Abstract
BACKGROUND:
A novel anti-immunoglobulin E (anti-IgE) therapy for asthma, omalizumab, has been approved for use in Canada.
OBJECTIVE:
To review the basic and clinical data for omalizumab, and to examine its possible role for asthma management in Canada.
METHODS:
A literature search from 1960 to 2006 was conducted in MEDLINE to identify studies of omalizumab. In addition, abstracts from recent respiratory and allergy scientific meetings were sought, and any unpublished data were requested from the manufacturer. A consensus panel of respiratory and allergy specialists reviewed and summarized the data, and derived a set of recommendations for omalizumab use.
RESULTS:
Omalizumab is a humanized monoclonal antibody designed to bind to the C epsilon 3 domain of the IgE molecule, forming soluble immune complexes that are cleared by the reticuloendothelial system. Subcutaneous injections, given at two- or fourweek intervals at the recommended dose, result in a rapid decrease in free circulating IgE levels. In two phase III clinical trials of 1405 adult and adolescent patients with moderate to severe asthma maintained on moderate doses of inhaled corticosteroids (ICS), omalizumab reduced exacerbation rates compared with placebo, and was associated with improved symptoms and a greater corticosteroid-sparing effect. In a trial of 419 patients with severe disease that was uncontrolled despite the use of high-dose ICS and concurrent long-acting beta2-agonists, severe exacerbations were 50% less frequent in omalizumab-treated patients than in control subjects. Retrospective analyses have identified the characteristics of patients most likely to respond to omalizumab treatment.
RECOMMENDATIONS:
Omalizumab may be considered as a potential adjunctive therapy in atopic patients with severe asthma uncontrolled by conventional therapy with optimal doses of ICS and appropriate adjunctive therapy (eg, long-acting beta2-agonists). Typically, patients are identified by the need for frequent short course or continuous oral corticosteroids. Therapy should be initiated only after review by a specialist to confirm the diagnosis and that conventional therapy is optimal.
Keywords: Asthma severity, Atopy, IgE, Monoclonal antibodies
Abstract
CONTEXTE :
Un nouveau traitement anti-immunoglobuline E (anti-IgE) contre l’asthme, l’omalizumab, a été approuvé au Canada.
OBJECTIF :
Passer en revue les données fondamentales et cliniques sur l’omalizumab et examiner le rôle possible de ce médicament dans la prise en charge de l’asthme au Canada.
MÉTHODOLOGIE :
Une recherche documentaire a été effectuée dans MEDLINE afin de repérer les études menées de 1960 à 2006 sur l’omalizumab. La recherche a également porté sur les résumés de réunions scientifiques récentes dans le domaine des maladies respiratoires et des allergies; par ailleurs, toute donnée non publiée a été demandée au fabricant. Après avoir revu et résumé les données, un comité mixte constitué de spécialistes des maladies respiratoires et des allergies a rédigé un ensemble de recommandations relatives à l’utilisation de l’omalizumab.
RÉSULTATS :
L’omalizumab est un anticorps monoclonal humanisé qui se lie au domaine C epsilon 3 de la molécule d’IgE pour former des complexes immuns solubles qui sont éliminés par le système réticulo-endothélial. L’administration d’injections sous-cutanées espacées de deux ou de quatre semaines à la dose recommandée entraîne une diminution rapide des taux d’IgE circulantes libres. Lors de deux essais cliniques de phase III menés auprès de 1 405 adultes et adolescents atteints d’asthme modéré à grave qui recevaient des doses moyennes stables de corticostéroïdes en inhalation (CSI), l’omalizumab a diminué les taux d’exacerbation par rapport au placebo et a été associé à une amélioration des symptômes ainsi qu’à une épargne plus importante des corticostéroïdes. Dans un essai mené auprès de 419 patients atteints d’asthme grave non maîtrisé malgré l’utilisation de doses élevées de CSI et de la prise concomitante d’agonistes bêta-2 à action prolongée, les exacerbations graves étaient de 50 % moins fréquentes chez les patients traités par l’omalizumab que chez les sujets témoins. Des analyses rétrospectives ont permis d’identifier les caractéristiques des patients les plus susceptibles de répondre au traitement par l’omalizumab.
RECOMMANDATIONS :
L’omalizumab pourrait être envisagé comme traitement d’appoint dans les cas atopiques d’asthme grave non maîtrisé avec des traitements classiques par des doses optimales de CSI et un traitement d’appoint approprié (p.ex. : agonistes bêta-2 à action prolongée). En général, les patients sont classés en fonction de leur recours – traitement court et fréquent ou continu et oral – aux corticostéroïdes. Il ne faut amorcer le traitement qu’après avoir consulté un spécialiste pour confirmer le diagnostic et s’assurer que le traitement classique est optimal.
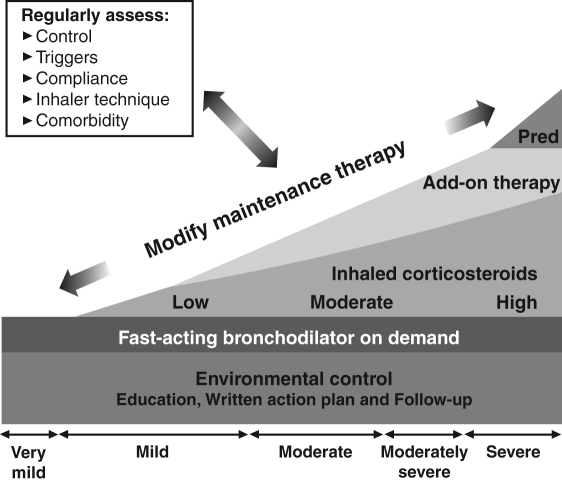
Various national and international guidelines for the management of asthma have been remarkably consistent in their recommendations (1–4) (see the Canadian Asthma Consensus Guidelines [CACGs]) (Figure 1). For all patients in whom the diagnosis of asthma has been established, education is fundamental, with emphasis on the identification and elimination of environmental triggering factors.
Figure 1).
Continuum of treatments for asthma management. Pred Prednisone. Reproduced with permission from reference 60
When patients have only occasional symptoms of wheezing and breathlessness, quick-relief bronchodilators are used only when needed. When symptoms are more frequent or persistent, the foundation of maintenance care is an inhaled corticosteroid (ICS) taken regularly. With increasing disease severity, the ICS may be accompanied by adjunctive therapy, most commonly a long-acting beta2-agonist (LABA). Oral steroids are typically reserved for occasional short-term use in the treatment of exacerbations, but some patients require daily oral corticosteroids, an approach that is adopted reluctantly in the most severe of patients. Following this approach has apparently been successful in Canada. Because these treatments have found widespread use, the impact has been reductions in both the morbidity and mortality of asthma in Canada. Despite the increasing prevalence of asthma over the past 20 years, mortality has decreased from its peak in the mid-1980s. Moreover, hospitalization rates for asthma care in all age groups have fallen over the same period, with the possible exception of the youngest children (under four years of age) (5).
