Abstract
pH homeostasis is essential for life, yet it remains unclear how animals sense their systemic acid/base (A/B) status. Soluble adenylyl cyclase (sAC) is an evolutionary conserved signaling enzyme that produces the second messenger cAMP in response to bicarbonate ions (HCO3−). We cloned the sAC ortholog from the dogfish, a shark that regulates blood A/B by absorbing and secreting protons (H+) and HCO3− at its gills. Similar to mammalian sAC, dogfish soluble adenylyl cyclase (dfsAC) is activated by HCO3− and can be inhibited by two structurally and mechanistically distinct small molecule inhibitors. dfsAC is expressed in the gill epithelium, where the subset of base-secreting cells resides. Injection of inhibitors into animals under alkaline stress confirmed that dfsAC is essential for maintaining systemic pH and HCO3− levels in the whole organism. One of the downstream effects of dfsAC is to promote the insertion of vacuolar proton pumps into the basolateral membrane to absorb H+ into the blood. sAC orthologs are present throughout metazoans, and mammalian sAC is expressed in A/B regulatory organs, suggesting that systemic A/B sensing via sAC is widespread in the animal kingdom.
Keywords: cAMP, pH, proton pump, dogfish, gill
Protons (H+) and bicarbonate ions (HCO3−) play central roles in biology. Cellular enzymes are sensitive to pH, and CO2/HCO3− buffer animal physiological fluids. To compensate for major acid/base (A/B) metabolic and environmental disturbances, animals have developed specialized epithelia to regulate pH and HCO3− concentrations of their extracellular fluids. The ion-transporting mechanisms in cells of A/B regulatory organs show remarkable similarities throughout animal phyla. Yet, a fundamental question has remained unsolved: How is A/B stress sensed? Like all biological sensors, an A/B sensor must be able to detect deviations from a set point and induce compensatory responses. As a signaling enzyme directly modulated by bicarbonate, soluble adenylyl cyclase (sAC) (1–3) represents a potential A/B sensor. sAC is distinct from the more widely studied, G protein-regulated sources of the ubiquitous second messenger cAMP, the transmembrane adenylyl cyclases (tmACs). sAC is not regulated by heterotrimeric G proteins and is distributed throughout the cell (1, 4). Consistent with a role as a putative A/B sensor, cAMP production by sAC (hereafter referred to as “sAC activity”) inside cells has been shown to reflect extracellular CO2/HCO3− levels in mammalian sperm (5, 6), epididymis (7), and motile airway cilia (8).
Elasmobranchs (sharks, rays, and relatives) are cartilaginous fish that evolved ∼400 million years ago (9). Their gills perform essentially all (>97%) systemic A/B relevant ion transport; neither kidney nor ventilatory adjustments play a major role (10). Elasmobranch gills (Fig. S1 A and B) have numerous acid- and base-secreting cells, which are functionally analogous to those in the mammalian kidney (11, 12). In the dogfish shark, the vacuolar proton pump (V-H+-ATPase; VHA) translocates from the cell cytoplasm into the basolateral membrane of gill base-secreting cells in response to blood alkalosis (12) (Fig. S1C). Basolateral VHA secretes protons into the blood and energizes apical bicarbonate secretion into seawater to counteract the alkalosis (12–14). This VHA translocation is essential for regulating blood A/B during the alkaline tide (14), a natural postfeeding elevation in blood pH and bicarbonate that results from H+ secretion into the stomach (for food digestion) and concomitant bicarbonate absorption into the blood (15, 16). Consistent with a potential role for a sAC ortholog, the VHA translocation was shown to be dependent on generation of intracellular bicarbonate by carbonic anhydrase (CA) (14).
The aim of the current study was to identify the systemic A/B sensor in an animal. We demonstrate that an ortholog of sAC is expressed in dogfish gill and that its inhibition prevents compensatory responses to blood alkalosis both in isolated gills and in whole animals. Therefore, sAC represents a systemic A/B sensor in dogfish.
Results
Cloning Dogfish sAC.
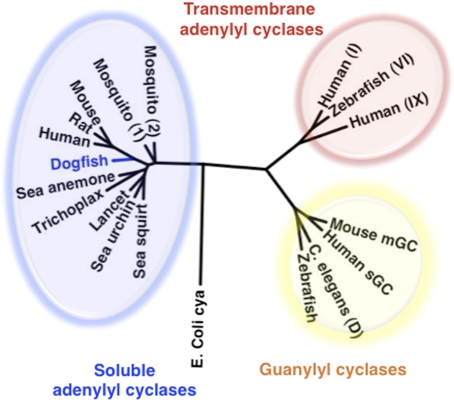
An EST from dogfish encoding dogfish soluble adenylyl cyclase (dfsAC) was identified by BLAST search using human sAC. The cDNA contained an ORF of 2,946 bp predicting a 985-aa protein of ≈110 kDa (GenBank database accession no. ACA52542.1). dfsAC shares greatest sequence homology with the catalytic domains and presumptive P-loop regions of other sAC-like proteins (1, 17). Phylogenetic analysis comparing dfsAC with other nucleotidyl cyclases, including guanylyl cyclases and tmACs (Fig. 1), confirms dfsAC to be a sAC ortholog.
Fig. 1.
