Abstract
The sea anemone, Nematostella vectensis, has become an attractive new model organism for comparative genomics and evolutionary developmental biology. Over the last few years, many genes have been isolated and their expression patterns studied to gain insight into their function. More recently, functional tools have been developed to manipulate gene function; however, most of these approaches rely on microinjection and are limited to early stages of development. Transgenic lines would significantly enhance the tractability of the system. In particular, the study of gene- or tissue-specific promoters would be most useful. Here we report the stable establishment of a transgenic line using the I-SceI meganuclease system to facilitate integration into the genome. We isolated a 1.6-kb fragment of the regulatory upstream region of the Myosin Heavy Chain1 (MyHC1) gene and found that the transgene is specifically expressed in the retractor and tentacle muscles of Nematostella polyps, faithfully reproducing the expression of the endogenous MyHC1 gene. This demonstrates that the 1.6-kb fragment contains all of the regulatory elements necessary to drive correct expression and suggests that retractor and tentacle muscles in Nematostella are distinct from other myoepithelial cells. The transgene is transmitted through the germline at high frequency, and G1 transgenic polyps have only one integration site. The relatively high frequency of transgenesis, in combination with gene- or tissue-specific promoters, will foster experimental possibilities for studying in vivo gene functions in gene regulatory networks and developmental processes in the nonbilaterian sea anemone, Nematostella vectensis.
Keywords: meganuclease, myosin heavy chain, muscle development
The sea anemone, Nematostella vectensis, has developed into one of the most attractive model organisms among nonbilaterian animals. It can be easily reared in the laboratory (1), and its spawning can be induced reproducibly (2), providing daily access to thousands of embryos. The expression patterns of numerous genes have been determined, and recently, functional approaches of overexpression and Morpholino oligonucleotide–mediated gene knockdown have been established (3, 4). The genome of Nematostella was the first non-bilaterian metazoan to be sequenced (5), which revealed a remarkably slow evolutionary rate, reflected by a high level of conservation of individual gene sequences, exon–intron boundaries, and genomic organization. This corroborates the view that the common ancestor of Cnidaria and Bilateria was genetically complex, and that much of that complexity has been maintained in the sea anemone, Nematostella (5, 6). Thus, Nematostella is a prime model for comparative developmental biology aimed at reconstructing the last common ancestor of Bilateria and Cnidaria and identifying ancestral gene functions in animals.
A key question is the regulation of muscle cell differentiation in the absence of the third germ layer, the mesoderm. Morpholino-mediated gene knockdowns relies on the microinjection into the zygote and thus can target early, but not late, gene functions, yet muscles start to differentiate only during early metamorphosis. Transgenesis might provide not only a tool to overcome these limitations, but also an excellent way to monitor differentiation and movement of transgenic cell populations in vivo. Recently, stable somatic transgenic lines have been reported for Hydra that express GFP under the control of an actin promoter in specific cell lineages, depending on the site and timing of integration (7); however, to date no germline transmission has been reported for any cnidarian. Here we report the stable generation and germline transmission of transgenic lines of Nematostella expressing fluorescent proteins under the control of a muscle-specific promoter. The transgenic technology allows the dissection of gene promoters of interest and monitoring of the development of the specific cell populations in vivo in a non-bilaterian metazoan model system.
Results and Discussion
Generation of the Transgenesis Vector.
In contrast to Hydra, in Nematostella, the injection of circular or linear plasmid DNA inefficiently integrates into the genome. Usually, integration is strongly facilitated by flanking transposable elements or other sequences that lead to excision and incision into the genome. A successful approach to achieving transgenesis that has proven highly successful in vertebrate and ascidian systems is the use of flanking binding sites for homing endonucleases from yeast, such as the I-SceI meganuclease (8–11). I-SceI recognizes an 18-bp-long nonpalindromic stretch of nucleotides, cuts in a sequence-specific manner, and integrates the inserted sequence into the genome by not-yet fully understood mechanisms (12). We constructed a versatile vector system in which both the promotor sequence and the reporter gene, flanked by inverted I-SceI sites, can be independently exchanged in a single cloning step (Fig. 1A). Recombinant I-SceI enzyme efficiently cuts at the inverted recognition sites and releases the insert.