Unfortunately, many patients with asthma continue to suffer from disabling symptoms. The pivotal Asthma in Canada survey (6) revealed that more than one-half of Canadians treated for asthma failed to enjoy adequate control of their disease as recommended by consensus guidelines. Although many of these patients have relatively mild disability, there is great concern about the small percentage with severe or ‘refractory’ asthma, despite apparently optimal management with multiple medications. These patients often require urgent care, including hospitalization, despite the use of high-dose inhaled steroids, LABAs, other adjuncts and prednisone. They may be repeatedly absent from work or school as a consequence and suffer a markedly impaired quality of life.
Patients with apparently severe asthma may fall into several categories. Some patients have asthma that, although controllable, is uncontrolled for prosaic reasons that are revealed by a return to basic management principles. Such patients may not be complying with potentially useful therapy (7,8), may not be using their inhaled medication properly (9), may be living or working with a potent antigen, or may be smokers (10,11).
Still, closer examination may show that other patients do not suffer from asthma at all. Chronic obstructive pulmonary disease may be mistaken for asthma, particularly if there is an early onset variant such as alpha1-antitrypsin deficiency (12,13). Endobronchial tumours and other causes of large airway obstruction or collapse may mimic asthma (14). Hyperventilation syndrome and vocal cord dysfunction may lead to the mistaken diagnosis of severe and refractory asthma (15).
Some patients have a variant of asthma that complicates care; these variants include allergic bronchopulmonary aspergillosis, Churg-Strauss vasculitis and asthma associated with various immunoglobulin (Ig) deficiencies (16). Still, other patients are resistant to standard asthma treatment, namely, corticosteroids. Such patients have asthma that is termed either difficult to control (if they respond to steroids at higher than usual doses) or steroid resistant (if corticosteroids produce no effect) (17). Finally, some patients have asthma that is refractory for no single identifiable reason, with terms such as refractory asthma, brittle asthma or severe asthma used to describe various perceived patterns of disease.
There is no single established treatment approach to severe asthma. The use of long-term oral steroid therapy is common, with an attempt made to keep dosages to the minimum so as to reduce the inevitable systemic side effects, such as osteoporosis, cataract formation, weight gain, glucose intolerance and immunosuppression. Agents such as troleandomycin have been recommended but appear to offer little or no advantage over systemic steroids alone (18). Alternative immunosuppressive agents have been recommended as replacements for or adjuncts to corticosteroids. These agents include methotrexate, azathioprine and cyclophosphamide (19,20), of which none are commonly used. Despite initial optimism for intravenous Ig in the management of severe asthma, controlled studies have failed to show a benefit (21).
A 2003 update to the CACGs noted that targeting specific immune-mediated pathophysiological mechanisms of airway inflammation “may herald the future for asthma treatment” (2). The following year, Health Canada approved an agent with one such mechanism for the treatment of moderate to severe allergic asthma, namely, the recombinant humanized anti-IgE monoclonal antibody omalizumab. Anti-IgE therapy has been included as an option for severe persistent allergic asthma – step 4 in the current iteration of the Global Initiative for Asthma (GINA) guidelines (evidence level B [subject to revision to level A depending on possible updates to the GINA guidelines]) (4). Omalizumab is the first available ‘biological’ therapy of asthma, although other monoclonal therapies are under development and may become available (17). The availability of a new therapeutic class will need to be placed in the context of current management practices. Therefore, we believed it timely, in anticipation of further discussion and revision of Canadian guidelines, to summarize the rationale for anti-IgE therapy, our review of current clinical data on the efficacy and safety of omalizumab, and our recommendations for its appropriate use by Canadian clinicians.
RATIONALE FOR ANTI-IgE THERAPY IN ASTHMA
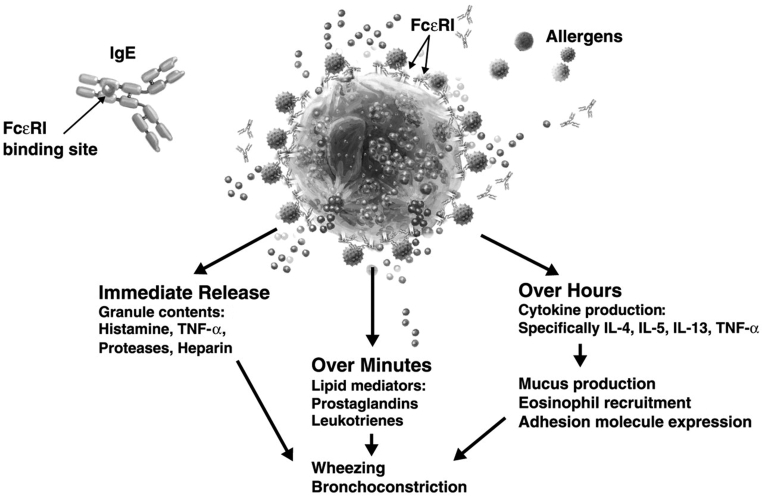
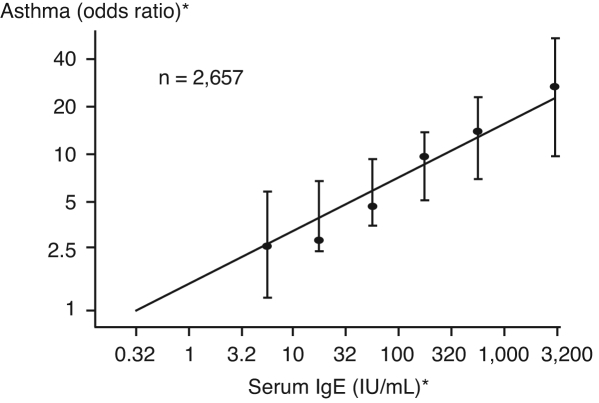
IgE plays a key role in the pathogenesis of type I hypersensitivity reactions, including allergic asthma, rhinitis and food allergy. In susceptible patients, exposure to an allergen promotes the release of IgE antibodies by B cells and plasma cells, which bind to high-affinity Fc epsilon RI (FcɛRI) on effector cells such as basophils and mast cells (22). Cross-linking of allergen and cell-bound IgE leads to the release of mediators of early- and late-phase allergic reactions (Figure 2) (23). In patients with allergic asthma, the IgE-mediated inflammatory response is believed to contribute to persistent airway hyperresponsiveness and symptoms (24). Limitations in air flow and episodic exacerbations signal chronic, IgE-mediated tissue inflammation (25). Epidemiological data have confirmed a link between increasing serum IgE and asthma prevalence (Figure 3) and severity in both adults and children, and suggest that a predisposition to produce IgE is inherited (22,26). Higher IgE levels in the serum correlate with increased numbers of high-affinity FcɛRI on mast cells and basophils (22). Elevated IgE may also correlate with asthma outcomes; postmortem analyses have shown that lung tissue from patients who died from asthma had higher numbers of high-affinity IgE receptors than individuals with mild asthma at the time of death and were also higher than numbers found in asthmatic individuals who died of nonpulmonary causes (27). Such clinical and pathological findings have led to the concept that lowering IgE levels may blunt or inhibit IgE-associated inflammatory responses in patients whose asthma remains uncontrolled with our current best management practices. Such an approach targets the inflammatory cascade at its inception rather than after inflammatory changes are present and well established (22).
