Abstract
Cell survival and death-inducing signals are tightly associated with each other, and the decision as to whether a cell survives or dies is determined by controlling the relationship between these signals. However, the mechanism underlying the reciprocal regulation of such signals remains unclear. In this study, we reveal a functional association between PDK1 (3-phosphoinositide-dependent protein kinase 1), a critical mediator of cell survival, and ASK1 (apoptosis signal-regulating kinase 1), an apoptotic stress-activated MAPKKK. The physical association between PDK1 and ASK1 is mediated through the pleckstrin homology domain of PDK1 and the C-terminal regulatory domain of ASK1 and is decreased by ASK1-activating stimuli, such as H2O2, tumor necrosis factor α, thapsigargin, and ionomycin, as well as insulin, a PDK1 stimulator. Wild-type PDK1, but not kinase-dead PDK1, negatively regulates ASK1 activity by phosphorylating Ser967, a binding site for 14-3-3 protein, on ASK1. PDK1 functionally suppresses ASK1-mediated AP-1 transactivation and H2O2-mediated apoptosis in a kinase-dependent manner. On the other hand, ASK1 has been shown to inhibit PDK1 functions, including PDK1-mediated regulation of apoptosis and cell growth, by phosphorylating PDK1 at Ser394 and Ser398, indicating that these putative phosphorylation sites are involved in the negative regulation of PDK1 activity. These results provide evidence that PDK1 and ASK1 directly interact and phosphorylate each other and act as negative regulators of their respective kinases in resting cells.
Keywords: Apoptosis, Enzymes/Kinase, Phosphorylation/Kinases/Serine-Threonine, Protein/Protein-Protein Interactions, Signal Transduction/Protein Kinases/Serine/Threonine, Transcription/AP1
Introduction
Evidence is accumulating that the cellular decision between either cell survival or death is determined by the integration of multiple survival and death signals. Akt, also known as protein kinase B (PKB),3 plays a key role in cell survival signaling and has been shown to phosphorylate a number of its proapoptotic substrates directly, including GSK-3 (glycogen synthase kinase 3), Bad (Bcl-2-associated death promoter), caspase-9, and forkhead transcription factor, through protein-protein interactions (1–6). It is also involved in suppressing cellular proapoptotic signaling. Akt directly phosphorylates ASK1 (apoptosis signal-regulating kinase 1) at Ser83 and inhibits its function (7). Raf-1, a Ser/Thr kinase involved in normal cell growth, is also thought to inhibit ASK1-induced apoptosis via direct interaction, although the precise phosphorylation site on ASK1 has not been identified (8). These findings suggest that the inhibition of proapoptotic signaling pathways by proteins involved in survival signaling mechanisms may be part of the cellular machinery that maintains cell survival and vice versa.
ASK1 is a member of a MAPKKK family and functions as an upstream kinase engaged in c-Jun NH2-terminal kinase (JNK)/p38 signaling via the phosphorylation and activation of MAPKKs, such as MKK3, -4, -6, and -7 (9–11). A number of cellular proteins that interact with ASK1 have been identified, and their roles in the regulation of ASK1 function have been investigated (11–16). Among these proteins, it was discovered that tumor necrosis factor receptor-associated factor, Daxx, JSAP1 (JNK/stress-activated protein kinase-associated protein 1)/JIP3 (JNK-interacting protein 3), and MPK38 (murine protein serine/threonine kinase 38) stimulated ASK1 function through direct interaction, whereas thioredoxin, glutaredoxin, 14-3-3, Hsp72 (heat shock protein 72), Raf-1, Akt/PKB, and PP5 (protein serine/threonine phosphatase 5) all contributed to the inhibition of ASK1 function. However, the mechanisms by which ASK1 phosphorylation prevents cells from undergoing cell death and induces cell survival remain unclear, despite the involvement of Akt/PKB, apoptosis signal-regulating kinase 2 (ASK2), and MPK38 in the regulation of ASK1 function through direct phosphorylation (7, 17, 18). Thus, it is possible that a search for the unidentified kinases responsible for ASK1 phosphorylation will contribute toward a better understanding of the mechanism of AKS1 regulation in response to various cellular stresses.