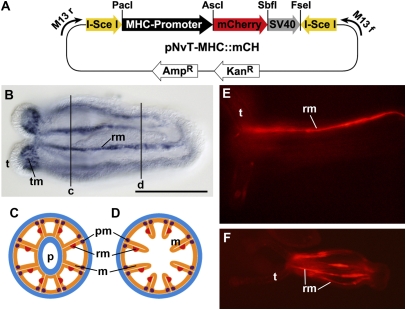
Fig. 1.
Generation of transgenesis vector and establishment of G0 transgenic animals. (A) Transgenesis vector consisting of a 1.6-kb fragment of MyHC1 promoter region upstream of the start codon and an mCherry reporter gene, flanked by inverted binding sites of meganuclease I-SceI. (B) Whole-mount in situ hybridization detecting MyHC1 expression in tentacle and retractor muscle cells in all eight mesenteries of a primary polyp. (C and D) Schematic cross-section through the body of a polyp at pharynx (p) position (C) and gastric position, as indicated in B. Endoderm is depicted in orange, ectoderm in blue, retractor muscles in red, and parietal muscles in purple. (E) mCherry reporter expression (red) in one mesentery of a primary polyp, reflecting a restricted transgenic patch. (F) mCherry reporter expression (red) in most mesenteries, demonstrating that large patches of transgenic tissue can be obtained in G0. B, E, and F show a lateral view, with oral to the left. m, mesentery; P, pharynx opening; pm, parietal muscle; rm, retractor muscles; t, tentacle; tm, tentacle muscles. (Scale bar: 200 μm.)
Identification of the Myosin Heavy Chain1 Gene Promoter.
To gain insight into the regulation of muscle cell differentiation in a diploblastic animal, we aimed to identify a muscle-specific gene and isolate its promoter. Toward this end, we searched our EST collection (6) and found a partial cDNA clone of the Myosin Heavy Chain type II gene (MyHC1). Expression studies by whole-mount in situ hybridization in primary polyps and adult stages demonstrated that this gene is strongly expressed in retractor and tentacle muscles (Fig. 1 B–D). Thus, this gene is an excellent marker gene for studying the differentiation of muscle cells. To isolate the DNA sequence regulating the muscle-specific expression, a 1.6-kb genomic fragment upstream of the start codon was amplified from genomic DNA and cloned into the transgenesis vector to drive the expression of the fluorescent reporter protein mCherry (13). This construct exhibited consistent and strong expression and was used in subsequent experiments.
Transgenesis and Expression of Reporter Genes in G0 Embryos and Primary Polyps.
After the injection of a mixture of I-SceI enzyme and the transgenesis vector into zygotes, the onset of mCherry expression could be detected in somatic patches at about 24 h of development. These early patches of strong expression were most likely the result of transient expression from nonintegrated plasmid DNA. mCherry expression subsequently became restricted to smaller clones with clear cellular borders of expression after 5–9 days of development. Because mCherry protein is relatively stable, its transient expression can be detected for several days, longer than that for eGFP. MyHC1::mCherry expression in the mesenteries was restricted to longitudinal stripes, reflecting differentiating retractor muscles. In the tentacles, expression was specific to spindle-shaped retractor cells along the proximodistal axis. The number of mCherry-expressing mesenteries corresponded to the size of the initial somatic patch. Interestingly, even relatively small transgenic patches resulted in longitudinal stripes of mCherry expression spanning almost the entire body axis, suggesting that individual muscle cells have very long protrusions (Fig. 1 E and F).
Germline Transmission and Maintenance of the Transgenic Line in G1 and G2
In primary polyps, somatic patches of MyHC1::mCherry expression could be found in about 7.5% of the injected embryos (120/1,610) (Table 1) and were restricted to the forming mesenteries and tentacles (Figs. 1 E and F and 2 A and B). In Nematostella, all germ cells arise within the eight mesenteries in the endoderm; therefore, the chance of germline transmission is highest in animals with large endodermal patches. But because it is improbable that all germ cells within an animal are transgenic, only a subset of the gametes from transgenic G0 individuals will carry the transgene. Consistent with these considerations, we found germline transmission in 8%–40% of all polyps that exhibited a somatic transgenic expression in the endoderm. Thus, the number of transgenic G1 offspring was highly variable, indicating a mosaic germline (Table 1).