Figure 2).
Immunoglobulin E (IgE)-dependent release of inflammatory mediators. Reproduced with permission from reference 61. FcɛR1 Fc epsilon RI; IL Interleukin; TNF-αTumour necrosis factor-alpha
Figure 3).
Prevalence of asthma is related to the level of serum immunoglobulin E (IgE). *Logarithmic scale. Reproduced with permission from reference 62. Copyright ©1989 Massachusetts Medical Society. All rights reserved
OMALIZUMAB: PHARMACOLOGY
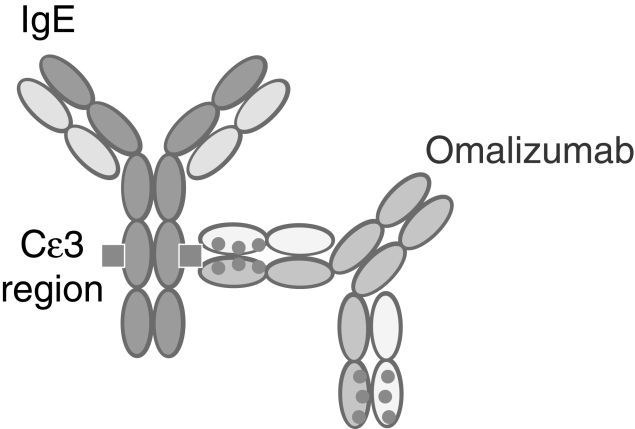
Omalizumab is a recombinant, DNA-derived, humanized monoclonal antibody with approximately 5% murine sequences attached to a human IgG framework (Figure 4). By binding to IgE at its Cɛ3 domain, omalizumab inhibits the binding of IgE to its high-affinity Fcɛ receptor on effector cells. In this way, it reduces the amount of free IgE available for recognition by effector cells that trigger the allergic inflammatory cascade. Omalizumab does not bind to cell-bound IgE (25,28). The reduction in free IgE (at least 96% at recommended dosing) is observed within 1 h of subcutaneous administration. Omalizumab also significantly downregulates/decreases the number of FcɛRI on effector cells in peripheral blood and target organs (25). In one phase I study, receptor density on basophils was decreased by 50% of baseline by day 3 and by 97% by day 90 (29). Histamine release subsequent to allergen exposure is also significantly reduced (28). Pharmacodynamic studies indicate that there is no rebound in free IgE associated with withdrawal of omalizumab, although total IgE levels return to baseline levels within one year of treatment discontinuation. Omalizumab/IgE complexes are cleared by the reticuloendothelial system. In patients with asthma, the clearance half-life averages 26 days.
Figure 4).
The humanized monoclonal anti-immunoglobulin E (IgE) antibody omalizumab. Cɛ3 C epsilon 3. Reproduced with permission from reference 58
PROOF OF CONCEPT TRIALS
Initial evidence of the antiasthmatic efficacy of omalizumab was provided in the late 1990s, when investigators demonstrated that intravenous administration of the agent (initially called anti-IgE antibody E25) inhibited allergen-induced early-and late-phase asthmatic responses (30–32). Investigators had less success with aerosolized administration of E25, which was both ineffective at reducing the asthmatic response and more immunogenic than parenteral administration (33). In key trials confirming its efficacy (28), omalizumab has been administered subcutaneously.
More recent studies have clarified how reducing cellular inflammation with omalizumab plays a role in clinical outcomes in severe asthma. Djukanovic et al (34) reported that treatment with omalizumab produces a marked decrease in serum IgE and IgE-positive cells in the airway mucosa, as well as a significant reduction in sputum and tissue eosinophils, cells that are positive for high-affinity Fcɛ receptors, and other inflammatory cell types. However, it did not influence the bronchial response to methacholine in the study subjects, who had mild to moderate persistent asthma (34). Furthermore, anti-IgE treatment may have a limited impact on forced expiratory volume in 1 s (FEV1) due to irreversible airway damage and obstruction caused by chronic inflammation (35).
EFFICACY IN MODERATE TO SEVEREPERSISTENT ASTHMA
Numerous studies have examined the impact of omalizumab added to conventional therapy in patients with severe and uncontrolled asthma. These studies are listed and summarized in Table 1. The patient populations differed somewhat in the definition of severity used and in the concomitant medications used, with a trend toward later studies focusing on more severe disease and more aggressive concomitant therapy. The usual primary efficacy variable in these trials was the number or rate of asthma exacerbations experienced by the trial subjects. Overall, the annualized rate of exacerbations was reduced by 38.3% in patients receiving omalizumab compared with controls (36). Specific study results are discussed below.
TABLE 1.
