Summary
CXXC finger protein 1 (Cfp1), encoded by the CXXC1 gene, is a component of the euchromatic Setd1A histone H3-Lys4 methyltransferase complex and is a critical regulator of histone methylation, cytosine methylation, cellular differentiation, and vertebrate development. Murine embryonic stem (ES) cells lacking Cfp1 (CXXC1−/−) are viable but exhibit increased levels of global histone H3-Lys4 methylation, suggesting that Cfp1 functions to inhibit or restrict the activity of the Setd1A histone H3-Lys4 methyltransferase complex. The studies reported here reveal that ES cells lacking Cfp1 contain decreased levels of Setd1A protein and exhibit a subnuclear mis-localization of both Setd1A protein and tri-methylation of histone H3-Lys4 with regions of heterochromatin. Remarkably, structure-function studies reveal that expression of either the amino terminal fragment of Cfp1 (amino acids 1-367) or the carboxyl terminal fragment of Cfp1 (amino acids 361-656) is sufficient to restore appropriate levels of Setd1A protein in CXXC1−/− ES cells. Furthermore, functional analysis of various Cfp1 point mutations reveals that retention of either Cfp1 DNA-binding activity or association with the Setd1 histone H3-Lys4 methyltransferase complex is required to restore normal Setd1A protein levels. In contrast, expression of full-length Cfp1 in CXXC1−/− ES cells is required to restrict Setd1A and histone H3-Lys4 tri-methylation to euchromatin, indicating that both Cfp1 DNA-binding activity and interaction with the Setd1A complex are required for appropriate genomic targeting of the Setd1A complex. These studies illustrate the complexity of Cfp1 function and identify Cfp1 as a regulator of Setd1A genomic targeting.
Keywords: chromatin, epigenetics, histone methylation, sub-nuclear targeting
Introduction
DNA in eukaryotic cells is complexed with histones and other proteins in the form of chromatin. The core histone tails are subject to a variety of covalent modifications, including acetylation, phosphorylation, methylation, ubiquitination, sumoylation, and ADP-ribosylation [1, 2]. Histone methylation plays critical roles in gene expression, epigenetic regulation, and disease [3]. Histone methylation is catalyzed by a family of histone methyltransferase (HMT) enzymes, many of which are characterized by an evolutionarily conserved catalytic SET (Su(var)3–9, Enhancer of Zeste, Trithorax) domain [4]. A major function of the SET domain-containing proteins is to modulate gene activity [5]. Lysine (K) residues of histones can be mono-, di-, or tri-methylated, and the functional relevance of these modifications depends on their amino acid position. For example, di- and tri-methylated histone H3K4 is found associated with promoters and 5′ regions of active genes [6], whereas di- and tri-methylated histone H3K9 is present at transcriptionally inactive chromatin sites [7–9]. Yeast express a single H3K4 HMT, Set1, which associates with a complex known as COMPASS (Complex Proteins Associated with Set1) [10] and is required for telomeric and rDNA silencing [11, 12]. In contrast, mammalian cells contain numerous HMTs that exhibit specificity for histone H3K4, including Setd1A, Setd1B, Mll1, Mll2, Mll3/Halr, Mll4/Alr, Ash1L, Smyd1, Smyd2, Smyd3, and Set7/9 that are present as distinct multi-protein complexes and play critical roles in gene expression and development [4, 13–16].
The molecular mechanisms that control the targeting and activity of HMT complexes are not well understood. Methylation at histone H3K4 correlates with transcriptional activation and is directly coupled to the transcription process [17]. In yeast and mammals, Set1/Setd1A localize to the 5′ end of actively transcribed genes and interacts with the RNA polymerase II (RNAP II) C-terminal domain phosphorylated at serine 5 (Ser5-P CTD), a mark associated with transcription initiation [18–20]. In yeast, Paf1C interaction with RNAP II is required for recruitment of the Set1/COMPASS H3K4 HMT complex to actively transcribed genes [19]. In mammals, Setd1A is tethered to RNAP II by Wdr82, an integral component of the Setd1A complex [18]. Wdr82 associates with the RNA recognition motif (RRM) within Setd1A and directly recognizes Ser5-P CTD of RNAP II [18]. In mammals, Mll1 interacts with RNAP II containing Ser5-P CTD and mediates histone H3K4 methylation at a subset of transcriptionally active genes [21]. In addition, menin, a component of the Mll2 H3K4 HMT complex, associates with RNAP II containing Ser5-P CTD [22]. In yeast and mammals, the Setd2 H3K36 HMT primarily associates with the elongating hyper-phosphorylated form of RNAP II [23, 24]. Therefore, histone methylation mediated by HMTs is involved in regulating both transcription initiation and elongation.
Although generally widely expressed, mammalian H3K4 HMTs provide non-redundant functions. For example, Mll2 is important for expression of the HOXB gene cluster but not the HOXA cluster [13], whereas HOXA9 and HOXC8 are exclusive Mll1 targets [22, 25]. The HMTs Ash1L and Mll1 occupy the 5′ regions of active genes, and their localization is nearly indistinguishable, which suggests redundancy of function [14]. However, in vivo depletion of either enzyme results in diminished methylation of histone H3K4 at active HOXA genes [14]. In addition, loss of a single member of the H3K4 HMT family can lead to disease or death [26, 27]. MLL1 is frequently the target of chromosomal translocations involved in acute lymphoid and myeloid leukemias [28–31]. In addition, genetic disruption of the murine MLL1 or MLL2 genes leads to embryonic lethality [13, 32]. In addition, Smyd3 expression is up-regulated in colorectal and hepatocellular carcinomas, and its H3K4 HMT activity activates oncogenes and other genes associated with the cell cycle; whereas depletion of Smyd3 by small interfering RNA treatment leads to suppression of cell growth [27].
With the exception of the enzymatic Setd1 component, the subunit composition of the mammalian Setd1A and Setd1B HMTase complexes are identical [16], each containing CXXC finger protein 1 (Cfp1), Rbbp5, Wdr5, Ash2, and Wdr82 [15, 16]. Setd1A and Setd1B mRNA are ubiquitously expressed in murine tissues, and Setd1A and Setd1B proteins do not exhibit differential cell type expression [16]. However, confocal immunofluorescence reveals that endogenous Setd1A and Setd1B proteins exhibit a largely non-overlapping subnuclear localization [16]. This suggests that Setd1A and Setd1B are targeted to a unique set of genomic sites and that each provides unique functions toward the regulation of chromatin structure and gene expression. Consequently, it is likely that the non-redundant function of each H3K4 HMT is a result of distinct target gene specificity [16].