Phylogenetic relation of dfsAC to other nucleotidyl cyclases. The GenBank accession numbers are as follows: (i) sACs: dogfish: S. acanthias, ACA52542.1; mouse: Mus musculus, NP_766617.1; rat: Rattus norvegicus, NP_067716.1; human: Homo sapiens, NP_060887.2; Trichoplax: Trichoplax adhaerens, XP_002117857.1 (hypothetical); Starlet sea anemone: Nematostella vectensis, XP_001623318.1 (predicted); mosquito (1): Aedes aegypti, XP_001661592.1 (hypothetical); mosquito (2): Culex quinquefasciatus, XP_001842661.1 (hypothetical); sea urchin: Strongylocentrotus purpuratus, NP_001020380.1; sea squirt: Ciona intestinalis, XP_002121952.1 (predicted); lancet: Branchiostoma floridae, XP_002214797.1 (hypothetical); (ii) guanylyl cyclases: Caenorhabditis elegans: guanylyl cyclase protein 28, isoform D AAC78238.2; zebrafish: Danio rerio, XP_001337045.2 (predicted); mouse: NP_001124165.1 guanylate cyclase 2d; human: H. sapiens, AAA60547.1 soluble retinal isoform; and (iii) tmACs: zebrafish (VI): XP_001922749.1 (predicted, isoform VI); human (I): H. sapiens, Q08828.2 (ADCY1); human (IX): H. sapiens O60503.4 (ADCY9); E. coli cya: AAA67602.1 (adenylate cyclase). Interestingly, sAC orthologs have not been identified in the genomes of some model organisms (e.g., Drosophila melanogaster, D. rerio, C. elegans), suggesting that they may use fundamentally distinct mechanisms for A/B sensing.
Recombinant dfsAC Is Bicarbonate-Responsive and Inhibited by sAC-Selective Small Molecule Inhibitors.
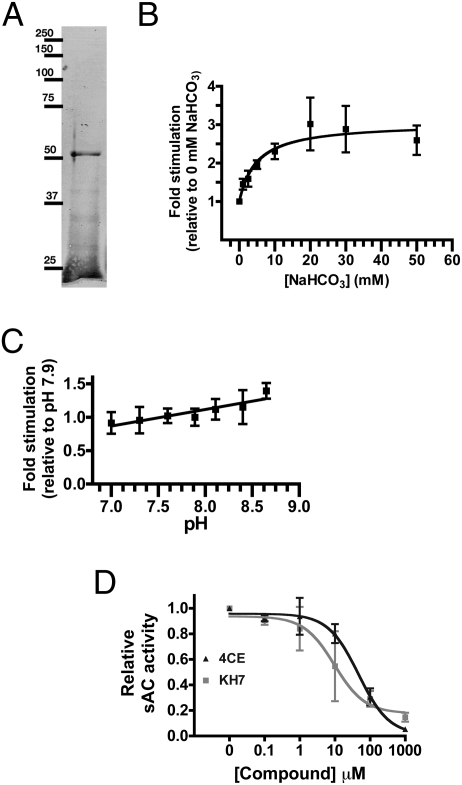
We expressed and purified an N-terminal His-tagged recombinant protein encoding the putative two catalytic domains of dfsAC (amino acids 1–485) (Fig. 2A).
Fig. 2.
Activity of recombinant dfsAC. (A) Coomassie blue-stained SDS/PAGE gel of His-tagged dfsAC (53 KDa expected) purified from E. coli. (B) dfsAC activity assayed at a constant pH of 7.75 in the presence of 2.5 mM ATP, 20 mM MgCl2, 0.5 mM MnCl2, and the indicated concentrations of NaHCO3. EC50 = 4.9 mM NaHCO3. (C) dfsAC activity at the indicated pH. (D) dfsAC activity in the presence of 2.5 mM ATP, 5 mM MnCl2, and the indicated concentrations of KH7 or 4CE. IC50 for KH7 = 9.6 μM and IC50 for 4CE = 46.9 μM. Values are expressed as fold stimulation relative to the baseline in each experiment (0 mM HCO3−, pH 7.75, or no drug, respectively). Data points reflect the averages of four independent assays for each condition.
Recombinant dfsAC activity was stimulated by HCO3− with a half-maximal effect (EC50) of ∼5 mM (Fig. 2B). This EC50 is consistent with the physiological [HCO3−] in blood plasma of normal dogfish (∼4 mM; Table 1). The difference in bicarbonate EC50 between dogfish and mammalian sAC (11–25 mM) (2, 3, 18) reflects the differences in plasma [HCO3−] between air and water breathers. Recombinant dfsAC showed a slight stimulation at alkaline pH (Fig. 2C); however, the pH dose response was nearly insignificant compared with the stimulation that would be induced by physiologically relevant increases in HCO3−.
Table 1.
sAC inhibition prevents compensation of blood alkalosis in whole animals
| 0 | 12 h | |||||
| Control | KH7 | 4CE | Control | KH7 | 4CE | |
| pH | 7.77 ± 0.02 (a) | 7.82 ± 0.06 (a) | 7.84 ± 0.03 (a) | 7.97 ± 0.03 (b) | 8.22 ± 0.06 (c) | 8.12 ± 0.04 (c) |
| [HCO3−] | 4.1 ± 0.4 (a) | 3.5 ± 1.1 (a) | 3.4 ± 0.6 (a) | 8.3 ± 0.8 (b) | 17.9 ± 3.4 (c) | 17.1 ± 4.4 (c) |
| PCO2 | 1.5 ± 0.2 (a) | 0.9 ± 0.2 (a) | 1.2 ± 0.3 (a) | 2.1 ± 0.4 (b) | 2.2 ± 0.4 (b) | 2.8 ± 0.6 (b) |
Blood pH, plasma [HCO3−] (mM), and PCO2 (torr) of dogfish infused i.v. with NaHCO3 (1,000 μEq·kg−1·h−1) and injected with DMSO (control, n = 11), KH7 (n = 9), or 4CE (n = 7). KH7 and 4CE were injected as a 5-μmol·kg−1 bolus at t = −0.5 and 6 h. Data were analyzed using repeated-measures two-way ANOVA. Different letters indicate significant differences (P < 0.05) within each variable using Bonferroni’s posttest (if the interaction between treatment and time was significant) and the Tukey–Kramer planned least square means test (if the interaction was not significant). A summary of the statistics is shown in Table S1.