Summary of studies examining the impact of omalizumab added to conventional therapy in patients with uncontrolled asthma
| Study (n) | Characteristics of patients at baseline | Study duration and type | Concomitant therapy | Primary efficacy end point | Rate with treatment vs control | Additional significant results (omalizumab vs comparator) |
|---|---|---|---|---|---|---|
| Solèr et al (24) (n=546) | Age 12 to 75 years Moderate to severe allergic asthma BDP 769/772 μg/day (treatment/placebo groups) |
28 weeks (SSP 16, SRP 8, MP 4) Multicentre, randomized, double-blind, placebo-controlled, parallel group |
BDP at dose required for stability in SSP In SRP, BDP reduced by 25% of baseline at each visit* Salbutamol 100 μg/puff for rescue |
Exacerbation rate (episodes per subject per year) | SSP: 0.28 vs 0.66, P<0.001 SRP: 0.36 vs 0.75, P<0.001 |
Median daily ICS dose 100 μg vs 300 μg, P<0.001 ICS dose reduced by ≥50% in 79% vs 55% of patients Withdrawal of ICS in 43% vs 19% Significantly lower use of rescue medication, P<0.001 |
| Buhl et al (40)† (n=483) | Age 12 to 75 years Moderate to severe allergic asthma BDP 766/773 μg/day (treatment/placebo groups) |
24 weeks Multicentre, randomized, double-blind, placebo-controlled, parallel group |
BDP or other ICS at lowest effective dose Other asthma medications as required |
Exacerbation rate (episodes per subject per year) | 0.48 vs 1.14, P<0.001 | 24% vs 40.6% of patients had ≥1 exacerbation, P<0.001 Mean BDP equivalent dose 253 μg/day vs 434 μg/day ~35% vs ~15% required no ICS Lower use of concomitant therapies |
| Busse et al (37) (n=525) | Age 12 to 75 years Moderate to severe allergic asthma BDP 568/570 μg/day (treatment/placebo groups) |
28 weeks (SSP 16, SRP 8, MP 4) Randomized, double-blind, placebo-controlled, multicentre, parallel group |
BDP at dose required for stability in SSP; in SRP, dose reduced by 25% of baseline at each visit* Albuterol: 2 puffs (90 μg/puff) as needed (maximum 8 puffs daily) for rescue Immunotherapy and nonasthma medication maintained |
Exacerbation rate (episodes per subject per year) | SSP: 0.28 vs 0.54, P=0.006 SRP: 0.39 vs 0.66, P=0.009 |
Median reduction in ICS dose 75% vs 50%, P<0.001 ≥ 50% reduction in BDP dose achieved by 72.4% vs 54.9%, P<0.001 BDP discontinued in 39.6% vs 19.1%, P<0.001 |
| Lanier et al (41)‡ (n=460) | Age 12 to 75 years Moderate to severe allergic asthma BDP 565/552 μg/day (treatment/placebo groups) |
24 weeks Double-blind, placebo-controlled |
BDP or other ICS at lowest effective dose Other asthma medications as required |
Exacerbation rate (episodes per subject per year) | 0.6 vs 0.83, P=0.023 | 31.8% vs 42.8% of patients had ≥1 exacerbation, P=0.015 Mean BDP equivalent dose 227 μg/day vs 335 g/day, P<0.001 Significantly more treated patients achieved =50% ICS reduction or stopped ICS Lower use of concomitant therapies |
| INNOVATE (38) (n=419) | Age 12 to 75 years Recent history of exacerbations Inadequately controlled despite high-dose ICS (>2300 μg/day) and LABA |
28 weeks Randomized, double-blind, parallel group, multicentre Omalizumab added to existing treatment |
High-dose ICS and LABA 2/3 of patients received additional controller medications, including oral corticosteroids in 22% |
Exacerbation rate (episodes per subject per year) | 0.68 vs 0.91, P=0.042 | 50% reduction in severe exacerbations, P=0.002 44% reduction in total emergency visits, P=0.038 |
| ETOPA (39) (n=312) | Age 12 to 73 years Persistent, moderate to severe allergic asthma ICS ≥400 μg/day (adolescents) or ≥800 μg/day (adults) |
52 weeks Randomized, open-label, multicentre, parallel group Omalizumab added to best standard care |
ICS daily: 1000 μg (30.1%), 2000 μg (38.8%), 4000 μg (16%) LABA 78% Antileukotrienes 28% SCS 21.2% Salbutamol for rescue |
Rate of asthma-related deterioration incidents per patient-year | 4.92 vs 9.76, P<0.001 | Clinically significant exacerbations reduced by 60.8%, P<0.001 Change in mean daily dose of ICS: −342 μg/day vs +68 μg/day, P<0.001 Median rescue bronchodilator use 0.6 vs 3 puffs/day, P<0.001 |
| Holgate et al (43) (n=246) | Age 12 to 75 years Severe allergic asthma ≥ 1000 μg/dayfluticasone Mean daily dose of1362/1375 μg/day (placebo/treatment groups) |
32 weeks (16 add-on, 16 SRP) Randomized, double-blind, multicentre, placebo-controlled Omalizumab added to existing therapy |
LABA (43.3% and 49.2% in treatment and placebo groups, respectively) Short-acting beta-agonists as needed |
Per cent reduction in ICS use from baseline | Median ICS reduction 60% vs 50%, P=0.003 Mean 57.2% vs 43.3%, P=0.003 |
≥50% ICS reduction in 73.8% vs 50.8% of patients, P=0.001 Reduction to ≤500 μg/day in 60.3% vs 45.8% of patients, P=0.026 |
For eight weeks until total elimination or until forced expiratory volume in 1 s declined by 20% or more, or asthma worsened;
Extension of study by Solèr et al (24);
Extension of study by Busse et al (37). BDP Beclomethasone dipropionate; ETOPA Efficacy and Tolerability of Omalizumab in Poorly controlled Asthma; ICS Inhaled corticosteroids; INNOVATE Investigation of Omalizumab in Severe Asthma Treatment; LABA Long-acting beta2-agonists; MP Maintenance phase; SCS Systemic corticosteroids; SRP Steroid reduction phase; SSP Stable steroid phase; vs Versus
Dosing in the trials discussed below followed the practice used currently in clinical settings; specifically, patients received an amount of omalizumab calculated to reduce free circulating levels of IgE by more than 90% from baseline (this effect can be measured only by special techniques because clinical laboratory measurements of IgE do not distinguish between serum IgE that is free and IgE that is bound to omalizumab, and thus, omalizumab’s IgE binding effect cannot be quantified in routine clinical practice). The amount of omalizumab given is approximately 0.016 mg/kg for every 1 U/mL of IgE – a calculation made simple by determining a patient’s weight and IgE level, and then consulting a dosing table. Tables calculate doses that are given subcutaneously every two to four weeks.
In two phase III, randomized, double-blind trials conducted by Solèr et al (24) and Busse et al (37), 1071 patients (aged 12 years or older) with allergic asthma who remained symptomatic despite 500 μg/day to 1200 μg/day of ICS were randomly assigned to placebo or omalizumab (approximately 0.016 mg/kg for every 1 U/mL of IgE) administered subcutaneously every two or four weeks. During the first 16 weeks of these 28-week studies (the ‘stable steroid phase’), patients took a constant dose of beclomethasone dipropionate (BDP). In the ‘ICS reduction phase’, their ICS dose was progressively reduced over the subsequent eight weeks (by 25% of baseline dose every two weeks until elimination of ICS or until the patient experienced a decrease in FEV1 greater than 20% compared with the last measurement). For the final four weeks of the study, patients used the lowest ICS dose possible for asthma control. After 28 weeks, reduction of BDP use was significantly greater with omalizumab than with placebo (a median prescribed dose of 100 μg/day versus 300 μg/day, P<0.001). In the Solèr et al study (24), patients receiving active treatment had 58% fewer asthma exacerbations than placebo-administered patients during the stable steroid phase, and 52% fewer during the ICS reduction phase (both P<0.001). Similarly, in the study by Busse et al (37), the use of omalizumab versus placebo reduced the percentage of patients experiencing asthma exacerbations in both the stable steroid phase and the ICS reduction phase (14.6% versus 23.3% and 21.3% versus 32.3%, respectively). In addition to these results, there was a greater likelihood of ICS dose reduction or withdrawal among patients receiving omalizumab versus placebo. In these two studies, IgE reduction in the omalizumab-treated patients ranged between 89% and 98% to 99%. Rates of treatment discontinuation (6.9% versus 14.7% in the Solèr et al study [24]), asthma symptom scores and the use of rescue medication in all phases of treatment also favoured omalizumab.