Cfp1 is a critical epigenetic regulator of both cytosine methylation and histone methylation, and interacts with both the maintenance DNA methyltransferase Dnmt1 [33] and with the Setd1A H3K4 HMT complex [15]. Cfp1 localizes nearly exclusively to euchromatic nuclear speckles and associates with the nuclear matrix [34]. Cfp1 contains two cysteine-rich plant homeodomains (PHD), a cysteine-rich CXXC DNA-binding domain that exhibits specificity for unmethylated CpG dinucleotides, an acidic domain, a basic domain, a coiled-coil domain, and a cysteine-rich Set1 interaction domain (SID), which is required for interaction with the Setd1A and Setd1B H3K4 HMT complexes [33, 35, 36].
Disruption of the murine CXXC1 gene results in embryonic lethality shortly following implantation [37]. Murine embryonic stem (ES) cell lines lacking Cfp1 (CXXC1−/−) are viable but exhibit a variety of defects, including an increased population doubling time due to increased apoptosis, a ~70% decrease in global cytosine methylation, decreased Dnmt1 protein expression and maintenance DNMT activity, and an inability to achieve in vitro differentiation [38]. In addition, CXXC1−/− ES cells express elevated levels of histone H3K4 di- and tri-methylation, and reduced levels of histone H3K9 di-methylation [15]. Consequently, Cfp1 plays an important role in the regulation of cytosine methylation, histone methylation, and cellular differentiation.
The purpose of this study was to gain insight into the molecular mechanisms regulating the activity and targeting of the Setd1A H3K4 HMT complex. The results reported here reveal that CXXC1−/− ES cells contain reduced levels of the Setd1A protein and exhibit mis-localization of both Setd1A protein and histone H3K4 tri-methylation (H3K4Me3) to areas of heterochromatin. Surprisingly, expression in CXXC1−/− ES cells of either the amino half of Cfp1 (amino acids [aa] 1-367) or carboxyl half of Cfp1 (aa 361-656) is sufficient to restore appropriate levels of Setd1A protein. However, full-length Cfp1 is required to restrict the subnuclear localization of both Setd1A and histone H3K4me3 to euchromatin.
Results
ES cells lacking Cfp1 contain decreased levels of Setd1A protein
Exogenous expression of Setd1A fragments in HEK-293 (human embryonic kidney) cells competes with endogenous Setd1A binding with the Setd1A H3K4 HMT complex, resulting in decreased stability of endogenous Setd1A [16]. To examine whether loss of Cfp1 has a similar effect, Western blot analysis was performed to determine protein levels of Setd1A complex components in wild-type ES cells (CXXC1+/+), ES cells heterozygous for the disrupted CXXC1 allele (CXXC1+/−), ES cells lacking Cfp1 (CXXC1−/−), CXXC1−/− ES cells transfected with a full-length Cfp1 expression vector (Rescue), and CXXC1−/− ES cells carrying the empty expression vector (Vector). A significant decrease (~50%) in the level of Setd1A protein was observed in CXXC1−/− ES cells (Fig. 1A). Appropriate levels of Setd1A protein were restored upon introduction of a Cfp1 expression vector (Rescue), but not in ES cells carrying the empty expression vector (Vector). CXXC1+/− ES cells express approximately 50% of Cfp1 protein compared to CXXC1+/+ ES cells [38], and exhibit a slight decrease in Setd1A protein levels. In contrast, no difference in protein levels was observed for the other Setd1A HMT complex components (Rbbp5, Wdr5, Wdr82, and Ash2) in CXXC1−/− ES cells (Fig. 1A).
Fig. 1. ES cells lacking Cfp1 contain decreased levels of Setd1A protein.
A. Whole cell protein extracts were isolated from the following ES cell lines; CXXC1+/+, CXXC1+/−, CXXC1−/−, CXXC1−/− expressing full-length Cfp1 (Rescue), and CXXC1−/− carrying the empty expression vector (Vector). Extracts were subjected to Western blot analysis using antisera directed against the Setd1A HMT complex components Setd1A, Cfp1, Ash2, Rbbp5, Wdr5, and Wdr82. The graph presents the relative level of Setd1A protein normalized to β-actin expression from at least three independent experiments, and error bars indicate standard error. Asterisks denote statistically significant (p<0.05) differences compared to CXXC1+/+ ES cells.
B. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to assess Setd1A mRNA levels in the indicated ES cell lines. The graph presents Setd1A transcript levels relative to Gapdh from three independent experiments, and error bars indicate standard error. Asterisks denote statistically significant differences (p<0.05) compared to CXXC1+/+ ES cells.
C. Western blot analysis was performed as described in (A) to assess Setd1A protein levels in CXXC1−/− ES cells following treatment with 5 μM MG132 for 6 hours.
Previous work demonstrated that Cfp1 functions as a transcriptional activator in co-transfection assays [34, 36]. Thus, further studies were performed to examine whether reduced Setd1A levels in ES cells lacking Cfp1 is due to reduced transcription of the cognate gene. Surprisingly, quantitative real-time PCR analysis demonstrates that Setd1A mRNA levels are elevated 4 to 5-fold in CXXC1−/− ES cells compared to CXXC1+/+ and CXXC1+/− ES cells, and are restored to wild-type levels in rescued ES cells but not in CXXC1−/− ES cells carrying the empty expression vector (Fig. 1B). Therefore, the decreased levels of Setd1A protein observed in CXXC1−/− ES cells is not explained by reduced transcription of the SETD1A gene.
Previous work by our laboratory demonstrated that disruption of the interaction between endogenous Setd1A and other components of the intact histone methyltransferase complex led to a reduction of Setd1A protein levels as a consequence of reduced Setd1A half-life [16]. Additional studies were therefore performed to assess the role of protein stability in Setd1A protein levels in CXXC1−/− ES cells. These experiments reveal that treatment of CXXC1−/− ES cells with the proteosome inhibitor MG132 leads to an elevation of Setd1A proteins levels to near wild type levels (Fig. 1C).