PDK1 was first identified as an enzyme responsible for the phosphatidylinositol 3,4,5-trisphosphate-dependent phosphorylation of the activation loop of Akt/PKB, a downstream target of phosphatidylinositol 3-kinase (PI3K) (19). PDK1 is a member of the AGC (protein kinase A, G, and C) subfamily of protein kinases and is a master kinase involved in controlling a diverse set of kinases that have been implicated in many cellular signaling processes, including cell survival, proliferation, and differentiation (20–23). A post-translational modification, such as phosphorylation, is one of the major mechanisms regulating protein function, although the mechanism of how PDK1 phosphorylation regulates its activity remains controversial and is less well understood. It has been shown that PDK1, like other members of the AGC family of protein kinases, is capable of self-activation by phosphorylating Ser241 within its own activation loop (24). Recent studies have provided evidence that the tyrosine phosphorylation of PDK1 also plays an important role in stimulating PDK1 activity (25–27). These observations suggest that PDK1 phosphorylation is involved in the regulation of PDK1 activity, although the inhibition of PDK1 function, mediated by phosphorylation, has not yet been reported.
In this study, we show that PDK1 directly phosphorylates ASK1 on Ser967 through physical interaction and inactivates ASK1. This interaction contributes to the suppression of JNK-mediated AP-1 (activator protein 1) transactivation and ASK1-induced apoptosis. On the other hand, ASK1 suppresses PDK1 functions in a kinase-dependent manner by phosphorylating PDK1 at Ser394 and Ser398, suggesting a novel mechanism for the regulation of PDK1 activity.
MATERIALS AND METHODS
Cell Culture and Transient Transfection
HEK293, HeLa, 293T, and HaCaT cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen) in a 5% CO2 atmosphere at 37 °C as described (28). Each plasmid DNA indicated under “Results” was transfected into HEK293, 293T, or HaCaT cells with WelFect-ExTM Plus (WelGENE, Daegu, Korea) according to the manufacturer's instructions. The transfection efficiency as assessed by green fluorescent protein (GFP) was about 30–40%.
Plasmids, Cell Line Construction, and PDK1 Mutants
Wild-type (WT) and kinase-dead (K709R) ASK1, ASK1(N), ASK1(K), ASK1(C), ASK1(K709R) mutants (S83A, T838A, S967A, and S1034A), MKK6(K82A), p38, ATF2, c-fos, and the AP-1-Lux reporter have all been described previously (17). WT and kinase-dead (KD) PDK1, PDK1(PH), and PDK1(CA) have also been described previously (28). HEK293 cells (PDK1 shRNA) stably expressing PDK1-specific shRNA were screened in the presence of 1.5 μg/ml puromycin until all control parental HEK293 cells died. HEK293 cells (inducible PDK1 shRNA) transfected with pSingle-tTS-shRNA harboring PDK1-specific shRNA were screened in the presence of 500 μg/ml G418. Stable clones were isolated and treated with 1 μg/ml doxycycline (Sigma), a tetracycline analogue, for 72 h, and endogenous PDK1 knockdown was determined by immunoblot analysis using an anti-PDK1 antibody. HEK293 cells (PDK1(OE)) stably expressing PDK1 were screened in the presence of 600 μg/ml G418. The generation of ASK1+/+ and ASK1−/− mouse embryonic fibroblasts (MEFs) were reported previously (29). PDK1 mutants were generated by PCR mutagenesis, as described previously (17), on PDK1-C (amino acids 249–556) or wild-type PDK1 using the primers described in the supplemental material.
Antibodies and Reagents
The anti-FLAG (M2) and anti-β-actin antibodies were obtained from Sigma. The antibodies against hemagglutinin (HA), ASK1, MKK3, and ATF2 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The phosphoantibodies against MKK3/6(Ser189/207), p38(Thr180/Tyr182), ATF2(Thr71), PDK1(Ser241) and ASK1(Thr845), PKA C(Thr197), PKCδ(Thr505), and the anti-p38 antibody were from Cell Signaling Technology (Danvers, MA). The anti-PDK1, anti-phospho-Akt(Thr308), anti-phospho-Bad(Ser136), and anti-GST antibodies were described previously (30). Akt inhibitors VIII and SH-5, PKA inhibitor H-89, and PKC inhibitor staurosporine were obtained from Calbiochem.