A third trial, the Investigation of Omalizumab in Severe Asthma Treatment (INNOVATE) (38), explored the hypothesis that omalizumab may be appropriate for patients whose asthma remains uncontrolled despite optimal modern (GINA step 4) therapy. Unlike the foregoing studies, all patients received regular therapy with high-dose ICS and LABA in combination. The study included 419 individuals aged 12 years or older with proven allergic (to at least one perennial allergen) asthma, poor lung function (FEV1 40% or greater to less than 80% predicted) and a recent history of clinically significant exacerbations despite high-dose ICS, LABA and other agents. The subjects were given omalizumab or placebo for 28 weeks. During this treatment period, the rate of clinically significant exacerbations was 26% lower with active treatment than with placebo (0.68 versus 0.91 exacerbations per subject per year, P=0.0002). Severe asthma exacerbations and emergency department visits were also significantly less frequent among omalizumab-treated individuals (0.24 versus 0.48 exacerbations per subject per year, P=0.002; and 0.24 versus 0.43 visits per subject per year, P=0.038, resepectively). Asthma symptom scores and morning peak expiratory flow improved significantly with active treatment.
Similarly, a 12-month, randomized, open-label, controlled trial involving 312 patients with poorly controlled, moderate to severe asthma determined that adding omalizumab (given at four-week intervals) to current optimal therapies, as defined by the National Heart, Lung, and Blood Institute, reduced by one-half the annualized mean number of asthma deteriorationrelated incidents (4.92 versus 9.76 incidents per patient-year with omalizumab compared with placebo, P<0.001) and clinically significant exacerbations (1.12 versus 2.86 exacerbations per patient-year, P<0.001). The use of rescue medications and health care resources was lower, and lung function improved to a greater extent with the anti-IgE antibody treatment (39).
These results and those of double-blind extensions of the trials by Solèr et al (24) and Busse et al (37) suggest that the reduction in exacerbation frequency observed with omalizumab treatment is maintained for at least one year (40,41). Recent data from approximately 150 patients who had taken the agent for at least three years indicate that the agent’s benefits on asthma control (as measured by physician assessment) and lung function (FEV1) are maintained for this period, and that there may be continued gradual reduction in the use of concomitant ICS (42).
A study by Holgate et al (43) assessed reduction of ICS as a primary efficacy end point within a design similar to those of the trials described above. In this analysis, the mean reduction in ICS was 60% among omalizumab-treated individuals and 50% (P=0.003) for those receiving standard therapy (fluticasone and short- and long-acting beta-agonists, as required). Approximately 74% of those taking omalizumab were able to reduce ICS by 50% or more; the corresponding rate was 51% in those receiving standard therapy (P=0.001).
An anticipated result of enhanced treatment efficacy –including a reduction in exacerbations and the associated need for medical care – is improvement in quality of life. Data compiled in at least six studies point to an increase in quality of life with omalizumab treatment associated with marked improvement in control (24,35,37–41,43).
Pediatric patients
Although their primary study variable was safety, Milgrom et al (44) assessed the impact of omalizumab on steroid use and asthma exacerbations in 334 children aged six to 12 years. As in the studies reviewed above, active treatment given at four-week intervals increased the likelihood and degree of BDP reduction compared with placebo (median reduction 100% versus 66.7%). Fifty-five per cent of omalizumab-treated children and 39% of the placebo group were able to stop BDP use. Moreover, asthma exacerbations during the steroid reduction phase of this trial occurred in 18.2% of patients receiving omalizumab and 38.5% of those receiving placebo; the mean numbers of episodes per patient were 0.42 versus 2.72, respectively. The use of rescue medications taken by patients in the omalizumab group was lower in the stable steroid and dose reduction phases; specifically, at 28 weeks, the median number of daily rescue puffs was zero in the omalizumab group versus 0.46 in the placebo group.
SAFETY
Omalizumab was well tolerated in the trials discussed above. The incidence of adverse events in clinical trials of asthmatic patients of up to one year’s duration has been consistently similar (difference in incidence not more than 1%) in subjects treated with omalizumab, placebo or other control medications. Among patients treated with omalizumab for up to one year, the adverse reactions most commonly observed include injection site reaction (45%), viral infections (24%), upper respiratory tract infection (19%), sinusitis (16%), headache (15%) and pharyngitis (10%). Adverse events seldom (0.1% or less) require clinical intervention and are usually considered mild to moderate in intensity (28).
Recent long-term assessments in adult and pediatric patients indicate that the agent’s safety and tolerability profile is maintained for a period of at least three years. Among 149 adults who completed approximately 3.5 years of omalizumab therapy, no new safety issues arose. The most frequent adverse events reported were infections and infestations (52.8%), musculoskeletal and connective tissue disorders (14%), and gastrointestinal complaints (11.8%). Asthma was the most frequent individual adverse event (27.5%) (45). Similarly, among approximately 100 children aged six to 12 years who completed a three-year, open-label extension study of omalizumab treatment, the most frequently reported adverse events were mild to moderate in severity, and included upper respiratory tract infection (51.6%), viral infection (35.1%), asthma (34%), pharyngitis (26.6%), headache (24.5%) and sinusitis (22.3%) (46).
Following the clinical development program for omalizumab, a review of safety data documented a slightly increased rate of malignancy in omalizumab-treated patients versus controls (0.5% versus 0.2%) (28). This information was available before licensure and was examined closely by regulatory authorities in the countries where omalizumab has been approved. Several factors have prompted these authorities to regard the malignancies as almost certainly being unrelated to omalizumab, while reflecting the findings stated in the product monograph. These factors include the observations that the malignancies documented were of widely differing types and occurred in various organs. Moreover, the tumours were typically documented in the course of one-year trials with known tumour biology, suggesting that the tumours had been present before the patients’ enrollment and exposure to the medication. Finally, pre-existing malignancy was not an exclusion factor in omalizumab trials. The prevalence of malignancies has been tracked closely in postmarketing surveillance trials, with no worrisome trends emerging. The data available are extensive; omalizumab has now been administered to more than 25,000 adolescent and adult patients in Canada and the United States, some of whom who have used it for at least five years as part of the manufacturer’s clinical trial program.
The product monograph also notes that there have been rare cases of anaphylaxis and development of antibodies to omalizumab. To date, surveillance data from the United States have produced no evidence of an increased incidence of malignancy in treated patients, and observed rates of anaphylaxis and immunogenicity are less than 0.1% in both treated individuals and controls. Similarly, there have been no cases of neoplasia or immunological reactions in children followed for four years (46).
COST-EFFECTIVENESS
Severe asthma remains a prevalent and costly health problem. Compared with intermittent or mild disease, severe asthma is associated with an increased incidence of emergency department visits, hospitalization and mortality (47,48). Patients with severe asthma account for the bulk of total health care costs related to the disease (49–51). According to one study, severe persistent asthma accounts for more than US$2,700 annually in direct costs other than hospitalization, an amount more than twice that for moderate persistent asthma and four and 10 times higher than costs associated with mild persistent and intermittent disease, respectively (52). Indirect costs, including those related to decreased quality of life, either as a result of the disease itself or emanating from adverse effects of medications, may also be considerable.