Cfp1 is required to restrict Setd1A protein and H3K4me3 to euchromatin
The molecular mechanisms regulating HMT activity and genomic targeting remain largely unknown. Previous studies revealed the paradoxical finding that ES cells lacking the Cfp1 component of the Setd1A H3K4 HMT complex carry increased levels of histone H3K4 methylation. These findings suggest that Cfp1 may inhibit or restrict the activity of the Setd1A HMT complex. To examine this issue further, subnuclear localization of Setd1A relative to DAPI staining was examined by confocal immunofluorescence. DAPI is a fluorescent DNA stain that preferentially binds to the condensed structure of pericentromeric heterochromatin [39]. Quantification of co-localization reveals that Setd1A protein exhibits only a slight (~4%) overlap with DAPI-bright heterochromatin in wild-type ES cells. However, a significant (4 to 5-fold) increase in co-localization of Setd1A with DAPI-bright heterochromatin is observed in CXXC1−/− ES cells (Fig. 2A). Rescue of appropriate restriction of Setd1A to euchromatin is observed in CXXC1−/− ES cells expressing full-length Cfp1 (1-656), but not in cells carrying the empty expression vector (Fig. 2A).
Fig. 2. Cfp1 is required to restrict Setd1A and H3K4me3 to euchromatin.
A. Subnuclear distribution of endogenous Setd1A was determined in CXXC1+/+, CXXC1−/−, and CXXC1−/− ES cells expressing full-length Cfp1 (1-656) or carrying the empty expression vector, using rabbit anti-Setd1A antibody and bovine anti-rabbit IgG-FITC-conjugated secondary antibody. Nuclei were counterstained with DAPI and observed by confocal microscopy. Co-localization is indicated by a yellow color in the merged and co-localization image. The numbers inside the co-localization image indicate the percent co-localized signal for the presented nucleus. The numbers outside of the image summarize the average percent overlap of Setd1A with DAPI-bright heterochromatin and standard error for at least 30 nuclei. Asterisks denote a statistically significant difference (p<0.05) compared to CXXC1+/+ ES cells.
B. Subnuclear distribution of endogenous histone H3K4me3 was detected in the indicated ES cell lines using rabbit anti-H3K4me3 antibody and bovine anti-rabbit IgG-FITC-conjugated secondary antibody, as described above. The asterisks denote a statistically significant difference (p<0.05) compared to CXXC1+/+ ES cells.
The subnuclear localization of histone H3K4me3, a product of Setd1A HMT activity, was similarly analyzed by confocal immunofluorescence. Consistent with the findings of Setd1A mis-localization in CXXC1−/− ES cells, quantification of overlap between histone H3K4me3 and DAPI-bright heterochromatin indicates that histone H3K4me3 exhibits only a slight overlap with DAPI-bright heteochromatin in wild-type ES cells. However, a significant (5 to 6-fold) increase in co-localization of histone H3K4me3 with DAPI-bright heterochromatin regions is observed in CXXC1−/− ES cells (Fig. 2B). Rescue of appropriate subnuclear localization of histone H3K4me3 is observed in CXXC1−/− ES cells expressing full-length Cfp1 (1-656), but not in cells carrying the empty expression vector (Fig. 2B). These results demonstrate that ES cells lacking Cfp1 exhibit partial mis-localization of both Setd1A and histone H3K4me3 to DAPI-bright regions of heterochromatin, and reveal that Cfp1 restricts the Setd1A H3K4 HMT complex to euchromatin.
Retention of either Cfp1 DNA-binding activity or association with the Setd1A HMT complex is required to restore appropriate levels of Setd1A protein
Defects in Setd1A protein level and localization observed in CXXC1−/− ES cells are corrected upon introduction of a full-length Cfp1 expression vector (Fig. 1 and 2), thus providing a convenient method to assess structure-function relationships of Cfp1. Various cDNA expression constructs encoding FLAG-tagged Cfp1 truncations and mutations were stably expressed in CXXC1−/− ES cells to identify the functional domains of Cfp1 necessary and sufficient to restore normal levels of Setd1A protein (Fig. 3A). Isolated ES cell lines were screened for protein expression by Western blot analysis using an anti-Cfp1 antibody. CXXC1+/− ES cells express ~50% of Cfp1 protein compared to CXXC1+/+ ES cells, but exhibit normal levels of cytosine methylation and histone methylation and are able to differentiate in vitro [38]. Consequently, clones were selected for analysis that carry at least 50% of the level of Cfp1 observed in CXXC1+/+ ES cells (44).
Fig. 3. Cfp1 DNA-binding activity or association with the Setd1A complex is required for appropriate levels of Setd1A protein.
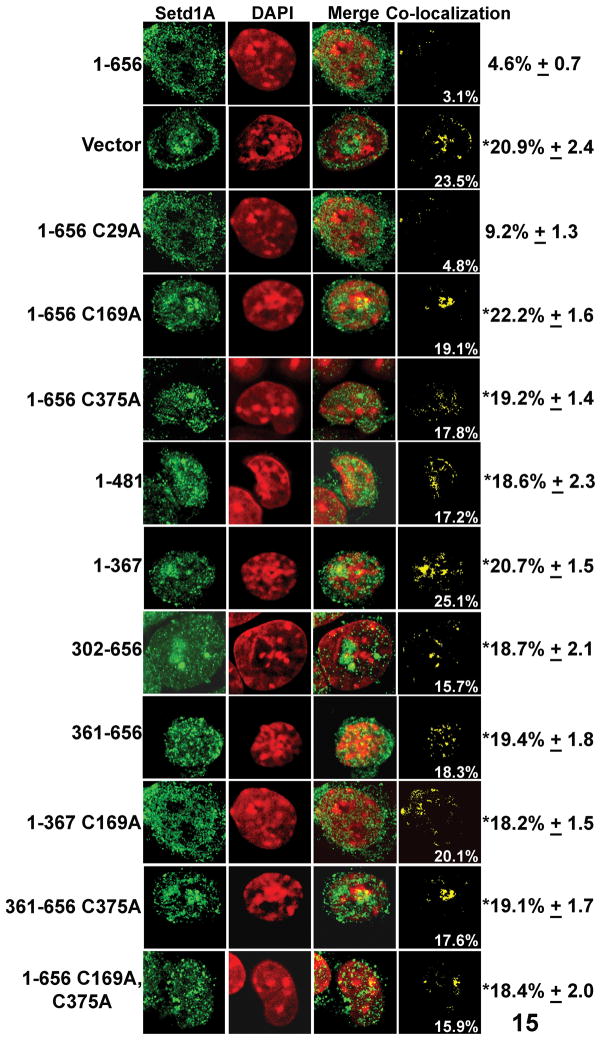
A. Schematic representation of full-length Cfp1 (1-656) and Cfp1 truncations and mutations that were stably expressed in CXXC1−/− ES cells. The filled circle at the N-terminus of Cfp1 represents the FLAG epitope, while NLS represents a nuclear localization signal. Mutations that ablate DNA-binding activity (C169A) or interaction with Setd1A (C375A) are indicated by “X”.