Table 1.
Efficiency of I-SceI–mediated transgenesis of MyHC::mCherry
| Total injected MyHC::mCherry | % | |
| Injected zygotes | 1,610 | 100 |
| 24-h survival | 1,442 | 90 |
| 24-h expression | 582 | 40 |
| Recovered primary polyps | 539 | 34 |
| Expressing primary polyps | 120 | 7.5 |
| Germline transmission* | 8–40 |
*Germline transmission is given as the percentage of primary polyps with transgenic patches, which varies greatly because of the mosaicism of the transgenic germline.
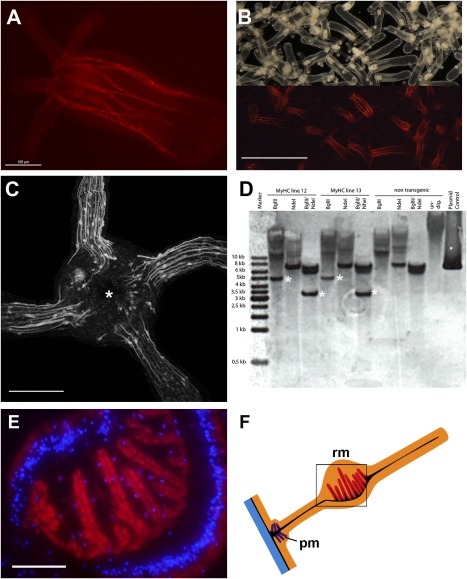
Fig. 2.
Germline transmission in transgenic G1 polyps. (A) A G1 MyHC1::mCherry transgenic primary polyp showing red mCherry expression in eight mesenteries. (B) Live image of offspring of G0 transgenic polyps with mosaic germline. Lower: an epifluorescence light picture of the same sample. G1 transgenic primary polyps can be easily identified under the epifluorescence stereomicroscope. (C) Reconstruction of confocal stacks showing the oral view on the head of a primary polyp with a mouth opening (asterisk) and four tentacles. Note the long extensions of individual mononuclear muscle cells in the tentacles. (D) Southern blot analysis of single G1 transgenic adult polyps and nontransgenic control animals. Both individual polyps have a single integration site for the transgene (marked by asterisks). (E) Cryotome cross-section of retractor muscles in the mesentery stained with an antibody against mCherry (red). Nuclear staining was done with DAPI (blue). (F) Schematic representation of a mesentery with the retractor muscle on one side in the middle of the mesentery. The color code and abbreviations are as in Fig. 1. [Scale bars: 100 μm (A), 800 μm (B), 50 μm (C), and 50 μm (E)].
Transgenic G1 polyps display eight longitudinal stripes of mCherry expression that correspond to the position of the retractor muscles within the eight mesenteries (Fig. 2A). Offspring of a cross of somatic transgenic animals with nontransgenic polyps can be easily identified by fluorescent stereomicroscopy (Fig. 2B), demonstrating that MyHC1::mCherry transgenics can be easily used for muscle mutant screens. Confocal images of tentacles of G1 MyHC1::mCherry primary polyps revealed individual mononuclear muscle cells with long extensions expressing mCherry (Fig. 2C). This allows in vivo monitoring of individual contracting muscle cells as well as the differentiation of muscle cells; for instance, continuous peristaltic movements of the body column can be followed in vivo (Movie S1).
Primary polyps need 3–6 months to reach sexual maturity in adult polyps. Whereas expression of the transgene in the mesenteries was maintained throughout the complete life cycle, including sexually mature polyps, expression in the tentacles appeared to be much weaker or was barely detectable. Whether this indicates a shift of muscle differentiation in the tentacle between the primary polyp and the adult polyp is unclear. To study the expression of the transgene in adult polyps in more detail, we prepared cryo cross-sections and performed immunostaining against the expressed mCherry protein. The results confirm that the MyHC1 promoter drives transgene expression in the retractor muscles of the mesenteries (Fig. 2 E and F). The endoderm of the body wall and the proximal mesentery also may express the transgene at a low level, detectable only by antibody staining against the stable mCherry protein (Fig. 2E).