Protein Interactions, Immunoprecipitation, and Immunoblot Analysis
In Vitro Kinase Assay
Cells transfected with the appropriate plasmids were collected and lysed with buffer (20 mm Hepes, pH 7.9, 10 mm EDTA, 0.1 m KCl, and 0.3 m NaCl). The immunoprecipitates or GST precipitates, purified on glutathione-Sepharose beads, were assayed for the indicated protein kinase activities in reaction mixtures containing each kinase buffer (17) and 5 μg of recombinant GST-tagged substrates (for ASK1, MKK6(K82A); for MKK3/MKK6, p38; for p38, ATF2; for PDK1, serum/glucocorticoid-regulated kinase; and for JNK, c-Jun) in a volume of 30 μl, followed by SDS-PAGE and autoradiography. Protein concentration was determined by the Bradford assay.
Reporter Assay
Cells transfected with the plasmids indicated plus the AP-1-Luc reporter were lysed, and luciferase activity was detected by the luciferase assay kit (Promega) according to the manufacturer's instructions (17). The plasmid expressing β-galactosidase was cotransfected as an internal control, and the total DNA employed in each transfection was kept constant by supplementing with empty vector DNAs. Experiments were repeated independently at least three times.
Small Interfering RNA (siRNA) Experiments
The siRNA experiments were performed using the PDK1 siRNAs described in the supplemental material (28, 30). ASK1 siRNA was described previously (31).
Apoptosis Assay
HEK293 cells were transfected with the expression vectors indicated in the presence or absence of H2O2. 293T cells undergoing apoptosis, after treatment with TNF-α (20 ng/ml) and cycloheximide (10 μg/ml), were quantitated by the GFP expression system as well as by staining with the terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) system, according to the manufacturer's instructions (Roche Molecular Biochemicals). Apoptosis analysis using the GFP system was performed as described previously (32).
Cell Cycle Analysis
Assays were performed as described previously (30). Briefly, HaCaT cells (2 × 105/60-mm dish), transfected with the indicated combinations of plasmid vectors, were washed with ice-cold phosphate-buffered saline and then synchronized in G0/G1 by 2 mm hydroxyurea treatment for 20 h. The number of cells in each stage of the cell cycle was analyzed after 10% serum treatment for 24 h. The trypsinized cells were washed twice with ice-cold phosphate-buffered saline and incubated at 37 °C for 30 min with a solution (1 mm Tris-HCl, pH 7.5) containing 50 μg/ml propidium iodide and 1 mg/ml RNase A. The cells present in each phase of the cell cycle were analyzed by flow cytometry performed on a FACSCalibur-S system (BD Biosciences).
Statistical Analysis
Data shown represent the means ± S.E. of at least three independent experiments; p < 0.05 relative to control, significance calculated by Student's t test.