B. Western blot analysis was performed on whole cell extracts collected from CXXC1+/+, CXXC1−/−, and CXXC1−/− ES cells expressing full-length Cfp1 (1-656) or the indicated Cfp1 mutations (or carrying the empty expression vector), using antisera directed against Setd1A [16]. The level of β-actin serves as a loading control. The graph represents relative Setd1A protein levels normalized to β-actin from at least three independent experiments, and error bars indicate standard error. Asterisks denote statistically significant (p<0.05) differences compared to CXXC1−/− ES cells expressing full-length Cfp1 (1-656).
Expression of a carboxyl terminal deletion fragment of Cfp1 that lacks the PHD2 domain (aa 1-481), or an amino terminal deletion fragment that lacks the PHD1, CXXC, and acidic domains (aa 302-656) leads to restoration of normal levels of Setd1A protein, indicating that none of these Cfp1 protein domains are necessary for this rescue activity (Fig. 3B). Surprisingly, expression of either the amino half of Cfp1 (1-367; containing the PHD1, CXXC, acidic, and basic domains) or the carboxyl half of Cfp1 (361-656; containing the coiled-coil, SID, and PHD2 domain) is sufficient to restore appropriate levels of Setd1A protein, indicating that Cfp1 contains redundant functional domains that support Setd1A protein levels, and that no single Cfp1 domain is essential for this function (Fig. 3B).
The amino terminal fragment of Cfp1 (aa 1-367) contains the CXXC DNA-binding domain, and the carboxyl terminal Cfp1 fragment (aa 361-656) contains the Setd1 interaction domain (SID) [33]. Previous work determined that a mutation of a conserved cysteine residue (C169A) within the CXXC domain ablates Cfp1 DNA-binding activity [35], and mutation of a conserved cysteine residue within the SID domain (C375A) ablates interaction of Cfp1 with the Setd1A HMT complex [33]. Additional studies were performed to assess the functional significance of these Cfp1 properties on the ability to restore normal levels of Setd1A protein. CXXC1−/− ES cells expressing full-length Cfp1 that lacks DNA-binding activity (1-656 C169A) or interaction with the Setd1A H3K4 HMT complex (1-656 C375A) contain normal levels of Setd1A protein. This was expected, given that expression of either half of Cfp1 is sufficient to restore normal Setd1A protein levels. However, ablation of DNA-binding activity within the amino terminal fragment of Cfp1 (1-367 C169A), or disruption of Setd1A interaction with the carboxyl terminal Cfp1 fragment (361-656 C375A), results in loss of Setd1A protein rescue activity (Fig. 3B). Lastly, rescue activity was lost upon introduction of both point mutations into full-length Cfp1 (1-656 C169A, C375A). These data indicate that retention of either Cfp1 DNA-binding activity or interaction with the Setd1A H3K4 HMT complex is required to restore appropriate Setd1A protein levels in CXXC1−/− ES cells.
Full-length Cfp1 is required to restrict Setd1A protein and histone H3K4me3 to euchromatin
CXXC1−/− ES cells expressing various Cfp1 truncations and mutations were analyzed by confocal immunofluorescence to determine the functional domains of Cfp1 required to restrict the subnuclear localization of Setd1A and histone H3K4me3 to euchromatin. The vast majority of Setd1A protein and histone H3K4me3 is localized to DAPI-dim euchromatic regions in CXXC1−/− ES cells expressing full-length Cfp1 (1-656) (Fig. 4 and 5). In contrast to the pattern of Cfp1 rescue activity exhibited for Setd1A protein levels, however, expression of the amino (1-481 or 1-367) or carboxyl (302-656 or 361-656) fragments of Cfp1 in CXXC1−/− ES cells is not sufficient to exclude Setd1A and histone H3K4me3 from DAPI-bright heterochromatin (Fig. 4 and 5). In addition, CXXC1−/− ES cells expressing full-length Cfp1 that lacks DNA-binding activity (1-656 C169A) or fails to interact with the Setd1A H3K4 HMT complex (1-656 C375A) also fail to restrict Setd1A and histone H3K4me3 to euchromatin (Fig. 4 and 5). As expected, ablation of the DNA-binding activity within the amino terminal fragment of Cfp1 (1-367 C169A), disruption of the Setd1A interaction with the carboxyl terminal fragment of Cfp1 (361-656 C375A), or introduction of both mutations within full-length Cfp1 (1-656 C169A, C375A) also results in a failure to exclude Setd1A and histone H3K4me3 from DAPI-bright heterochromatin (Fig. 4 and 5). Therefore, full-length Cfp1 is required to restrict Setd1A and histone H3K4me3 localization to euchromatin, and Cfp1 DNA-binding activity and interaction with the Setd1A H3K4 HMT complex are both required for proper restriction of Setd1A and histone H3K4me3 to euchromatin.
Fig. 4.
Full-length Cfp1 is required to restrict Setd1A protein to euchromatin. The subnuclear distribution of endogenous Setd1A was detected in CXXC1−/− ES cells expressing full-length Cfp1 (1-656) or the indicated Cfp1 truncations and mutations, using rabbit anti-Setd1A antibody and bovine anti-rabbit IgG-FITC-conjugated secondary antibody as described for figure 2. Asterisks denote a statistically significant difference (p<0.05) compared to CXXC1−/− ES cells expressing full-length Cfp1 (1-656).
Fig. 5.
Full-length Cfp1 is required to restrict histone H3K4me3 to euchromatin. The subnuclear distribution of histone H3K4me3 was detected in CXXC1−/− ES cells expressing full-length Cfp1 (1-656) or the indicated Cfp1 truncations and mutations, using rabbit anti-H3K4me3 antibody and bovine anti-rabbit IgG-FITC-conjugated secondary antibody as described for figure 2. Asterisks denote statistically significant differences (p<0.05) compared to CXXC1−/− ES cells expressing full-length Cfp1 (1-656).
Discussion
The results of the studies reported here reveal that ES cells lacking the epigenetic regulator Cfp1 contain decreased levels of the histone H3K4 methyltransferase Setd1A. Yeast cells lacking Spp1, the Cfp1 homologue, also express reduced amounts of Set1 [40], and Spp1 is thought to stabilize the Set1 protein [40]. Furthermore, expression of Cfp1-interacting Setd1A protein fragments in human cells disrupts the association of endogenous Setd1A with the intact HMT complex, resulting in reduced Setd1A protein levels as a consequence of reduced Setd1A half-life [16]. Thus, the decreased levels of Setd1A protein observed in ES cells lacking Cfp1 may be due to decreased Setd1A protein stability. The observed increase of Setd1A protein in CXXC1−/− ES cells following treatment with the proteosome inhibitor MG132 supports this hypothesis. In contrast, the levels of the other components of the Setd1A complex (Ash2, Rbbp5, Wdr5, Wdr82) are not altered in CXXC1−/− ES cells, which may due to their association with additional H3K4 HMT complexes (Setd1B, Mll1, Mll2, and Mll3) [16, 18, 22, 28, 41–43]. Despite reduced Setd1A protein levels, CXXC1−/− ES cells express a ~5-fold increased level of Setd1A mRNA, suggesting that these cells increase transcription of the SETD1A gene to compensate for reduced levels of Setd1A protein.