Historically, mammalian sAC activity was thought to be dependent on the divalent cation manganese (19), and Mn2+ sensitivity can be used to distinguish sAC-like cyclases from tmACs (19, 20). As expected, recombinant dfsAC activity is highest with millimolar Mn2+ as the divalent cation. Inhibitor profiles can also be used to distinguish sAC-like cyclases from tmACs; sAC-like cyclases are specifically inhibited by the small molecule KH7 (5, 21) and are more sensitive to catechol derivatives of estrogen (21, 22). Recombinant dfsAC activity was inhibited by KH7 and 4-catechol estrogen (4CE) with IC50s of 9.6 and 46.9 μM, respectively (Fig. 2D). These values are similar to those for mammalian sAC (6, 7).
dfsAC Is Present and Active in Dogfish Gills.
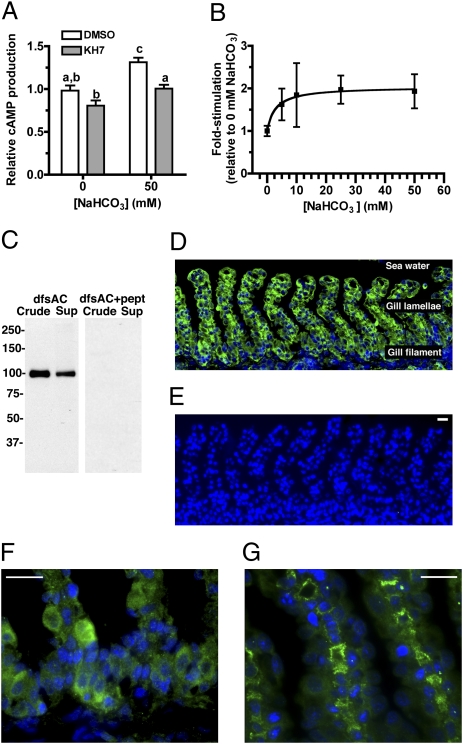
We detected dfsAC mRNA in dogfish gill using RT-PCR. To confirm the presence of dfsAC protein in gill, we characterized the adenylyl cyclase activity in gill extracts and generated antipeptide polyclonal antisera. A subset of the total cAMP-forming activity in gill extracts qualitatively matched the biochemical characteristics of recombinant dfsAC (Fig. 3 A and B): It is stimulated by HCO3− with an EC50 of ∼3 mM and is inhibited by 4CE and KH7. Western blotting using antipeptide antiserum specific for dfsAC identified a single ∼110-kDa band in dogfish gills (Fig. 3C). dfsAC was detected in gill crude homogenates and also in gill supernatants. This anti-dfsAC antisera gave specific immunofluorescent staining in the cytoplasm of epithelial cells in the gill filament and lamellae (Fig. 3 D–G). Some gill sections showed strong staining in pillar cells (Fig. 3G), which have been implicated with reversible hydration of CO2 via extracellular CA and with gas exchange (23). Thus, dfsAC might be involved in regulating multiple physiological mechanisms in response to A/B disturbances. This widespread distribution of dfsAC at the gill is distinct from mammalian epididymis, where sAC is highly expressed only in the VHA-rich cells (7), but it is similar to mammalian nephron, where sAC is abundant in several segments and potentially has multiple functions (7, 24, 25).
Fig. 3.
dfsAC is present in the gill, and cAMP-producing activity in the gill matches recombinant dfsAC. (A) Adenylyl cyclase activity in gill homogenates measured in the presence 50 mM NaCl or NaHCO3 in the presence or absence of 50 μM KH7. Shown are the averages of quadruple determinations with SEM. Equivalent results were obtained with 4CE. Lowercase letters denote levels of activity that are not significantly different from each other by repeated-measures two-way ANOVA (P < 0.05 for treatment, time, and interaction), Bonferroni’s posttest. (B) KH7-sensitive cAMP activity in gill homogenates measured as indicated in A. EC50 = 2.9 mM NaHCO3. (C) Western blot of dogfish shark gill homogenate (crude) and high-speed supernatant (sup) using anti-dfsAC in the absence or presence of immunizing peptide. (D) Immunofluorescence using anti-dfsAC (green) in dogfish gill. Nuclei were labeled with DAPI (blue). (E) Staining using anti-dfsAC antisera preabsorbed with excess immunizing peptide; similar results were obtained with preimmune serum. (F and G) Higher magnification pictures of dfsAC immunolabeling. (G) Immunolabeling in pillar cells. (Scale bars = 10 μm.)
Pharmacological Inhibition of dfsAC Impairs Blood A/B Regulation In Vivo and in Isolated Gills.
We used the two structurally and mechanistically unrelated inhibitors 4CE and KH7 to characterize dfsAC function in vivo. Basal blood pH and plasma [HCO3−] were ∼7.8 units and ∼4 mM, respectively. After 12 h of i.v. NaHCO3 infusion (1,000 μEq·kg−1·h−1), control (DMSO-injected) fish reached steady-state values of pH = 7.97 and 8.3 mM [HCO3−] (Table 1), similar to previously reported values (12–14). In fish injected with 4CE or KH7, blood pH increased to pH = 8.12 with 4CE and to pH = 8.22 with KH7 and plasma [HCO3−] rose to 17 mM and 18 mM, respectively (Table 1). Thus, inhibition of dfsAC prevents dogfish from efficiently regulating their blood A/B. There was a trend for increased PCO2 during dfsAC inhibition; however, it was not statistically different from that of control fish (Table 1). These experiments also demonstrate the effectiveness of sAC inhibitors in a whole-animal context.
sAC Inhibition Prevented VHA Translocation in Whole Animals and in Isolated Gills.