RESULTS
PDK1 and ASK1 Interact Directly with Each Other in Vivo and in Vitro
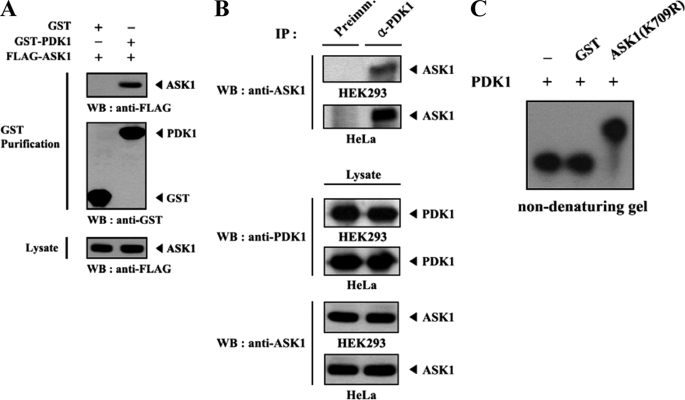
Given that Akt, a downstream target of PDK1, phosphorylates Ser83 of ASK1 and inhibits signaling downstream of ASK1 through direct interaction and that ASK1 has been previously identified as a PDK1-interacting protein in the yeast two-hybrid system (7) (data not shown), we speculated that PDK1, an upstream kinase of Akt/PKB, might regulate the function of ASK1 through phosphorylation. To determine whether ASK1 can act as a physiological substrate for PDK1 in cells, we first performed cotransfection experiments using GST-PDK1 and FLAG-ASK1 expression vectors. The interaction between PDK1 and ASK1 was detected by immunoblotting with an anti-FLAG antibody (Fig. 1A). However, this interaction was not dependent on the kinase activity of either enzyme (data not shown). An endogenous interaction between PDK1 and ASK1 was confirmed in both HEK293 and HeLa cells by coimmunoprecipitation experiments (Fig. 1B). To demonstrate further the direct interaction between PDK1 and ASK1, we also performed non-denaturing PAGE analysis using purified, recombinant ASK1 with PDK1 proteins. Autophosphorylated recombinant PDK1 was incubated with an unlabeled, recombinant kinase-dead form (K709R) of ASK1 or with GST as a nonspecific control. The mobility of 32P-labeled PDK1 in the presence of ASK1(K709R) was clearly different from that of GST alone or in the absence of AKS1(K709R), which provided evidence of a physical association between PDK1 and ASK1 in vitro (Fig. 1C). These results demonstrated that a physical association between PDK1 and ASK1 can occur both in vivo and in vitro and suggested that either ASK1 or PDK1 can act as a substrate for the other protein.
FIGURE 1.
PDK1 forms a complex with ASK1 in vivo and in vitro. A, HEK293 cells were transiently transfected with the indicated combinations of expression vectors encoding GST-PDK1 and FLAG-ASK1. GST fusion proteins were purified with glutathione-Sepharose beads (GST Purification). Complex formation between PDK1 and ASK1 was determined by immunoblot analysis with an anti-FLAG antibody. B, proteins immunoprecipitated from HEK293 and HeLa cell lysates, with either rabbit preimmune serum (Preimm.) or anti-PDK1 antibody (α-PDK1), were immunoblotted with an anti-ASK1 antibody to determine the association between endogenous PDK1 and ASK1. C, for the in vitro association between PDK1 and ASK1, autophosphorylated recombinant PDK1 (2–3 μg) was incubated with unlabeled recombinant GST or GST-ASK1(K709R) (5 μg each) at room temperature for 1 h and analyzed on an 8% native polyacrylamide gel. WB, Western blot; IP, immunoprecipitation.