Expression of either an amino terminal fragment (aa 1-367) or carboxyl terminal fragment (aa 361-656) of Cfp1 is sufficient to restore normal levels of Setd1A protein in CXXC1−/− ES cells. These results are consistent with previous findings that expression in CXXC1−/− ES cells of either Cfp1 1-367 or Cfp1 361-656 is sufficient to rescue defects in ES cell plating efficiency, cytosine methylation, and in vitro differentiation [44]. Interestingly, Cfp1 1-367 fails to interact with the Setd1A complex [33], but still restores appropriate levels of Setd1A protein, indicating that a physical interaction of Cfp1 with Setd1A is not required for appropriate levels of Setd1A protein. In addition, analysis of point mutations within the CXXC (C169A) or SID (C375A) domains reveals that retention of either Cfp1 DNA-binding activity or interaction with the Setd1A H3K4 HMT complex is necessary to restore normal levels of Setd1A protein in CXXC1−/− ES cells.
ES cells that lack Cfp1 exhibit increased levels of histone H3K4 di- and tri-methylation [15] despite expressing decreased levels of Setd1A, suggesting that Cfp1 restricts the activity of the Setd1A H3K4 HMT complex. Consistent with this model, confocal immunofluorescence reveals that both Setd1A protein and histone H3K4me3 are partially mis-localized to DAPI-bright regions of heterochromatin in CXXC1−/− ES cells. In contrast to the pattern of Cfp1 rescue activity observed for Setd1A protein levels, expression of full-length Cfp1 in CXXC1−/− ES cells is required to properly restrict subnuclear localization of Setd1A and histone H3K4me3 to euchromatin. These studies further indicate that Cfp1 DNA-binding activity and interaction with the Setd1A H3K4 HMT complex are both required for proper subnuclear localization of Setd1A. The requirement for an intact Cfp1 CXXC domain for proper genomic localization may indicate that Cfp1 DNA-binding activity restricts the Setd1A HMT complex to euchromatin by binding to unmethylated CpG dinucleotides in euchromatin.
Individual CXXC1−/− ES cell nuclei exhibit a range (5%–30%) of co-localization between Setd1A protein and histone H3K4me3 with DAPI-bright heterochromatin, and 20–30% mis-localization of Setd1A and histone H3K4me3 is observed in 35–40% of CXXC1−/− ES cell nuclei. It is possible that cell-to-cell variation in the degree of co-localization may be cell cycle-dependent. However, significant mis-localization of Setd1A and H3K4me3 is never observed in wild-type ES cells nor in rescued CXXC1−/− ES cells expressing full-length Cfp1. The persistence of DAPI-bright staining co-localizing with histone H3K4me3 indicates that deposition of this euchromatin epigenetic mark is insufficient to induce a general chromatin remodeling at these heterochromatin regions.
Little is known regarding the relative contributions of each mammalian H3K4 HMT complex. However, Cfp1 has been shown to be an integral component of only the Setd1A and Setd1B HMT complexes [15, 16]. The localization of Setd1B in the absence of Cfp1 has not been determined, but the finding that the extent of Setd1A mis-localization is similar to that of mis-localized H3K4me3 suggests that the Setd1 HMT complexes are responsible for the bulk of histone H3K4me3. This conclusion is consistent with a recent report that siRNA-mediated depletion of Setd1A and Setd1B leads to a dramatic global reduction of histone H3K4me3 [45].
The full-length Cfp1 protein that is required to restrict subnuclear localization of Setd1A and histone H3K4me3 to euchromatin contains two PHD domains. PHD domains are thought to be involved in chromatin-mediated transcriptional control [46], and can serve as binding modules for unmodified and methylated histone H3K4and methylated histone H3K36 [17, 47–51]. For example, the PHD1 domain of Spp1, the yeast homologue of Cfp1, binds di- and tri-methylated histone H3K4[51]. In addition, the PHD finger of the tumor suppressor Ing2 directly associates with histone H3K4me3, and this interaction is critical for proper occupancy of the Ing2-HDAC1 complex at target promoters during the DNA damage response and active transcriptional repression [48]. Therefore, the PHD domains of Cfp1 may be important for binding modified histone H3K4 and targeting the Cfp1/Setd1A complex to specific genomic sites.
The mechanisms responsible for appropriate subnuclear localization of histone H3K4 HMTs are complex and involve gene-specific recruitment by DNA-binding factors. For example, the insulator DNA-binding protein Boris recruits Setd1A to the MYC and BRCA1 genes [52]; NF-E2 recruits Mll2 to the β-globin locus [53]; the Ap2δ transcription factor recruits Ash2L and Mll2 to the HOXC8 locus [54]; and the paired-box transcription factor Pax7 recruits Mll2 to the MYF5 gene [55].
In addition, several integral components of the mammalian Set1-like histone H3K4 HMT complexes have been implicated in genomic targeting. The Wdr5 protein, which is common to each member of the mammalian Set1-like HMT complex family, has been reported to bind directly to histone H3 [56–59]. In addition, the Wdr82 component of the Setd1A and Setd1B HMT complexes binds to RNA polymerase II containing Ser5-phosphorylated CTD, thus recruiting these complexes to sites of transciption initiation [18]. Furthermore, the composition of the Setd1A and Setd1B HMT complexes are identical except for the identity of the enzymatic (Setd1) component [15, 16], yet confocal microscopy reveals that these complexes exhibit a nearly non-overlapping euchromatic subnuclear localization [16]. This finding strongly suggests that these closely related complexes regulate distinct sets of target genes, and that this specificity is mediated by each Setd1 protein, presumably through interactions with distinct targeting effector molecules. The data reported here reveals that Cfp1 plays a novel role in restricting the subnuclear localization of Setd1A and histone H3K4me3 to euchromatin, thus identifying Cfp1 as another critical regulator of histone H3K4 HMT genomic targeting.