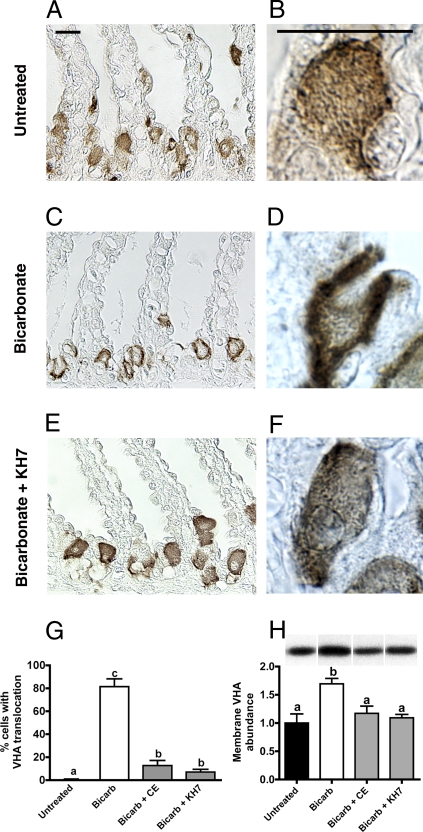
To explore why inhibition of dfsAC deregulates blood A/B, we examined VHA translocation in gill cells by immunohistochemistry and Western blotting in membrane-enriched samples. In untreated fish, the predominant VHA immunostaining was cytoplasmic (Fig. 4 A and B). On blood alkalosis, there was significant VHA translocation to the basolateral membrane (Fig. 4 C and D) to reabsorb H+ into the blood and compensate alkalosis (12–14). This VHA translocation was prevented by inhibition of dfsAC (Fig. 4 E and F) by either of two structurally unrelated inhibitors, KH7 or 4CE. Inhibition of sAC during alkalosis resulted in fewer cells with VHA translocation (Fig. 4G) and less abundant VHA in cell membranes (Fig. 4H) compared with fish injected with DMSO alone.
Fig. 4.
sAC-dependent VHA translocation during systemic alkalosis in whole fish. Anti-VHA immunolabeling in dogfish gills. Low-power (A) and high-power (B) magnification of gills from untreated dogfish sharks. Low-power (C) and high-power (D) magnification of gills taken from dogfish shark after 12 h of continuous NaHCO3 infusion. Low-power (E) and high-power (F) magnification of gills taken from dogfish shark after 12 h of continuous NaHCO3 infusion and injected with KH7 (5 μmol·kg−1 bolus at t = −0.5 and 6 h). (Scale bars = 10 μm.) (G) Percentage of cells showing VHA translocation in gill fragments (n = 4). (H) VHA abundance in membrane-enriched gill samples (n = 5–8). (Top) Bands are representative of each treatment. In G and H, letters indicate different levels of statistical significance by one-way ANOVA, Bonferroni’s multiple comparison test.
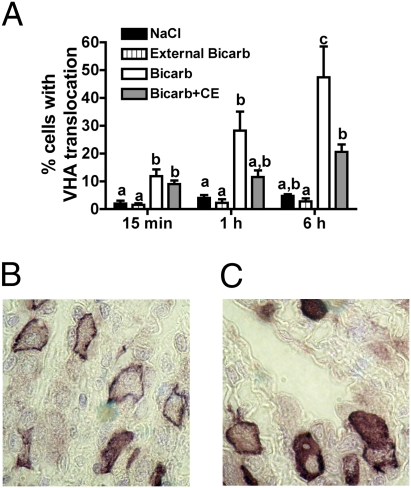
To test whether VHA translocation in gills from alkalotic animals is the result of autonomous sensing at the gill or a response to signals originating at another organ, we selectively perfused and isolated gill fragments. As in the whole-animal experiments, VHA translocated to the basolateral membrane in response to perfusion of alkaline solutions (50 mM HCO3−, pH 8.50) on the blood side but not on the seawater side (Fig. 5 A and B). Also similar to the whole-animal experiments, VHA translocation was significantly reduced by sAC inhibition (Fig. 5 A and C). Therefore, sAC-dependent A/B sensing appears to be autonomously controlled within the gill.
Fig. 5.
sAC-dependent VHA translocation during localized alkalosis in isolated gill. (A) Percentage of cells showing VHA translocation in gill fragments internally perfused and incubated in alkaline saline with or without 100 μM 4CE (n = 5). A repeated-measures two-way ANOVA (P < 0.05 for treatment, time, and interaction) or the Tukey–Kramer planned least square means test was used. Letters indicate different levels of statistical significance. Bicarb, bicarbonate. Representative VHA immunostaining of gills internally perfused and incubated in alkaline saline and DMSO (B) or 4CE (C).
Discussion
In this paper, we have identified sAC to be a sensor for systemic A/B status in the dogfish shark by means of the following criteria:
HCO3−-responsive dfsAC is abundantly expressed in the gill, the organ almost exclusively responsible for systemic A/B regulation in dogfish. In particular, it is expressed in the external epithelial layer, where the base-secreting and H+-reabsorbing VHA-rich cells are located.
dfsAC in vitro affinity for HCO3− predicts that its activity will reflect physiologically relevant changes in plasma [HCO3−].
Two structurally and mechanistically unrelated pharmacological inhibitors prevented recovery from alkalosis in whole animals. This effect can be attributed at least partially to diminished translocation of VHA into the basolateral membrane of gill cells, as observed in whole animals and isolated gills.
In the absence of genetic manipulation, which is not yet possible in the dogfish shark, these data represent the strongest possible evidence pointing to sAC as an intrinsic gill sensor essential for systemic A/B homeostasis.