Transgenesis using transposons often leads to multiple genomic integrations that segregate by subsequent outcrossing, whereas injection of plasmid DNA can lead to concatemeric integration (7, 14, 15). The 18-bp specific binding sequence of I-SceI (16) was not found in the Nematostella genome, as in the genome of any vertebrate where the meganuclease has been used for transgenesis (17). Southern blot analyses of single transgenic G1 polyps were performed to determine the number of integration sites in the genome. Because an antisense probe against the mCherry transgene could possibly cross-react with the GFP-like genes present in the Nematostella genome, we used the endogenous MyHC1 promoter region as a probe. The Southern blot analyses clearly showed that our transgenic line resulted from a single integration site, and that concatemerization did not occur (Fig. 2D). These findings are in line with previous observations in fish showing that meganuclease-mediated transgenesis predominantly results in one or few (i.e., 1–10) integrations (reviewed in ref. 17). Single and nonconcatemeric integrations are less prone to subsequent silencing than high copy numbers generated by plasmid injection (18, 19). Furthermore, the expression levels are somewhat weaker (depending on the promoter used), yet more uniform and reproducible.
In summary, the MyHC1::mCherry transgenic line faithfully reproduces the expression pattern of the MyHC1 gene in the retractor and tentacle muscles as detected by in situ hybridization. This shows that the 1.6-kb genomic fragment contains all of the regulatory elements necessary to drive proper expression. Whereas all epithelial cells in Cnidaria have more or less contractile elements at their base and thus are considered myoepithelial cells, the specific expression of mCherry in the retractor muscles reveals that the retractor muscle cells in sea anemones are distinct from other myoepithelial cells of the animal (e.g., circumferential myoepithelial cells in the endoderm).
Monitoring Muscle Differentiation and Reorganization During Head Regeneration.
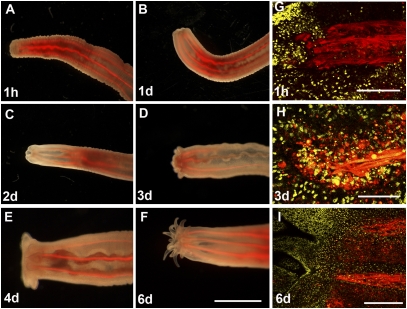
All Cnidaria have a high capacity for regeneration. When an adult polyp is bisected, the remaining part will regenerate the missing part via a morphallactic process (i.e., restructuring of the remaining part through a proportion-regulation mechanism) within a few days. In Nematostella, head regeneration requires the formation of a pharynx and new tentacles. The pharynx is an inverted structure, consisting of a thick ectodermal cell layer and a thin endodermal layer in continuation to the endoderm in the mesenteries carrying the retractor muscles (20). We followed head regeneration in MyHC1::mCherry transgenic animals for 6 days after bisection below the pharynx region and found that immediately after cutting, the muscles retracted from the wound site (Fig. 3A). The tissue at the wound site bent inward, in preparation for differentiation of the pharynx (Fig. 3 B–D). The first tentacle buds appeared about 3 days after bisection (Fig. 3D). Notably, unlike primary polyps, which always develop four tentacles, regenerates develop more than 14 tentacles at once (Fig. 3 D–F). A more detailed analysis of the regenerating pharynx region by confocal microscopy revealed the accumulation of numerous cells at the regenerating site expressing the MyHC1::mCherry transgene, suggesting that this region is an active site of new differentiation and reorganization (Fig. 3 G–I).
Fig. 3.
Muscle reorganization during head regeneration. (A–F) A live MyHC11::mCherry G1 transgenic polyp regenerating a head after bisection below the pharynx. The ectoderm at the wound bends inward and starts to form a new pharynx. The first tentacle buds appear after 3 days of regeneration. Note that more than 14 tentacles appear at once. (G–I) Confocal closeups of the regenerating tip (same orientation as in A–F). ToPro-3–stained nuclei are shown in yellow. (G) Retractor muscles retract immediately after being cut from the wound. (H) At day 3 after bisection, numerous cells expressing the mCherry transgene under control of the MyHC1 promoter accumulate at the wound site. (I) After 6 days, mesenteries are attached near the differentiated pharynx close to the base of the tentacles. [Scale bars: 500 μm (A–F), 20 μm (G and H), and 40 μm (I).]
Future Prospects.