Given the impact of severe asthma on the use of health care resources and as a cause of disability, the addition of an effective therapeutic agent shown to reduce exacerbations, hospitalizations, and patients’ requirements for inhaled and oral steroid therapies may reasonably be expected to reduce direct and indirect costs. According to one study, treatment of 4.6 patients for one year with omalizumab ensures that one patient remains free of serious exacerbations for that period (53).
The indirect cost benefits of omalizumab have not yet been calculated. However, as noted above, there is some evidence that omalizumab-treated individuals experience a clinically significant improvement in quality of life, and this finding has the potential to be associated with economic benefits. Solèr et al (24) indicate that, in their study, asthma-related absenteeism was significantly reduced in patients receiving omalizumab versus placebo. In addition, young children whose treatment regimen included omalizumab missed approximately one-half as many school days as those receiving ICS (P=0.04) (44).
In Canada, the current cost per vial of omalizumab is $600; the projected annual cost per patient is approximately $12,000 (54). Direct cost savings are most likely to be achieved if this medication is reserved for patients whose severe allergic asthma remains symptomatic with frequent exacerbations in spite of appropriate preventive and management measures, including optimal use of proven conventional therapies. We stress the need for careful and responsible patient selection. This opinion is supported by a one-year retrospective analysis of direct costs to improve quality of life associated with omalizumab administration in two clinical trials in the United States. The authors of this analysis suggest that omalizumab saves costs if given to nonsmoking patients who are hospitalized at least five times or for at least 20 days despite maximal therapy (55). An evaluation of omalizumab from a managed care perspective suggests, similarly, that the high cost of the agent may be offset by savings in other areas of urgent and chronic asthma care if it is targeted to the patients who have been the most frequent or high-intensity users of medical resources (56).
PATIENT SELECTION AND RESPONSE
Characteristics of the patient’s history may help to determine the appropriateness of a trial of omalizumab. Using logistic regression analysis of baseline characteristics of 1070 individuals, Bousquet et al (57) determined that patients with allergic asthma were most likely to benefit from the addition of omalizumab to their treatment regimen if they had the following: a history of frequent need for emergency treatment (67% response rate to omalizumab versus 42% to placebo); required doses of BDP 800 μg/day or more, or fluticasone greater than 400 μg/day (65% versus 40% response rates); or had an FEV1 less than 65% predicted (60% versus 40% response rates). In their analysis, 76% of patients had at least one of these factors, which more than doubled the likelihood of response to active treatment. Of note, the authors’ composite definition of response included no exacerbation over 16 weeks of treatment and at least one of the following: reduced symptom score; reduced use of rescue medication; improved lung function; and improved quality of life.
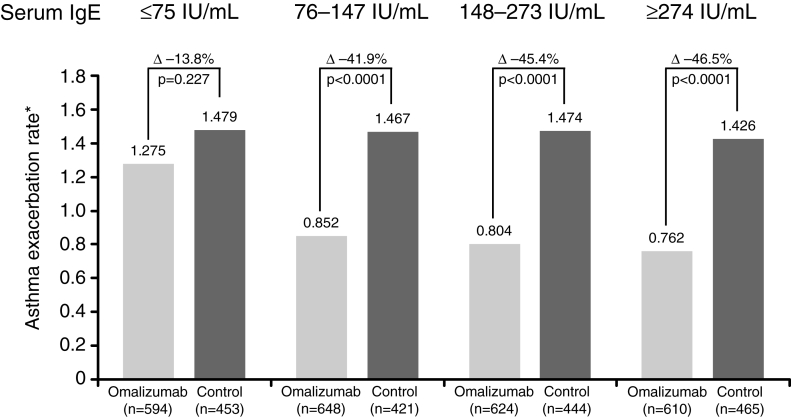
Along with asthma severity, the determination of IgE levels may become a recommended element of patient selection, although this is still an area of investigation. In the INNOVATE study, a baseline total IgE of greater than 76 U/mL was associated with the greatest likelihood of overall response and the greatest reduction in the rate of asthma exacerbation (Figure 5) (58).
Figure 5).
Greater reduction in exacerbation rate in patients with immunoglobulin E (IgE) of 76 U/mL or greater. *Annualized rate. Reproduced with permission from reference 58
Response to therapy should be assessed at four to six months, and omalizumab should be stopped if there is no improvement in asthma control. If effective, omalizumab should be continued indefinitively because IgE production is not altered.
Pregnancy
The safety and efficacy of omalizumab in pregnancy has not been determined. IgG does cross the placental barrier, but theoretically, anti-IgE should not be harmful to the fetus and may be of benefit. Reproduction studies in cynomolgus monkeys using 75 mg/kg omalizumab subcutaneously (12-fold maximal doses for humans) did not elicit maternal toxicity, embryotoxicity or teratogenicity when administered throughout organogenesis and did not elicit adverse effects on fetal or maternal growth when administered throughout late gestation, delivery or nursing (Xolair [Novartis Pharmaceuticals Canada Inc] monograph [28]). Xolair is classified by the Food and Drug Administration (FDA) as pregnancy category B. Blaiss (59) mentioned that “several women who participated in clinical studies with omalizumab before FDA approval became pregnant and delivered normal infants. Because of the newness of this agent, it is important to weigh the benefit to risk before use during pregnancy”.
AREAS FOR FUTURE STUDY
As described above, omalizumab has been studied as a potential therapy for asthma in children younger than 12 years of age. Although early results are positive and similar to those seen in adults, omalizumab is not currently indicated for use in this population. Its efficacy in allergic rhinitis, chronic obstructive pulmonary disease, and peanut and latex allergy is also being investigated, but no specific recommendations can be made at this time. Optimal long-term dosing strategies are also the subject of ongoing investigation. It is plausible but unproven that omalizumab doses or dosing frequency may be reduced over time without loss of efficacy, given downregulation of IgE production during therapy; however, no recommendations can be made at this time. Similarly, patients are not currently considered candidates for omalizumab therapy if their serum IgE levels and/or body weight exceed those specified by available dosing tables, implying that the maximal doses of omalizumab would fail to bind to the targeted 96% or more of circulating IgE. Is it possible that such patients will benefit from lower dose omalizumab therapy, and thus, a partial reduction in circulating free IgE levels? Again, this plausible hypothesis awaits further investigation, and no recommendations can be made at this time.
CONCLUSIONS AND RECOMMENDATIONS
Taken together, data from the studies summarized here support our assertion that omalizumab may fulfill a currently unmet need in the management of persistent, severe, allergic asthma. By inhibiting inflammation due to IgE, omalizumab may allow selected patients, aged 12 years or older with documented atopy to a perennial aeroallergen and severe asthma, to enjoy decreased frequency of exacerbations, safely reduce their reliance on ICS or oral steroids, and experience an improvement in quality of life. Table 2 summarizes the typical criteria for omalizumab use.