The His-tagged recombinant dfsAC that we generated consists almost exclusively of the two catalytic domains, which have been shown in all class III adenylyl cyclases to be important for conversion of ATP into cAMP. Because it lacks the C-terminus region present in full-length native dfsAC, it is possible that native dfsAC has additional regulatory properties compared with recombinant dfsAC. However, as seen with mammalian and bacterial sAC-like enzymes (2, 3, 17, 18, 22), the catalytic domains of dfsAC dictate its affinity for HCO3− and its sensitivity to the inhibitors used in this study.
Inhibition of dfsAC mimicked the effects on systemic A/B regulation and VHA translocation previously observed when CA was inhibited (14) or when microtubule assembly and vesicle translocation were interrupted (13). Both CA (14) and sAC are widespread throughout the gill epithelium and likely present in the cyotoplasm of the same cells. Therefore, we propose a combined role for CA, sAC, and VHA movement in A/B regulation. In our model (Fig. S1C), dfsAC is an intrinsic A/B sensor that detects elevations in intracellular [HCO3−] generated from CO2 by CA and dfsAC-generated cAMP triggers the microtubule-dependent translocation of VHA to the basolateral membrane. Other as yet unidentified mechanisms that are constitutively active and/or up-regulated during dfsAC inhibition (or incomplete dfsAC inhibition during our study) may also be present and could have moderated the A/B disturbances observed.
The cellular mechanism for A/B regulation in VHA cells of dogfish gills (Fig. S1C) is the mirror image (i.e., similar mechanism with opposite cellular polarity) to the one described in clear cells of isolated rat epididymis. Mammalian sAC inside clear cells senses elevations in luminal pH/HCO3− and, similar to dfsAC, triggers membrane accumulation of VHA (7). However, in the clear cells, sAC-generated cAMP induces VHA translocation to the apical membrane, where it secretes H+ to restore an acidic pH in the fluid of the lumen necessary to keep stored sperm quiescent (26). As in dogfish gill, inhibiting CA (7) or interrupting microtubule assembly (27) also blocked the A/B response in epididymis.
The kidney is the organ primarily responsible for metabolic A/B regulation in mammals, and it possesses both acid- and base-secreting cells (28). These cells are functionally analogous to epididymal clear cells and dogfish VHA-rich cells, respectively. Although there are no in vivo or in vitro functional studies reported for sAC in kidney, mammalian sAC has been shown to colocalize with VHA in both renal cell types (24). This association supports the model that CA, sAC, and VHA form a regulatory complex in diverse A/B sensing organs.
CA and VHA are implicated in A/B regulation throughout metazoans (28 –30), and the presence of sAC in metazoans ranging from placozoans to mammals (Fig. 1) suggests that systemic A/B homeostasis via CA-sAC-VHA is conserved throughout animal physiology.
Materials and Methods
Animals.
Pacific spiny dogfish Squalus acanthias L. were caught from the Trevor channel (Vancouver Island, BC, Canada) and immediately transferred to a 151,000-L circular tank provided with flowing seawater at the Bamfield Marine Sciences Centre (9–13°C, 30–32‰ salinity, normal photoperiod). Fish were offered dead hake, herring, and flatfish once a week until 3 days before the experiment, at which point fish were fasted. Forty-two dogfish were used for this study (mean ± SEM: 1.93 ± 0.12 kg). All experiments were performed following animal care protocol 531702 approved by the Biological Sciences Animal Care Committee at the University of Alberta.
Infusions.
Dogfish were catheterized in the caudal vein and artery under anesthesia (12–14). To induce blood alkalosis without producing major osmotic or ionic disturbances, a 250-mM NaHCO3 solution supplemented with 250 mM NaCl was continuously infused into the vein using a peristaltic pump at a rate of 1,000 μEq·kg−1·h−1 (12). 4CE and the small molecule inhibitor KH7 (5 μmol·kg−1) were also injected through the caudal vein catheter from a concentrated 5-mM stock in DMSO as a 1-mL·kg−1 bolus. The targeted final concentration in the blood was 100 μM [based on ∼5% blood volume per mass in marine elasmobranchs (31)]. A first dose was injected 30 min before the infusions, and a second dose was injected at t = 6 h to compensate for drug penetration into tissues, breakdown, and/or excretion. Control fish were injected with the same volume of DMSO alone (2% of blood volume, 0.1% of body mass).
Blood and Terminal Sampling.
Blood sampling was performed as described previously (12–14). Briefly, 200-μL blood samples were withdrawn via the caudal vein catheter; blood pH was measured immediately using a thermostated Accumet microsize pH electrode model 13-620-94 (Fischer Scientific), plasma was obtained after a 2-min spin, and total CO2 was determined in a chamber (37°C) equipped with a CO2 electrode (Radiometer) by the method of Cameron (32). [HCO3−] was calculated using the solubility of CO2, the apparent pK for dogfish at the experimental temperature, and rearrangements of the Henderson–Hasselbalch equation (33). Dogfish were killed by an overdose of tricaine methanesulphonate (TMS, AquaLife; Syndel Laboratories). Gill samples were snap-frozen in liquid N2 and kept at −80°C until they were used in Western blotting experiments. Additional gill samples were immersed in ice-cold 3% (weight/vol) paraformaldehyde in 0.1 M Na+-cacodylate (pH 7.4) overnight at 4°C and stored in 70% (vol/vol) ethanol for immunohistochemistry.
Isolated Gill Experiments.