Here we report the first transgenic line in Nematostella and the first germline transmission in a cnidarian using a meganuclease-mediated integration. The identification of a promoter expressed in a specific cell population is a promising example demonstrating that gene- and tissue-specific promoters can be readily identified in Nematostella. Such transgenic reporter lines for specific cell populations could be used in in vivo time-lapse studies, laser ablation of one or several transgenic cells, and mutagenesis screens. Current work is focusing on the isolation of other gene-specific promoters and their functional dissection in the endogenous background. Furthermore, the development of ubiquitously expressed or inducible promoters in conjunction with tagged fusion proteins will enhance the repertoire of functional approaches. It also should be feasible to target gene functions late in development or even in adults, which currently are not easily accessible to manipulation. The generation of other transgenic lines specific for neurons or other cell populations will allow the production of multitransgenic lines by genetic crosses and monitoring of the interaction of these cell populations in vivo and in response to environmental signals, stress, and experimental perturbations.
Currently, virtually nothing is known about the complexity of gene regulation in diploblastic animals in comparison with Bilateria. To address this question, basal endogenous promoters must be isolated to identify enhancers by random insertion into the genome. Such basal promoters also will allow the testing of regulatory elements that are conserved between Nematostella and different Bilateria. Thus, future applications of the transgenic technology are expected to significantly contribute to the functional aspects of gene regulatory networks and enhance our understanding of the cellular dynamics during morphogenesis and cellular differentiation.
Materials and Methods
Animal Culture.
The culture of Nematostella was maintained and induction of spawning was carried out as described previously (2).
Isolation of the Myosin Heavy-Chain Promoter.
A partial cDNA clone of the MyHC1 gene was found in an EST collection (6). 5′RACE was used to obtain the 5′ end of the transcript. A fragment of 1,626 bp upstream of the start codon of the MyHC1 gene was then amplified by PCR from genomic DNA, using primers designed on the base of the assembled genome sequence of Nematostella (MyHC5: CCGGATGGGAGCAAAGAAAAACTT; MyHC3: CATCTTGAAACAGTTATTCTAAA) (5). The sequence of the promoter was confirmed by overlap with the 5′ end of the cDNA clone.
Generation of Plasmids for Transgenics.
A pCRII-TOPO plasmid (Invitrogen) was used as a backbone for the transgenesis vector. The multicloning site was replaced by a cassette consisting of the MyHC1 promoter, and the mCherry reporter, flanked by inverted I-SceI sites, was inserted. The promoter, reporter gene, and meganuclease sites were separated by single rare cutters (Fig. 1A).
Microinjection.
Fertilized eggs were prepared for microinjection as described previously (2). Zygotes were injected with 50 ng/μL of DNA, 1× I-SceI buffer, 40 μg/mL of Alexa Dextran (Invitrogen), and 0.2 U/μL of I-SceI (New England Biolabs). The average injected volume was 45 pL. The mix was incubated for 30 min at 37°C before injection. Injected eggs were put into fresh medium and incubated at 20°C overnight. Animals were transferred into fresh medium daily and raised to primary polyps at 20°C in the dark. The first feeding was usually possible after 7–9 days. Animals that still expressed mCherry at day 9 were selected and raised as a pool of animals.
Cryosections and Immunohistochemistry.
Adult polyps were relaxed in 7% MgCl2 for 10 min, then cut in smaller pieces and fixed in 4% paraformaldehyde/PBS/0.1%Tween20 for 1 h. After washing in PBS, the specimens were transferred to infiltration solution [20% OCT compound (Sakura), 25% sucrose in PBS, pH 7.4]. After 12–16 h, the specimens were put into 80% OCT compound and 1.25% sucrose for freezing and cutting. Then 12-μm cryosections were incubated with an anti-DsRed (mCherry) antibody (Clontech) for 1 h, followed by anti-rabbit IgG (Alexa Fluor 456; Invitrogen). Nuclear counterstaining was carried out by incubation with ToPro-3 (Invitrogen). Images were obtained with a Leica SP2 confocal microscope and were overlaid in Adobe Photoshop.