TABLE 2.
Criteria for omalizumab administration
|
Despite participation of private payers, the cost of omalizumab may be an issue. Judicious patient selection helps to ensure that it is used in a cost-effective manner as a useful addition to the armamentarium of asthma care. Appropriate candidates are likely those whose severe asthma manifests frequent exacerbations or significant persistent asthma-related disability despite optimal therapy with multiple agents (as per the CACGs), compliance with the regimen prescribed, and the identification and treatment of any comorbid conditions. Such patients are likely to have received frequent bursts of prednisone or require daily oral steroids, and/or have experienced failure of, or serious adverse effects with ICS, LABA or other adjunctive therapies. Omalizumab is not indicated or justified for milder asthma that may be readily controlled with effective conventional inhaled therapies.
Before omalizumab therapy is initiated, the patient should be assessed by a specialist who should confirm the diagnosis, evaluate the patient’s asthma education, assess appropriateness and adherence to current therapies, and address issues such as smoking cessation and antigens in the home and work-place. Baseline lung function assessment is valuable because the likelihood of a response to omalizumab is increased by a low FEV1 level.
The dose of omalizumab is based on untreated serum IgE levels and body weight, a calculation that is made simple by reference to readily available dosing tables. Once therapy has begun, the dose is not adjusted and serum IgE levels are not remeasured. Although omalizumab reduces free IgE in serum, this reduction is not reflected by conventional laboratory measurements of serum IgE. As a safety precaution against severe hypersensitivity reactions, patients are monitored for 2 h following the first injection and for 1 h following subsequent injections. This precludes patient self-injection; injections must be given in a medical facility where equipment for resuscitation is readily available. For physicians unwilling or unable to provide such facilities, a network of clinics for injection is available to administer a prescribed course of therapy.
An adequate treatment duration before evaluation of therapeutic efficacy is at least four to six months (ie, sufficient time for elimination of cell-bound IgE). In the evaluation by Bousquet et al (36), 38% of patients had a response at four weeks and 64% responded by 16 weeks. Fewer than two-thirds of patients who responded at 16 weeks had responded at four weeks; 87% had shown a response by 12 weeks.
Consistent with the CACGs, sustained acceptable control on omalizumab should lead to a cautious, gradual reduction in chronic oral steroids. Complete withdrawal of oral corticosteroids is a reasonable treatment goal; the reduction of a very high dose of ICS may also be attractive, but we suggest caution because total withdrawal of ICS may not be possible, and it seems more reasonable to aim to reduce ICS doses to the low or medium range. Although there is evidence that IgE production may decrease over time, it is unknown at this time whether omalizumab can eventually be reduced or withdrawn.
REFERENCES
- 1.Boulet LP, Becker A, Berube D, Beveridge R, Ernst P. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. CMAJ. 1999;161(Suppl 11):S1–S61. [PMC free article] [PubMed] [Google Scholar]
- 2.Lemiere C, Bai T, Balter M, et al. Adult Asthma Consensus Guidelines Update 2003. Can Respir J. 2004;11(Suppl A):9A–18A. doi: 10.1155/2004/271362. [DOI] [PubMed] [Google Scholar]
- 3.National Institutes of Health and National Heart, Lung, and Blood Institute NAEPP Expert Panel Report. Guidelines for the Diagnosis and Management of Asthma Bethesda: NIH Publications; 97-5051 (1997) and 02-5075 (update 2002). [Google Scholar]
- 4.Global Initiative for Asthma Pocket Guide for Asthma Management and Prevention (update 2005)<www.ginasthma.org> (Version current at June 1, 2006).
- 5.Canadian Institute for Health Information, Canadian Lung Association, Health Canada, and Statistics Canada Respiratory Disease in Canada Ottawa: Health Canada; 2001. H39-593/2001E. [Google Scholar]
- 6.Chapman KR, Ernst P, Grenville A, Dewland P, Zimmerman S. Control of asthma in Canada: Failure to achieve guideline targets. Can Respir J. 2001;8(Suppl A):35A–40A. doi: 10.1155/2001/245261. [DOI] [PubMed] [Google Scholar]
- 7.Chapman KR, Walker L, Cluley S, Fabbri L. Improving patient compliance with asthma therapy. Respir Med. 2000;94:2–9. doi: 10.1053/rmed.1999.0667. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane GM.Compliance and outcomes in patients with asthma Drugs 199652Suppl 612-9. [DOI] [PubMed] [Google Scholar]
- 9.Dolovich MB, Ahrens RC, Hess DR, et al. American College of Chest Physicians; American College of Asthma, Allergy, and Immunology Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–71. doi: 10.1378/chest.127.1.335. [DOI] [PubMed] [Google Scholar]
- 10.Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children N Engl J Med 19933281665–9.(See comments) [DOI] [PubMed] [Google Scholar]
- 11.Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57:226–30. doi: 10.1136/thorax.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoller JK, Sandhaus RA, Turino G, Dickson R, Rodgers K, Strange C. Delay in diagnosis of alpha1-antitrypsin deficiency: A continuing problem. Chest. 2005;128:1989–94. doi: 10.1378/chest.128.4.1989. [DOI] [PubMed] [Google Scholar]
- 13.Campos MA, Wanner A, Zhang G, Sandhaus RA. Trends in the diagnosis of symptomatic patients with alpha1-antitrypsin deficiency between 1968 and 2003. Chest. 2005;128:1179–86. doi: 10.1378/chest.128.3.1179. [DOI] [PubMed] [Google Scholar]
- 14.Cicutto LC, Chapman KR, Chamberlain D, Downey GP. Difficult asthma: Consider all of the possibilities. Can Respir J. 2000;7:415–8. doi: 10.1155/2000/797306. [DOI] [PubMed] [Google Scholar]
- 15.Newman KB, Mason UG, III, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995;152:1382–6. doi: 10.1164/ajrccm.152.4.7551399. [DOI] [PubMed] [Google Scholar]
- 16.Chapman KR. Asthma unresponsive to usual therapy. In: FitzGerald JM, Ernst P, Boulet L-P, O’Byrne PM, editors. Evidence-Based Asthma Management. Hamilton: BC Decker; 2001. pp. 291–305. [Google Scholar]
- 17.Szefler SJ, Leung DY. Glucocorticoid-resistant asthma: Pathogenesis and clinical implications for management. Eur Respir J. 1997;10:1640–7. doi: 10.1183/09031936.97.10071640. [DOI] [PubMed] [Google Scholar]
- 18.Nelson HS, Hamilos DL, Corsello PR, Levesque NV, Buchmeier AD, Bucher BL. A double-blind study of troleandomycin and methylprednisolone in asthmatic subjects who require daily corticosteroids. Am Rev Respir Dis. 1993;147:398–404. doi: 10.1164/ajrccm/147.2.398. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez J, Szefler SJ. Alternative therapy in severe asthma. J Asthma. 1992;29:3–11. doi: 10.3109/02770909209110635. [DOI] [PubMed] [Google Scholar]
- 20.Moss RB. Alternative pharmacotherapies for steroid-dependent asthma. Chest. 1995;107:817–25. doi: 10.1378/chest.107.3.817. [DOI] [PubMed] [Google Scholar]
- 21.Kishiyama JL, Valacer D, Cunningham-Rundles C, et al. A multicenter, randomized, double-blind, placebo-controlled trial of high-dose intravenous immunoglobulin for oral corticosteroid-dependent asthma. Clin Immunol. 1999;91:126–33. doi: 10.1006/clim.1999.4714. [DOI] [PubMed] [Google Scholar]
- 22.Brownell J, Casale TB. Anti-IgE therapy. Immunol Allergy Clin North Am. 2004;24:551–68. doi: 10.1016/j.iac.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Davis L. Omalizumab: A novel therapy for allergic asthma. Ann Pharmacother. 2004;38:1236–42. doi: 10.1345/aph.1D626. [DOI] [PubMed] [Google Scholar]
- 24.Solèr M, Matz J, Townley R, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61. doi: 10.1183/09031936.01.00092101. (Erratum in 2001;18:739–40). [DOI] [PubMed] [Google Scholar]
- 25.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–65. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 26.Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. J Allergy Clin Immunol. 2001;107:429–40. doi: 10.1067/mai.2001.113759. [DOI] [PubMed] [Google Scholar]
- 27.Fregonese L, Patel A, van Schadewijk A, et al. Expression of the high-affinity IgE receptor (FcɛRI) is increased in fatal asthma. Am J Respir Crit Care Med. 2004;169:A297. (Abst) [Google Scholar]
- 28.Xolair product monograph. Novartis Pharmaceuticals Canada Inc, November 2004.