Dogfish were anesthetized with TMS (1 g·L−1), and the heart was exposed via pectoral/pericardial incision. Gills were perfused for at least 10 min via the bulbus arteriosus with ∼1.5 L of dogfish saline (280 mM NaCl, 6 mM KCl, 5 mM NaHCO3, 5 mM CaCl2, 3 mM MgCl2, 0.5 mM NaSO4, 1 mM NaHPO4, 4 mM NaHCO3, 350 mM urea, 70 mM Trimethylamine N-oxide, 5 mM glucose, pH 7.80), with either 50 mM NaCl (control) or 50 mM NaHCO3 (which raised pH to 8.43 ± 0.04) added. The final 50 mL contained 4CE (100 μM in DMSO) or carrier [DMSO alone, 2% (vol/vol)]. To test the effect of external [HCO3−] on VHA translocation, gills were perfused with normal dogfish saline (5 mM NaHCO3, pH 7.80) and incubated in dogfish saline with 50 mM NaHCO3 (pH: 8.42 ± 0.05). For cloning and activity experiments, gill samples were snap-frozen in liquid nitrogen. For quantification of VHA translocation in isolated gills, samples were dissected into three to four fragments of isolated filaments, which were placed in a small plastic basket and suspended inside an Erlenmeyer beaker almost completely filled with the corresponding saline solution (i.e., containing 4CE or DMSO). Beakers were sealed and maintained at 12°C, and the solutions were stirred using a magnetic agitator. At the end of the incubations (6 h), pH and TCO2 in the saline solutions did not vary significantly between treatments and incubation times (repeated-measures two-way ANOVA). Gill samples were fixed for immunohistochemistry at the indicated times.
Cloning of dfsAC.
A putative sAC homolog was identified via BLAST search from the S. acanthias multiple tissues EST library maintained by the Mount Desert Island Biological Laboratory (MDIBL). The putative dfsAC EST clone was sequenced by primer walking from a pcMV SPORT6.1 vector (Invitrogen). Sequences were aligned using Clustal W2 (34). Phylogenetic analysis on the alignment was performed using the maximum likelihood method from the PHYLIP software package (1,000 bootstrap replicates) (35). Treeillustrator (Department of Molecular Biotechnology, Ghent University, Belgium) was used to draw the phylogenetic tree in a radial logarithmic format.
To demonstrate dfsAC expression in gill, total RNA was extracted from frozen gill samples using TRIzol (Invitrogen). cDNA was prepared by standard methods using gene-specific as well as oligo-dT primers. An initial round of PCR (35 cycles) was performed using 5′-AGTAAGATGAAGTTCGGAAAGCT-3′ (base pairs 577–599) and 5′-ACAGAATAGCGGACCACTTGCAACG-3′ (base pairs 1219–1243) as primers. This PCR yielded a band of the expected size (666 bp) whose identity was confirmed to be dfsAC by nested PCR using 5′-CAACTTGAATCTGTCCTTCG-3′ (base pairs 840–859) and 5′-AGATGCTACACTGACATATC-3′ (base pairs 1173–1193) as primers (expected size of 353 bp) and direct nucleotide sequencing.
Production of Polyclonal Antibodies Against dfsAC.
Antiserum against the peptide INNEFRNYQGRINKC (amino acids 342–355, located in putative catalytic domain 2) was generated in rabbits and affinity-purified (GeneScript). Specificity of the antiserum was confirmed by immunoblots of Escherichia coli BL21-AI transfected with the His-dfsAC plasmid before (control) and after induction with arabinose. Further specificity controls were performed on gill samples using peptide preabsorption and preimmune serum in immunoblots and immunohistochemistry.
Heterologous Expression and Purification of Recombinant dfsAC.
All vectors used were from Invitrogen. The region corresponding to the putative catalytic domains of dfsAC (amino acids 1–485) was amplified from the pcMV SPORT6.1 vector containing full-length dfsAC by PCR, recombined into pENTR/TEV/D-TOPO vector, and shuttled into the pDEST17 vector, thus adding a 6× His tag to the N-terminus of the protein. Recombinant protein was produced in E. coli BL21-AI cells (pRARE) by induction with 0.1% arabinose for 3–4 h at 25°C. Cells were harvested by centrifugation, washed in 50 mM Tris and 1 mM EDTA (pH 8.0), and stored at −80°C. Pelleted cells were suspended in lysis buffer [50 mM Tris, 2 mM 3-thioglycerol, 300 mM NaCl, 2 mM imidazole, 1 mM PMSF, 20% (vol/vol) glycerol, pH 8.0] and lysed using an air-pressure cell disruptor (Avestin). The whole-cell extract was centrifuged at 31,000 × g for 40 min, and the supernatant was passed through agarose nickel-resin (Qiagen). Recombinant dfsAC was eluted by increasing concentrations of imidazole. The eluates were subjected to SDS/PAGE, and recombinant dfsAC was identified by Coomassie blue staining, immunoblotting using the anti-dfsAC antisera, and detection with Ni2+-HRP (HisProbe; Thermoscientific).
cAMP Assays Using Recombinant dfsAC and Gill Homogenates.
cAMP production by purified recombinant protein and in gill homogenates was determined using the Correlate-EIA Direct Assay (Assay Designs, Inc.).
Purified recombinant dfsAC protein was incubated in assay buffer (50 mM Tris, 2.5 mM ATP, 20 mM MgCl2, 0.5 mM MnCl2 or 5 mM MnCl2, 1 mM DTT) at 30°C for 30 min. Changes in pH produced by increasing [HCO3−] were compensated by altering the pH of the Tris buffer to ensure that added bicarbonate resulted in a final pH of 7.75 in all cases. Reactions were stopped by addition of an equal volume of 0.2 N of HCl. Recombinant dfsAC from two different protein purifications were each assayed twice at the indicated pH or in the presence or absence of the indicated concentrations of HCO3− and DMSO, KH7, or 4CE (both of the latter dissolved in DMSO).