Experimental procedures
Cell culture
Generation of murine CXXC1−/− ES cell lines was previously described [38]. ES cells were cultured on 0.1% gelatin-coated tissue culture dishes in high-glucose Dulbecco’s modified Eagle’s medium (GIBCO BRL, Life Technologies, Grand Island, NY) supplemented with 20% fetal bovine serum (GIBCO BRL), 100 Units/ml penicillin/streptomycin (Invitrogen, Carlsbad, CA), 2 mM L-glutamine (Invitrogen), 1% non-essential amino acids (Invitrogen), 0.2% leukemia inhibitory factor-conditioned medium, 100 nM β-mercaptoethanol, 0.025% HEPES pH 7.5, (Invitrogen), and 1% Hank’s balanced salt solution (Invitrogen).
Plasmid construction and transfection of ES cells
Murine Cfp1 cDNA [38, 60] was subcloned into pcDNA 3.1/Zeo (Invitrogen). The Cfp1 expression vector or the empty expression vector was electroporated into CXXC1−/− ES cells as previously described [38]. Single amino acid substitutions within Cfp1 were carried out using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) per the manufacturer’s protocol as previously described [33, 35]. For structure-function studies, cDNA constructs encoding full-length FLAG epitope-tagged human Cfp1 (aa 1-656) and various Cfp1 truncations and/or mutations, were subcloned into the pcDNA3.1/Hygro mammalian expression vector (Invitrogen). The amino terminal bipartite nuclear localization signal of Cfp1 (aa 109-121) was inserted between the FLAG epitope and Cfp1 sequence for constructs containing amino acids 302-656 and 361-656. Linearized DNA (25 μg) was electroporated into CXXC1−/− ES cells at 300 V and 500 μF capacitance. Cells were grown in selection medium containing 200 μg/ml hygromycin B (Sigma-Aldrich, St. Louis, MO) for approximately two weeks before single colonies were isolated for expansion and were subsequently maintained in medium containing 50 μg/ml hygromycin B (Sigma-Aldrich). Expression of FLAG-Cfp1 was verified by Western blot analysis using anti-FLAG (Sigma-Aldrich) or anti-Cfp1 antibodies [16]. Two or three independent clones carrying each construct were selected for analysis based on the protein expression level of FLAG-Cfp1, and data for a representative clone are presented.
Analysis of Setd1A mRNA Expression
Total RNA was isolated from ES cells using TriReagent solution (Molecular Research Center, Cincinnati, OH) and reverse transcribed as previously described [16]. Relative Setd1A gene expression was determined by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) using TaqMan gene expression assays containing a primer set and probe (FAM fluorescent reporter dye) purchased from PE Applied Biosystems specific for Setd1A (Exon 6–7, catalog number Mm00626143_m1). Mouse glyceraldehyde-3-phosphate (Gapdh)(catalog number 4352932E) served as an endogenous control. An Applied Biosystems 7500 Real-Time PCR System was used to detect PCR products following a standard amplification protocol recommended by the manufacturer. The comparative CT method was used to determine gene expression of Setd1A relative to the Gapdh control, which was averaged over three independent experiments.
Western blot analysis
Protein levels of Cfp1, Setd1A, Ash2, Wdr5, Wdr82, and Rbbp5 was assessed by isolating whole cell protein extracts as previously described [15, 16]. Protein extracts were quantified using the Bradford method [61], solubilized with 1X Laemmli sample buffer, and boiled for 5 min before being separated by electrophoresis on 7% (Cfp1) or 4–12% (Setd1A, Ash2, Wdr5, Wdr82, and Rbbp5) PAGEr-Gold pre-cast Tris-glycine gels (Lonza Group, Ltd., Switzerland), then transferred to nitrocellulose membranes (Amersham, GE Healthcare). Membranes were probed with primary antibodies, washed, and incubated with appropriate horseradish peroxidase-linked secondary antibodies as previously described [15, 16]. Signal was detected by ECL detection reagents (Amersham, GE Healthcare) and quantified by densitometry (Image J, NIH). The following antibodies were used for immunoblotting: mouse monoclonal β-actin and FLAG M2 (Sigma-Aldrich), rabbit polyclonal Setd1A, Wdr82, Cfp1, and Wdr5 [16], and rabbit polyclonal Ash2 and Rbbp5 (Bethyl Laboratories, Montgomery, TX).
Confocal immunofluorescence
Exponentially-growing ES cells were seeded onto sterilized glass coverslips at a density of 3 × 104 cells per well in 24-well tissue culture dishes. After 48 h of culture, cells were washed with ice-cold phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100 in PBS. Cells were incubated with blocking solution containing 5% normal donkey serum (Santa Cruz Biotechnology) in PBS containing 0.2% Tween-20 (PBS-T) before incubation with 1:500 dilutions of primary antibodies for histone H3K4me3 (Abcam, Cambridge, MA) or Setd1A [16]. Coverslips were washed with PBS-T, and incubated with 1:200 dilutions of fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Coverslips were then incubated with a solution of 0.1 μg/ml 4′,6-diaminidino-2-phenylindone (DAPI). Images were captured on a Zeiss UV LSM-510 Confocal Microscope (Carl Zeiss Inc., Thornwood, New York) at the Indiana University Center for Biological Microscopy using a UV argon laser (364 nm excitation) for DAPI, and visible argon laser (488 nm excitation) for FITC. The immunofluorescent images were analyzed with MetaMorph 6.0 (Universal Imaging Corporation, West Chester, PA). Threshold for FITC was adjusted to exclude background and non-specific staining, and threshold for DAPI was adjusted to include DAPI-bright regions. The same threshold values were used to analyze each image. Quantification of co-localization of positive fluorescent signals was analyzed using the MetaMorph co-localization module. At least 30 nuclei were analyzed for each cell line.
Acknowledgments
We thank Simon Atkinson for helpful discussions regarding confocal microscopy. This work was supported by the Riley Children’s Foundation, the Lilly Endowment, and National Science Foundation Grants NSF MCB-0344870 and MCB-0641851 (D.G.S.). C.M.T. was supported by a predoctoral fellowship from National Institutes of Health Grant T32 AI060519 and a Department of Education training grant in Graduate Assistance in Areas of National Need (GAANN).