PDK1-ASK1 Binding Is Mediated through Their C-terminal Domains and Is Modulated by ASK1 and PDK1 Signals
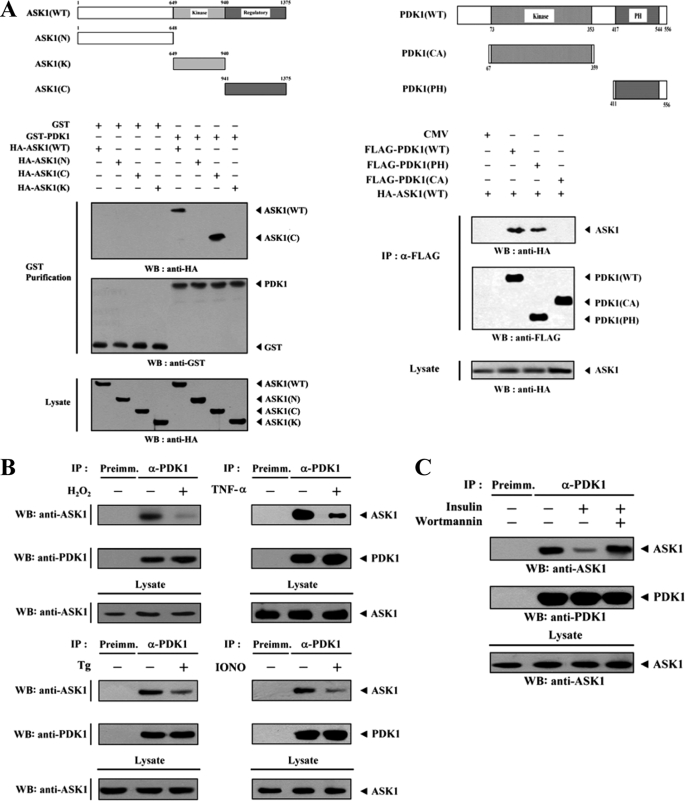
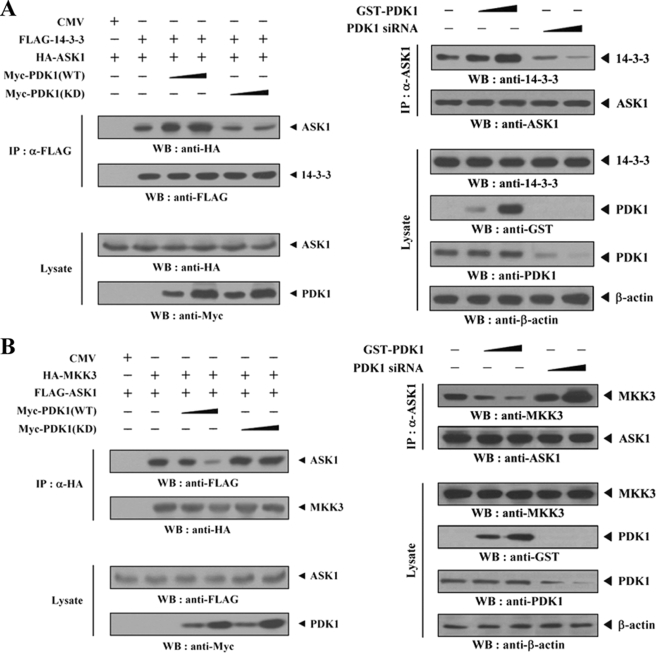
In an attempt to identify potential binding sites of PDK1, HEK293 cells were cotransfected with wild-type ASK1 or with one of its three deletion mutants (ASK1(N), ASK1(K), and ASK1(C)) together with PDK1. The C-terminal domain (amino acids 941–1375) of ASK1 was found to be responsible for PDK1 binding, whereas the N-terminal (amino acids 1–648) and kinase (amino acids 649–940) domains were unable to bind with PDK1 (Fig. 2A, left). This result suggested that the C-terminal domain of ASK1 is involved in PDK1 binding. The next step was to determine which region of PDK1 was required for ASK1 binding. Two PDK1 deletion constructs, FLAG-PDK1(PH), comprising the C-terminal pleckstrin homology (PH) domain (amino acids 411–556), and FLAG-PDK1(CA), harboring the catalytic domain (amino acids 67–359), as well as wild-type FLAG-PDK1 were cotransfected with HA-ASK1, and the ability of the constructs to form a complex with ASK1 was determined by in vivo binding assays. The binding of ASK1 was readily detected in the FLAG immunoprecipitate from HEK293 cells expressing wild-type FLAG-PDK1 and FLAG-PDK1(PH), whereas the binding of ASK1 was not detectable in the presence of FLAG-PDK1(CA) (Fig. 2A, right). This finding indicates that the C-terminal PH domain of PDK1 functions as an essential site for ASK1 binding in vivo. We next assessed whether ASK1 stimuli, including H2O2, TNF-α, endoplasmic reticulum stress (thapsigargin), and calcium overload (ionomycin), had an effect on PDK1-ASK1 complex formation in HEK293 cells. The presence of ASK1 in PDK1 immunoprecipitates was determined by immunoblotting with an anti-ASK1 antibody. The association between endogenous PDK1 and ASK1 was significantly decreased in the presence of H2O2, TNF-α, thapsigargin, or ionomycin when compared with the untreated control (Fig. 2B). Similarly, exposure of HEK293 cells to insulin resulted in a significant decrease in the PDK1-ASK1 complex formation, but this suppressive effect was alleviated by wortmannin, a PI3K/PDK1 inhibitor (Fig. 2C). This result suggested that PDK1 is linked to ASK1 signaling and vice versa.