- 29.McGlashan DW, Bochner BS, Adelman JC, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–45. [PubMed] [Google Scholar]
- 30.Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155:1828–34. doi: 10.1164/ajrccm.155.6.9196082. [DOI] [PubMed] [Google Scholar]
- 31.Boulet LP, Chapman KR, Côté J, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155:1835–40. doi: 10.1164/ajrccm.155.6.9196083. [DOI] [PubMed] [Google Scholar]
- 32.Milgrom H, Fick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med. 1999;341:1966–73. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 33.Fahy JV, Cockcroft DW, Boulet LP, et al. Effect of aerosolized anti-IgE (E25) on airway responses to inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1999;160:1023–7. doi: 10.1164/ajrccm.160.3.9810012. [DOI] [PubMed] [Google Scholar]
- 34.Djukanovic R, Wilson SJ, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170:583–93. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 35.Vignola AM, Mirabella F, Costanzo G, et al. Airway remodeling in asthma. Chest. 2003;123(Suppl 3):417S–22S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 36.Bousquet J, Cabrera P, Berkman N, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–8. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 37.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 38.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 39.Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59:701–8. doi: 10.1111/j.1398-9995.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 40.Buhl R, Solèr M, Matz J, et al. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur Respir J. 2002;20:73–8. doi: 10.1183/09031936.02.00278102. [DOI] [PubMed] [Google Scholar]
- 41.Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol. 2003;91:154–9. doi: 10.1016/S1081-1206(10)62170-9. [DOI] [PubMed] [Google Scholar]
- 42.Chung KF, Ankerst J, Rolli M, Gao J, Reisner C. Long-term asthma control with omalizumab, an anti-IgE monoclonal antibody, in patients with severe allergic asthma. Eur Respir J. 2005;26(Suppl 49):47S. (Abst) [Google Scholar]
- 43.Holgate ST, Chuchalin AG, Hebert J, et al. Omalizumab 011 International Study Group Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632–8. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 44.Milgrom H, Berger W, Nayak A, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. (Abst) [DOI] [PubMed] [Google Scholar]
- 45.Chuchalin A, Herbert J, Rolli M, et al. Long-term safety and tolerability of omalizumab, an anti-IgE monoclonal antibody, in patients with severe allergic asthma. Eur Respir J. 2005;26(Suppl 49):48S. (Abst) [Google Scholar]
- 46.Milgrom H, Miller SD, Lanier BQ, Fowler-Taylor A, Chen H, Gupta N. Long-term omalizumab therapy is well tolerated in children with moderate-to-severe IgE-mediated asthma. Proc Am Thorac Soc. 2005;2:A358. (Abst) [Google Scholar]
- 47.Dolan CM, Fraher KE, Bleecker ER, et al. TENOR Study Group Design and baseline characteristics of the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study: A large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2004;92:32–9. doi: 10.1016/S1081-1206(10)61707-3. [DOI] [PubMed] [Google Scholar]
- 48.Hartert TV, Speroff T, Togias A, et al. Risk factors for recurrent asthma hospital visits and death among a population of indigent older adults with asthma. Ann Allergy Asthma Immunol. 2002;89:467–73. doi: 10.1016/S1081-1206(10)62083-2. [DOI] [PubMed] [Google Scholar]
- 49.Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–9. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 50.Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–6. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 51.Barnes PJ, Jonsson B, Klim JB. The costs of asthma. Eur Respir J. 1996;9:636–42. doi: 10.1183/09031936.96.09040636. [DOI] [PubMed] [Google Scholar]
- 52.Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: A 1-yr prospective study. Eur Respir J. 2002;19:61–7. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 53.Holgate S, Chuchalin A, Herbert J, et al. Omalizumab improves asthma-specific quality of life in patients with severe allergic asthma. Eur Respir J. 2001;18:P348. (Abst) [Google Scholar]
- 54.Hadj Tahar A. Omalizumab as add-on therapy to inhaled steroids for asthma. Issues Emerg Health Technol. 2004;58:1–4. [PubMed] [Google Scholar]
- 55.Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9. doi: 10.1016/j.jaci.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 56.Belliveau PP, Lahoz MR. Evaluation of omalizumab from a health plan perspective. J Manag Care Pharm. 2005;11:735–45. doi: 10.18553/jmcp.2005.11.9.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–86. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 58.Holgate ST. Identifying the patients to treat: Implications for clinical practice. [Anti-IgE: A new era in the control of severe persistent allergic asthma?]. Annual Congress of the European Respiratory Society; Copenhagen, Denmark. September 19; 2005. [Google Scholar]
- 59.Blaiss MS, National Institute of Health Management of asthma during pregnancy. Allergy Asthma Proc. 2004;25:375–9. [PubMed] [Google Scholar]
- 60.Becker A, Watson W, Ferguson A, Dimich-Ward H, Chan-Yeung M. The Canadian asthma primary prevention study: Outcomes at 2 years of age. J Allergy Clin Immunol. 2004;113:650–6. doi: 10.1016/j.jaci.2004.01.754. [DOI] [PubMed] [Google Scholar]
- 61.Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med. 2001;164:S6–S11. doi: 10.1164/ajrccm.164.supplement_1.2103025. [DOI] [PubMed] [Google Scholar]
- 62.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]