The abbreviations used are
- aa
amino acid
- CFP
CXXC finger protein
- CTD
carboxyl terminal repeat domain
- DAPI
4′-6-diaminidino-2-phenylindone
- DNMT
DNA methyltransferase
- ES
embryonic stem
- FITC
fluorescein isothiocyanate
- H3K4me3
histone H3 lysine 4 tri-methylation
- HMT
histone methyltransferase
- K
lysine
- PBS
phosphate-buffered saline
- PHD
plant homeodomain
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- RNAP
RNA polymerase
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SID
Set1 interaction domain
References
- 1.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- 3.Kouzarides T. Chromatin modfications and their functions. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol. 2005;6:227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenuwein T, Laible G, Dorn R, Reuter G. SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol Life Sci. 1998;54:80–93. doi: 10.1007/s000180050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NCC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 7.Boggs BA, Cheung P, Heard E, Spector DL, Chinault AC, Allis CD. Differentially methylated forms of histone H3 show unique association patterns with inactive human X chromosomes. Nature Genet. 2002;30:73–76. doi: 10.1038/ng787. [DOI] [PubMed] [Google Scholar]
- 8.Noma K, Allis CD, Grewal SI. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science. 2001;293:1150–1155. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- 9.Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferase impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 10.Miller T, Krogan NJ, Dover J, Erdjument-Bromage H, Tempst P, Johnston M, Greenblatt JF, Shilatifard A. COMPASS: A complex of proteins associated with a trithorax-related SET domain protein. Proc Nat Acad Sci USA. 2001;28:12902–12907. doi: 10.1073/pnas.231473398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryk M, Briggs SD, Strahl BD, Curcio MJ, Allis CD, Winston F. Evidence that Set1, a factor required for methylation of histone H3, regulates rDNA silencing in S. cerevisiae by a Sir2-independent mechanism. Curr Biol. 2002;12:165–170. doi: 10.1016/s0960-9822(01)00652-2. [DOI] [PubMed] [Google Scholar]
- 12.Krogan NJ, Dover J, Khorrami S, Greenblatt JF, Schneider J, Johnston M, Shilatifard A. COMPASS, a histone H3 (Lysine 4) methyltransferase required for telomeric silencing of gene expression. J Biol Chem. 2002;277:10753–10755. doi: 10.1074/jbc.C200023200. [DOI] [PubMed] [Google Scholar]
- 13.Glaser S, Schaft J, Lubitz S, Vinterten K, van der Hoeven F, Tufteland KR, Asasland R, Anastassiadis K, Ang S-L, Stewart AF. Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development. 2006;133:1423–1432. doi: 10.1242/dev.02302. [DOI] [PubMed] [Google Scholar]
- 14.Gregory GD, Vakoc CR, Rozovskaia T, Zheng X, Patel S, Nakamura T, Canaani E, Blobel GA. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol. 2007;27:8466–8479. doi: 10.1128/MCB.00993-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Skalnik DG. CpG-binding protein (CXXC Finger Protein 1) is a component of the mammalian Set1 histone H3-Lys4 methyltransferase complex, the analogue of the yeast Set1/COMPASS complex. J Biol Chem. 2005;280:41725–41731. doi: 10.1074/jbc.M508312200. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Tate CM, You JY, Skalnik DG. Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. J Biol Chem. 2007;282:13419–13428. doi: 10.1074/jbc.M609809200. [DOI] [PubMed] [Google Scholar]
- 17.Martin DGE, Baetz K, Shi X, Walter KL, MacDonald VE, Wlodarski MJ, Gozani O, Hieter P, Howe L. The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol Cell Biol. 2006;26:7871–7879. doi: 10.1128/MCB.00573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Skalnik DG. Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol Cell Biol. 2008;28:609–618. doi: 10.1128/MCB.01356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng H-H, Robert F, Young RA, Struhl K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol Cell. 2003;11:709–719. doi: 10.1016/s1097-2765(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 20.Palancade B, Bensaude O. Investigating RNA polymerase II carboxyl-terminal domain (CTD) phosphorylation. Eur J Biochem. 2003;270:3859–3870. doi: 10.1046/j.1432-1033.2003.03794.x. [DOI] [PubMed] [Google Scholar]
- 21.Milne TA, Dou Y, Martin ME, Brock HW, Roeder RG, Hess JL. MLL associates specifically with a subset of transcriptionally active target genes. Proc Nat Acad Sci USA. 2005;102:14765–14770. doi: 10.1073/pnas.0503630102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, Kay GF, Hayward NK, Hess JL, Meyerson M. Menin associates with a trithorax famly histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 23.Edmunds JW, Mahadevan LC, Clayton AL. Dynamic histone H3 methylation during gene induction: HYPB/Setd2 mediates all H3K36 trimethylation. EMBO J. 2008;27:406. doi: 10.1038/sj.emboj.7601967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Howe L, Anderson S, Yates III, JR, Workman JL. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem. 2003;278:8897–8903. doi: 10.1074/jbc.M212134200. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domer PH, Fakharzadeh SS, Chen CS, Jockel J, Johansen L, Silverman GA, Kersey JH, Korsmeyer SJ. Acute mixed-lineage leukemia t(4;11)(q21;q23) generates an MLL-AF4 fusion product. Proc Nat Acad Sci USA. 2003;90:7884–7888. doi: 10.1073/pnas.90.16.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nature Cell Biol. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 28.Gu Y, Nakamura T, Alder H, Prasad R, Canaani O, Cimino G, Croce CM, Canaani E. The t(14;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- 29.Ma Q, Alder H, Nelson KK, Chatterjee D, Gu Y, Nakamura T, Canaani E, Croce CM, Siracusa LD, Buchberg AM. Analysis of the murine All-1 gene reveals conserved domains with human ALL-1 and identifies a motif shared with DNA methyltransferases. Proc Nat Acad Sci USA. 1993;90:6350–6354. doi: 10.1073/pnas.90.13.6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad R, Yano T, Sorio C, Nakamura T, Rallapalli R, Gu Y, Leshkowitz D, Croce CM, Canaani E. Domains with transcriptional regulatory activity within the ALL1 and AF4 proteins involved in acute leukemia. Proc Nat Acad Sci USA. 1995;92:12160–12164. doi: 10.1073/pnas.92.26.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992;71:701–708. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 32.Yu BD, Hell JL, Horning SE, Brown GAJ, Korsmeyer SJ. Altered Hox expression and segmental identity in MLL-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- 33.Butler JS, Lee JH, Skalnik DG. CFP1 interacts with DNMT1 independently of association with the Setd1 histone H3K4 methyltransferase complexes. DNA Cell Biol. 2008;27:533–543. doi: 10.1089/dna.2007.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JH, Skalnik DG. CpG-binding protein is a nuclear matrix- and euchromatin-associated protein localized to nuclear speckles containing human trithorax. J Biol Chem. 2002;277:42259–42267. doi: 10.1074/jbc.M205054200. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Voo KS, Skalnik DG. Identification and characterization of the DNA binding domain of CpG-binding protein. J Biol Chem. 2001;276:44669–44676. doi: 10.1074/jbc.M107179200. [DOI] [PubMed] [Google Scholar]