Aliquots of gill “crude homogenates” were incubated for 30 min at room temperature in an orbital shaker (500 rpm) in 150 mM Tris (pH 7.5), 2.5 mM ATP, 20 mM MgCl2, 0.25 mM isobutylmethylxanthine (IBMX), 3 mM DTT, 20 mM creatine phosphate, and 100 U·mL−1 creatine phosphokinase. NaHCO3 was added from freshly prepared concentrated stocks. The incubations were stopped by addition of HCl to 0.1 N, and cAMP produced was quantified as described above.
Immunohistochemistry.
Fixed gill samples were dehydrated in an increasing ethanol series, embedded in paraffin, and sectioned at 6 μm (12–14). Deparaffinized sections were treated with 1% SDS in PBS, followed by 2× 5-min washes in PBS. The anti-VHA antibodies (mouse A-subunit) were a kind gift from Dennis Brown (Massachusetts General Hospital and Harvard Medical School, Harvard University, Boston, MA). Incubation with the anti-VHA antibodies (1:1,000 dilution in 2% (vol/vol) normal goat serum in PBS) was performed overnight at 4°C; detection was performed using the diaminobenzidine-based kit ImmPress (Vector Labs). Qualitatively similar results were obtained with antibodies against killifish VHA A and B subunits (gifts from Fumi Katoh, University of Alberta, Edmonton, AB, Canada, and J.B. Claiborne, Georgia Southern University, Statesboro, GA, respectively), but no quantification was performed. For dfsAC detection by immunofluorescence, samples were incubated in 50 mM NH4Cl in PBS for 5 min (after 1% SDS treatment and wash in PBS) to quench autofluorescence. Sections were incubated with the anti-dfsAC antibodies (1:200) overnight at 4°C, detection was performed using highly cross-absorbed Alexa-fluor 488-conjugated goat anti-rabbit antibodies (1:500; Invitrogen), and sections were mounted using DAPI-containing medium (Vector Labs).
Western Blotting of dfsAC and Quantification of VHA Translocation.
Frozen gill samples were homogenized in 10 volumes of lysis buffer (250 mM sucrose, 1 mM EDTA, 30 mM Tris, 100 μg·mL−1 PMSF, 10 μg·mL−1 leupeptin, 10 μg·mL−1 aprotinin, pH 7.75). Tissue was ground in liquid N2, sonicated 2× 15 s, and centrifuged (3,000 × g, 10 min, 4°C) to remove debris. Supernatant aliquots (“crude homogenate”) were saved for cAMP assays (see above). The remaining supernatant was centrifuged (20,000 × g, 30 min, 4°C), and pellets were resuspended in lysis buffer and used for Western blotting. Samples were mixed with an equal volume of 2× Laemmli buffer (with 2 mM fresh DTT) and heated (15 min, 70°C). Twenty μg of total protein from each sample (estimated by Coomassie blue stain of SDS/PAGE) was separated by SDS/PAGE; transferred to PVDF membranes, which were blocked in 5% (weight/vol) milk-TBST for 30 min at room temperature; and incubated with anti-VHA antibodies (1:5,000 in 5% milk in TBST) or anti-dfsAC (1:5,000 in 5% milk in TBST) overnight (4°C), followed by 1 h of incubation with HRP-conjugated goat anti-rabbit antibodies. Membranes were washed (3× 20 min) between steps. Bands were detected by chemiluminescence, and VHA was quantified by densitometry.
Quantification of Cells with VHA Translocation.
Dogfish gill sections from the different experiments (whole animal and isolated gills) were immunostained for VHA, coded, and examined by an experimenter in a “blind” fashion. The total number of VHA-rich cells and those displaying distinct VHA translocation (defined as cells with strong basolateral VHA staining and a clear cytoplasm; see Fig. 4C for examples) were recorded. At least 500 cells from several gill filaments from five different fish from each treatment were analyzed. Percentages were transformed to the arcsine of the squared root of the value before statistical analysis.
Statistics.
All data are given as means ± SEM. Statistical significance was set at P < 0.05. Statistical analyses were performed using GraphPad Prism v.4.0a for Macintosh (Graphpad Software) and Statistica 7 (Statsoft Pacific).
Supplementary Material
Acknowledgments
We thank members of the Buck/Levin and Goss laboratories who helped at various stages of this work, particularly Ken Hess. We appreciate the assistance of the Director of the Bamfield Marine Sciences Centre, Bruce Cameron, and Igor the fisherman, who provided dogfish. We thank Dr. Dennis Brown, Dr. J.B. Claiborne, and Dr. Fumi Katoh for gifts of antibodies; Dr. Lucy Skrabanek for helping with the phylogenetic analysis; and Dr. Carlos Luquet for advice about statistical analyses. Dr. David Towle (Mount Desert Island Biological Laboratory) was extremely helpful by providing us with the original dfsAC cDNA clone. This work was supported by National Institutes of Health Grants GM62328 and NS55255 (to J.B. and L.R.L.) and a Natural Sciences and Engineering Research Council (Canada) Discovery grant to G.G.G.E.S. is supported by the Tri-Institutional MD-PhD Program. S.K.P. is funded by a Natural Sciences and Engineering Research Council (Canada) grant, a Canada Graduate Scholarship, and an Honorary Izaak Walton Killam memorial scholarship. The funding agencies had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. ACA52542.1).
This article contains supporting information online at www.pnas.org/cgi/content/full/0911790107/DCSupplemental.
