mammary gland with little or no effect on ovarian hormone receptors. Thus, combination treatments with agents targeting ER and PR in addition to curcumin could be a viable option.
In conclusion, in this preclinical study we have shown that curcumin has the ability to inhibit MPA-driven mammary tumorigenesis. Although we did not elucidate the specific mechanism by which curcumin exerts its suppressive effects, we did show that down-regulation of either PR or ER was not involved. We also showed that curcumin attenuates MPA-induced increases in VEGF levels in the mammary gland. Finally, we demonstrated that curcumin blocks pathological changes in mammary gland morphology arising as a consequence of the tumorigenesis brought about by exposure to MPA. The dose of curcumin administered to animals in our studies elicited no toxic effects as judged by lack of any loss in animal weight, demonstrating its safety even at high doses. Furthermore, a phase I clinical trial with curcumin reports that no toxicity was observed when patients were given up to 8000 mg/day (13). We propose therefore that curcumin be considered an excellent candidate for use as a chemopreventive agent in clinical trials involving postmenopausal women taking combined HRT containing both estrogens and progestins.
ACKNOWLEDGMENTS
This research was supported by NIH grant CA-86916 and in part by R56CA-86916 from the National Cancer Institute; PDF0600723 from the Susan Komen for Cure grants; Funds from Research Diagnostic Lab and a COR grant, both from the University of Missouri College of Veterinary Medicine; an NIH-funded Minority Biomedical Research Training Initiative grant from the Department of Veterinary Pathobiology, and a Phi Zeta, Pi Chapter grant. SMH is the Zalk Missouri Professor of Tumor Angiogenesis.
REFERENCES
- 1.Writing Group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestins in healthy postmenopausal women: principle results from the Women’s Health Initiative randomized control trial. JAMA. 2002;288:231–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Benakanakere I, Williford CB, Schnell J, Brandt S, Ellersieck MR, Molinolo A, Hyder SM. Natural and synthetic progestins accelerate 7,12-dimethylbenz[a]anthracene-initiated mammary tumors and increase angiogenesis in spargue-dawley rats. Clin Cancer Res. 2006;12:4062–4071. doi: 10.1158/1078-0432.CCR-06-0427. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y, Besch-Williford C, Brekken RA, Hyder SM. Progestin-dependent progression of human breast tumor xenografts: a novel model for evaluating anti-tumor therapeutics. Cancer Res. 2007;67:9929–9936. doi: 10.1158/0008-5472.CAN-07-1103. [DOI] [PubMed] [Google Scholar]
- 5.Hyder SM, Murthy L, Stancel GM. Progestin regulation of vascular endothelial growth factor in human breast cancer cells. Cancer Res. 1998;58:392–395. [PubMed] [Google Scholar]
- 6.Liang Y, Hyder SM. Proliferation of endothelial and tumor epithelial cells by progestin-induced VEGF from human breast cancer cells: paracrine and autocrine effects. Endocrinology. 2005;146:3632–3641. doi: 10.1210/en.2005-0103. [DOI] [PubMed] [Google Scholar]
- 7.Liang Y, Besch-Williford C, Benakanakere I, Hyder SM. Re-activation of p53 pathway inhibits in vivo and in vitro growth of hormone-dependent human breast cancer cells. Int J Oncology. 2007;31:777–784. [PubMed] [Google Scholar]
- 8.Hamdy RC. Women’s health initiative study. Southern Med Journal. 2002;95:951–965. doi: 10.1097/00007611-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Lin YG, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinomas by targeting the nuclear factor-kB pathway. Clin Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 11.Zava DT, Dollbaum CM, Blen M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc Soc Exp Biol Med. 1998;217:369–378. doi: 10.3181/00379727-217-44247. [DOI] [PubMed] [Google Scholar]
- 12.Verma SP, Goldin BR, Lin PS. The inhibition of the estrogen effects of pesticides and environmental chemicals by curcumin and isoflavonoids. Envion Health Prospect. 1998;106:807–812. doi: 10.1289/ehp.106-1533252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 14.Carroll CE, Ellersieck MR, Hyder SM. Curcumin inhibits MPA-induced secretion of VEGF from T47-D human breast cancer cells. Menopause. 2008;15:570–574. doi: 10.1097/gme.0b013e31814fae5d. [DOI] [PubMed] [Google Scholar]
- 15.Singletary K, MacDonald C, Wallig M, Fisher C. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and SMBA-DNA adduct formation by curcumin. Cancer Lett. 1996;106:137–141. doi: 10.1016/0304-3835(96)04224-3. [DOI] [PubMed] [Google Scholar]
- 16.Russo J, Russo IH. Atlas and histologic classification of tumors of the rat mammary gland. J Mammary Gland Biol Neoplasia. 2000;5:187–200. doi: 10.1023/a:1026443305758. [DOI] [PubMed] [Google Scholar]
- 17.Nardulli AM, Greene GL, O’Malley BW, Katzenellenbogen BS. Regulation of progesterone receptor message ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen’s effect on progesterone receptor synthesis and degradation. Endocrinology. 1988;122:935–944. doi: 10.1210/endo-122-3-935. [DOI] [PubMed] [Google Scholar]
- 18.Read LD, Snider CE, Miller JS, Greene GL, Katzenellenbogen BS. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol Endo. 1988;2:263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- 19.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J Biol Chem. 2005;280(20):20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 20.Shanker S, Ganapathy S, Chen Q, Srivastave RK. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer. 2008;7:16. doi: 10.1186/1476-4598-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Robinson JB, DeGuzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]
- 22.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-κB activity decreases the VEGF mRNA expression in MDA-MB-231. Breast Cancer Res and Treatment. 2002;73:237–243. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 23.Bachmeier BE, Nerlich AG, Iancu CM, Cilli M, Schleicher E, Bene R, Dell’Eva R, Jochum M, Albini A, Pfeffer U. The chemopreventive polyphenol curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell Physiol & Biochem. 2007;19:137–152. doi: 10.1159/000099202. [DOI] [PubMed] [Google Scholar]
- 24.Roger P, Sahla ME, Mäkelä S, Gustafsson JA, Baldet P, Rochefort H. Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 2001;61:2537–41. [PubMed] [Google Scholar]