- 36.Voo KS, Carlone DL, Jacobsen BM, Flodin A, Skalnik DG. Cloning of a mammalian transcriptional activator that binds unmethylated CpG motifs and shares a CXXC domain with DNA methyltransferase, human trithorax, and methyl-CpG binding domain protein 1. Mol Cell Biol. 2000;20:2108–2121. doi: 10.1128/mcb.20.6.2108-2121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlone DL, Skalnik DG. CpG binding protein is crucial for early embryonic development. Mol Cell Biol. 2001;21:7601–7606. doi: 10.1128/MCB.21.22.7601-7606.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlone DL, Lee JH, Young SRL, Dobrota E, Butler JS, Ruiz J, Skalnik DG. Reduced genomic cytosine methylation and defective cellular differentiation in embryonic stem cells lacking CpG binding protein. Mol Cell Biol. 2005;25:4881–4891. doi: 10.1128/MCB.25.12.4881-4891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guenatri M, Bailly D, Maison C, Almouzni G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol. 2004;166:493–505. doi: 10.1083/jcb.200403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dehe PM, Dichtl B, Schaft D, Roguev A, Pamblanco M, Lebrun R. Protein interactions within the Set1 complex and their roles in the regulation of histone 3 lysine 4 methylation and transcription termination. J Biol Chem. 2006;281:35404–35412. doi: 10.1074/jbc.M603099200. [DOI] [PubMed] [Google Scholar]
- 41.Goo YH, Sohn YC, Kim DH, Kim SW, Kang MJ, Jung DJ, Kwak E, Barlev NA, Berger SL, Chow VT, Roeder RG, Azorsa DO, Meltzer PS, Suh PG, Song EJ, Lee KJ, Lee C, Lee JW. Activating signal cointegrator 2 belongs to a novel steady-state complex that contains a subset of trithorax group proteins. Mol Cell Biol. 2003;23:140–149. doi: 10.1128/MCB.23.1.140-149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysocka J, Myers MP, Laherty CD, Eisenmann RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10:1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- 44.Tate CM, Lee J-H, Skalnik DG. CXXC finger protein 1 contains redundant functional domains that support embryonic stem cell cytosine methylation, histone methylation, and differentiation. Mol Cell Biol. 2009;29:3817–3831. doi: 10.1128/MCB.00243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M, Wang PF, Lee JS, Martin-Brown S, Florens L, Washburn M, Shilatifard A. Molecular regulation of H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS. Mol Cell Biol. 2008;28:7337–7344. doi: 10.1128/MCB.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halbach T, Scheer N, Werr W. Transcriptional activation by the PHD finger is inhibited through an adjacent leucine zipper that binds 14-3-3 proteins. Nucleic Acids Res. 2000;28:3542–3550. doi: 10.1093/nar/28.18.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Ilin S, Wqang W, Dunchan EM, Wsocka J, Allis CD, Patel DJ. Molecular basis for site-specific read-out of histone H3K4Me3 recognition by the BPTF PHD finger of NURF. Nature. 2006;442:91–95. doi: 10.1038/nature04802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pena PV, Davroaou F, Shi X, Walter KL, Verkhusha VV, Gozani O, Zhao R, Kutatladze TG. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature. 2006;442:100–103. doi: 10.1038/nature04814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. doi: 10.1038/nrm2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Pena P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shit Y, Cote J, Chua KF, Gozani O. ING2 PHD domain links histone H3 lysine 4 methylation to active gene respression. Nature. 2006;442:96–99. doi: 10.1038/nature04835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi X, Kachirskaia I, Walter KL, Kuo J-HA, Lake A, Davrazou F, Chan SM, Martin DGE, Fingerman IM, Briggs SD, Howe L, Utz PJ, Kutateladze TG, Lugovskoy AA, Bedford MT, Gozani O. Proteome-wide analysis in Saccaromyces cerevisiae identifies several PHD fingers as novel direct and selective binding modules of histone H3 methylated at either lysine 4 or lysine 36. J Biol Chem. 2007;282:2450–2455. doi: 10.1074/jbc.C600286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J, Saha PK, Yang Q-H, Lee S, Park JY, Suh Y, Lee S-K, Chan L, Roeder RG, Lee JW. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc Nat Acad Sci USA. 2008;105:19229–19234. doi: 10.1073/pnas.0810100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demers C, Chaturvedi C-P, Ranish JA, Juban G, Lai P, Morle F, Aebersold R, Dilworth FJ, Groudine M, Brand M. Activator-mediated recruitment of the MLL2 methyltransferase complex to the beta-globin locus. Mol Cell. 2007;27:573–584. doi: 10.1016/j.molcel.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan CC, Sindhu KV, Li S, Nishio H, Stoller JZ, Oishi K, Puttreddy S, Lee TJ, Epstein JA, Walsh MJ, Gelb BD. Transcription factor AP2delta associates with Ash2l and ALR, a trithorax family histone methyltransferase to activate Hoxc8 transcription. Proc Nat Acad Sci USA. 2008;105:7472–7477. doi: 10.1073/pnas.0711896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKinnel IW, Ishibahi J, Grand FL, Punch VGJ, Addicks GC, Greenblatt JF, Dilworth FJ, Rudnicki MA. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nature Cell Biol. 2008;10:77–83. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couture J-F, Collazo E, Trievel RC. Molecular recognition of histone H3 by the WD40 protein WDR5. Nature Struct Mol Biol. 2006;13:698–703. doi: 10.1038/nsmb1116. [DOI] [PubMed] [Google Scholar]
- 57.Han Z, Guo L, Wang H, Shen Y, Deng XW, Chai J. Structural basis for the specific recognition of methylated histone H3 lysine 4 by the WD-40 protein WDR5. Mol Cell. 2006;22:137–144. doi: 10.1016/j.molcel.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Ruthenburg AJ, wang W, Graybosch DM, Li H, Allis CD, Patel DJ, Verdine GL. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nature Struct Mol Biol. 2006;13:704–712. doi: 10.1038/nsmb1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Brurlingame AL, Roeder RG, Brivanlou AH, Allis CD. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 60.Carlone DL, Hart SRL, Ladd PD, Skalnik DG. Cloning and characterization of the gene encoding the mouse homologue of CpG binding protein. Gene. 2002;295:71–77. doi: 10.1016/s0378-1119(02)00820-x. [DOI] [PubMed] [Google Scholar]
- 61.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]