FIGURE 2.
ASK1 and PDK1 stimuli destabilize the PDK1-ASK1 association mediated via their C-terminal domains. A, schematic representations of ASK1 and PDK1 divided into smaller HA- and FLAG-tagged fusion proteins, respectively. The numbers indicate amino acid residues corresponding to the domain boundaries. HEK293 cells were transiently transfected with the indicated combinations of expression vectors. Complex formation between PDK1 and ASK1 was determined by immunoblot analysis with an anti-HA antibody using GST precipitates (GST Purification) or FLAG immunoprecipitates (α-FLAG). B, HEK293 cell lysates were treated with or without the following stimuli: H2O2 (2 mm, 30 min), TNF-α (500 ng/ml, 30 min), thapsigargin (Tg; 20 μm, 30 min), or ionomycin (IONO; 1 μm, 24 h). C, HEK293 cells were incubated for 30 min with or without wortmannin (100 nm) and then treated with insulin (100 nm) for 20 min. The cell lysates were then immunoprecipitated with either rabbit preimmune serum (Preimm.) or anti-PDK1 antibody (α-PDK1), followed by immunoblotting with an anti-ASK1 antibody to determine the association between endogenous PDK1 and ASK1. WB, Western blot; IP, immunoprecipitation.
PDK1 Inhibits ASK1 Kinase Activity by Phosphorylating Ser967 of ASK1
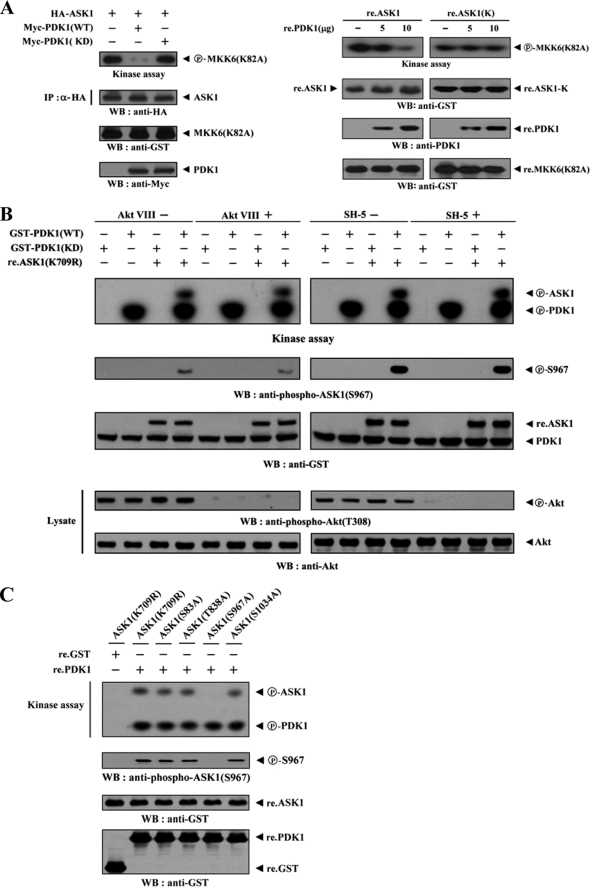
Because ASK1-interacting proteins can affect the function of ASK1 (11–16), we investigated the effect of PDK1 on ASK1 kinase activity using in vitro kinase assays in HEK293 cells. ASK1 kinase activity was decreased markedly when ASK1 was coexpressed with wild-type PDK1 but not with kinase-dead PDK1 (Fig. 3A, left). This result suggested that PDK1 inhibits ASK1 kinase activity in a kinase-dependent manner. In order to confirm the role of PDK1 in the regulation of ASK1 kinase activity, purified recombinant wild-type ASK1 was assayed in vitro for its kinase activity in the presence of increasing amounts of recombinant PDK1 protein. ASK1 kinase activity was substantially decreased in a dose-dependent manner (Fig. 3A, right). However, this inhibitory effect of recombinant PDK1 was not detectable in the presence of recombinant ASK1(K), which was defective in PDK1 binding (see Fig. 2A). We next examined whether PDK1 affects the phosphorylation of ASK1 and inhibits ASK1 activity. Extracts from HEK293 cells, expressing GST-tagged wild-type PDK1, were purified with glutathione-Sepharose beads and incubated with [γ-32P]ATP to allow the phosphorylation of recombinant non-phosphorylated forms of the ASK1 protein, ASK1(K709R) and ASK1(T838A). Coexpression of the recombinant ASK1 proteins with PDK1 induced a considerable level of ASK1 phosphorylation, suggesting that PDK1 phosphorylates ASK1 (supplemental Fig. 1). We then performed in vitro kinase assays in the presence of Akt, PKA, or PKC inhibitors to address the question of whether