Supplementary Material
Acknowledgments
We thank Joachim Wittbrodt for sending us a I-SceI site–containing vector, the Tsien laboratory for allowing the use of mCherry, the Lichtscheidl laboratory for providing access to and help with the confocal microscope, and all former and current members of the Technau laboratory for support and discussion. This work was funded by grants from Norges Forskningsrad, Österreichischer Forschungs- und Wissenschaftsfond, and the Deutsche Forschungsgemeinschaft (to U.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909148107/DCSupplemental.
References
- 1.Hand C, Uhlinger KR. The culture, sexual and asexual reproduction, and growth of the sea anemone, Nematostella vectensis. Biol Bull. 1992;182:169–176. doi: 10.2307/1542110. [DOI] [PubMed] [Google Scholar]
- 2.Fritzenwanker JH, Technau U. Induction of gametogenesis in the basal cnidarian Nematostella vectensis (Anthozoa) Dev Genes Evol. 2002;212:99–103. doi: 10.1007/s00427-002-0214-7. [DOI] [PubMed] [Google Scholar]
- 3.Rentzsch F, Fritzenwanker JH, Scholz CB, Technau U. FGF signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development. 2008;135:1761–1769. doi: 10.1242/dev.020784. [DOI] [PubMed] [Google Scholar]
- 4.Wikramanayake AH, et al. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature. 2003;426:446–450. doi: 10.1038/nature02113. [DOI] [PubMed] [Google Scholar]
- 5.Putnam NH, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 6.Technau U, et al. Maintenance of ancestral complexity and non-metazoan genes in two basal cnidarians. Trends Genet. 2005;21:633–639. doi: 10.1016/j.tig.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TC. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc Natl Acad Sci USA. 2006;103:6208–6211. doi: 10.1073/pnas.0510163103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobkow L, Epperlein HH, Herklotz S, Straube WL, Tanaka EM. A germline GFP transgenic axolotl and its use to track cell fate: Dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Dev Biol. 2006;290:386–397. doi: 10.1016/j.ydbio.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch N, et al. Xenopus tropicalis transgenic lines and their use in the study of embryonic induction. Dev Dyn. 2002;225:522–535. doi: 10.1002/dvdy.10188. [DOI] [PubMed] [Google Scholar]
- 10.Deschet K, Nakatani Y, Smith WC. Generation of Ci-Brachyury-GFP stable transgenic lines in the ascidian Ciona savignyi. Genesis. 2003;35:248–259. doi: 10.1002/gene.10195. [DOI] [PubMed] [Google Scholar]
- 11.Thermes V, et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 12.Monteilhet C, Perrin A, Thierry A, Colleaux L, Dujon B. Purification and characterization of the in vitro activity of I-Sce I, a novel and highly specific endonuclease encoded by a group I intron. Nucleic Acids Res. 1990;18:1407–1413. doi: 10.1093/nar/18.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaner NC, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 14.Pavlopoulos A, Averof M. Establishing genetic transformation for comparative developmental studies in the crustacean Parhyale hawaiensis. Proc Natl Acad Sci USA. 2005;102:7888–7893. doi: 10.1073/pnas.0501101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabher C, Wittbrodt J. Recent advances in meganuclease- and transposon-mediated transgenesis of medaka and zebrafish. Methods Mol Biol. 2008;461:521–539. doi: 10.1007/978-1-60327-483-8_36. [DOI] [PubMed] [Google Scholar]
- 16.Chevalier BS, Monnat RJ, Jr, Stoddard BL. The homing endonuclease I-CreI uses three metals, one of which is shared between the two active sites. Nat Struct Biol. 2001;8:312–316. doi: 10.1038/86181. [DOI] [PubMed] [Google Scholar]
- 17.Grabher C, Joly JS, Wittbrodt J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol. 2004;77:381–401. doi: 10.1016/s0091-679x(04)77021-1. [DOI] [PubMed] [Google Scholar]
- 18.Kelly WG, Xu S, Montgomery MK, Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrick D, Fiering S, Martin DI, Whitelaw E. Repeat-induced gene silencing in mammals. Nat Genet. 1998;18:56–59. doi: 10.1038/ng0198-56. [DOI] [PubMed] [Google Scholar]
- 20.Burton PM, Finnerty JR. Conserved and novel gene expression between regeneration and asexual fission in Nematostella vectensis. Dev Genes Evol. 2009;219:79–87. doi: 10.1007/s00427-009-0271-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.