References
- 1.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 3.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 4.Zippin JH, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess KC, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie F, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–362. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 7.Pastor-Soler N, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278(1):49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid A, et al. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J Gen Physiol. 2007;130(1):99–109. doi: 10.1085/jgp.200709784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RF, Cloutier R, Turner S. The oldest articulated chondrichthyan from the Early Devonian period. Nature. 2003;425:501–504. doi: 10.1038/nature02001. [DOI] [PubMed] [Google Scholar]
- 10.Heisler N. In: Physiology of Elasmobranch Fishes. Shuttleworth TJ, editor. Berlin: Springer; 1988. pp. 215–252. [Google Scholar]
- 11.Piermarini PM, Verlander JW, Royaux IE, Evans DH. Pendrin immunoreactivity in the gill epithelium of a euryhaline elasmobranch. Am J Physiol. 2002;283:R983–R992. doi: 10.1152/ajpregu.00178.2002. [DOI] [PubMed] [Google Scholar]
- 12.Tresguerres M, Katoh F, Fenton H, Jasinska E, Goss GG. Regulation of branchial V-H(+)-ATPase, Na(+)/K(+)-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias) J Exp Biol. 2005;208:345–354. doi: 10.1242/jeb.01382. [DOI] [PubMed] [Google Scholar]
- 13.Tresguerres M, Parks SK, Katoh F, Goss GG. Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): A role in base secretion. J Exp Biol. 2006;209:599–609. doi: 10.1242/jeb.02059. [DOI] [PubMed] [Google Scholar]
- 14.Tresguerres M, Parks SK, Wood CM, Goss GG. V-H+-ATPase translocation during blood alkalosis in dogfish gills: Interaction with carbonic anhydrase and involvement in the postfeeding alkaline tide. Am J Physiol. 2007;292:R2012–R2019. doi: 10.1152/ajpregu.00814.2006. [DOI] [PubMed] [Google Scholar]
- 15.Wood CM, Kajimura M, Mommsen TP, Walsh PJ. Alkaline tide and nitrogen conservation after feeding in an elasmobranch (Squalus acanthias) J Exp Biol. 2005;208:2693–2705. doi: 10.1242/jeb.01678. [DOI] [PubMed] [Google Scholar]
- 16.Wood CM, Schultz AG, Munger RS, Walsh PJ. Using omeprazole to link the components of the post-prandial alkaline tide in the spiny dogfish, Squalus acanthias. J Exp Biol. 2009;212:684–692. doi: 10.1242/jeb.026450. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M, Buck J, Levin LR. Conservation of functional domain structure in bicarbonate-regulated “soluble” adenylyl cyclases in bacteria and eukaryotes. Dev Genes Evol. 2004;214:503–509. doi: 10.1007/s00427-004-0432-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaloupka JA, Bullock SA, Iourgenko V, Levin LR, Buck J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol Reprod Dev. 2006;73:361–368. doi: 10.1002/mrd.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun T. Purification of soluble form of adenylyl cyclase from testes. Methods Enzymol. 1991;195:130–136. doi: 10.1016/0076-6879(91)95160-l. [DOI] [PubMed] [Google Scholar]
- 20.Neer EJ, Murad F. Separation of soluble adenylate and guanylate cyclases from the mature rat testis. Biochim Biophys Acta. 1979;583:531–534. doi: 10.1016/0304-4165(79)90070-9. [DOI] [PubMed] [Google Scholar]
- 21.Beltrán C, et al. Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem Biophys Res Commun. 2007;358:1128–1135. doi: 10.1016/j.bbrc.2007.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steegborn C, et al. A novel mechanism for adenylyl cyclase inhibition from the crystal structure of its complex with catechol estrogen. J Biol Chem. 2005;280:31754–31759. doi: 10.1074/jbc.M507144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmour KM, Bayaa M, Kenney L, McNeill B, Perry SF. Type IV carbonic anhydrase is present in the gills of spiny dogfish (Squalus acanthias) Am J Physiol. 2007;292:R556–R567. doi: 10.1152/ajpregu.00477.2006. [DOI] [PubMed] [Google Scholar]
- 24.Paunescu TG, et al. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am J Physiol Renal Physiol. 2008;294:F130–F138. doi: 10.1152/ajprenal.00406.2007. [DOI] [PubMed] [Google Scholar]
- 25.Hallows KR, et al. Regulation of epithelial Na+ transport by soluble adenylyl cyclase in kidney collecting duct cells. J Biol Chem. 2009;284:5774–5783. doi: 10.1074/jbc.M805501200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breton S, Smith PJS, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470–472. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- 27.Beaulieu V, et al. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem. 2005;280:8452–8463. doi: 10.1074/jbc.M412750200. [DOI] [PubMed] [Google Scholar]
- 28.Wagner CA, et al. Renal vacuolar H+-ATPase. Physiol Rev. 2004;84:1263–1314. doi: 10.1152/physrev.00045.2003. [DOI] [PubMed] [Google Scholar]
- 29.Okech BA, Boudko DY, Linser PJ, Harvey WR. Cationic pathway of pH regulation in larvae of Anopheles gambiae. J Exp Biol. 2008;211:957–968. doi: 10.1242/jeb.012021. [DOI] [PubMed] [Google Scholar]
- 30.Perry SF, Gilmour KM. Acid-base balance and CO2 excretion in fish: unanswered questions and emerging models. Respir Physiol Neurobiol. 2006;154:199–215. doi: 10.1016/j.resp.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Anderson WG, et al. Body fluid volume regulation in elasmobranch fish. Comp Biochem Physiol A Physiol. 2007;148(1):3–13. doi: 10.1016/j.cbpa.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Cameron JN. Rapid method for determination of total carbon dioxide in small blood samples. J Appl Physiol. 1971;31:632–634. doi: 10.1152/jappl.1971.31.4.632. [DOI] [PubMed] [Google Scholar]
- 33.Boutilier RG, Heming TA, Iwama GK. In: Fish Physiology. Hoar WS, Randall AD, editors. Orlando, FL: Academic; 1984. pp. 403–430. 10A. [Google Scholar]
- 34.Larkin MA, et al. Clustal W and Clustal X version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 35.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.