PDK1 directly phosphorylates ASK1. The PDK1-mediated phosphorylation of ASK1 was not affected by treatment of Akt, PKA, or PKC inhibitors when compared with the untreated control, indicating the direct involvement of PDK1 in the ASK1 phosphorylation (Fig. 3B and supplemental Fig. 2). Based on these results, our next step was to search for potential phosphorylation sites on ASK1. We investigated whether the four phosphorylation sites (Ser83, Thr838, Ser967, and Ser1034) on ASK1, which are known to be involved in the regulation of ASK1 activity (33), play a role in PDK1-mediated phosphorylation. To this end, we used an in vitro kinase assay and found that the mutation of serine 967 in ASK1(K709R) to alanine (ASK1(S967A)) completely abolished PDK1-mediated phosphorylation when compared with the positive control ASK1(K709R) and with the other substitution mutants (S83A, T838A, and S1034A) (Fig. 3C). This result indicated that Ser967 in ASK1, previously known as a 14-3-3 protein binding site (16), might represent a potential phosphorylation site for PDK1.
FIGURE 3.
PDK1 inhibits ASK1 kinase activity. A, requirement of PDK1 kinase activity for PDK1-mediated regulation of ASK1 kinase activity. HEK293 cells were transiently transfected with HA-ASK1 in the presence or absence of Myc-tagged PDK1 (WT and KD). Cell lysates were then subjected to immunoprecipitation with an anti-HA antibody, and the resulting immunoprecipitates were subjected to an in vitro kinase assay using GST-tagged MKK6(K82A) as the substrate to determine ASK1 kinase activity (left). Approximately 5 μg of recombinant GST-tagged wild-type ASK1 (re.ASK1) or ASK1(K) (re.ASK1(K)) proteins were incubated at room temperature for 1 h with the amount of recombinant GST-tagged PDK1 (re.PDK1) indicated in 30 μl of 20 mm Tris-HCl buffer (pH 7.5), and ASK1 kinase activity was determined by an in vitro kinase assay using GST-tagged MKK6(K82A) as a substrate (right). The circled letters P indicate phosphorylation. B, PDK1 phosphorylates ASK1. HEK293 cells transiently expressing GST-tagged PDK1 (WT and KD) were treated (+) or untreated (−) with specific Akt inhibitors (VIII, 20 μm; SH-5, 10 μm) for 30 min. GST-PDK1 (WT and KD), precipitated using glutathione-Sepharose beads, was assayed for its kinase activity in the presence of kinase buffer containing ∼3–4 μg of recombinant GST-tagged ASK1(K709R) substrate. C, identification of PDK1 phosphorylation sites on ASK1. The recombinant GST-tagged PDK1 (re.PDK1) was analyzed for its kinase activity by an in vitro kinase assay using recombinant GST-tagged ASK1(K709R) and one of its substitution mutants, ASK1(S83A), ASK1(T838A), ASK1(S967A), or ASK1(S1034A), as substrates. GST alone was used as a nonspecific control. WB, Western blot; IP, immunoprecipitation.
PDK1 Inhibits H2O2-induced ASK1-JNK/p38 Signaling
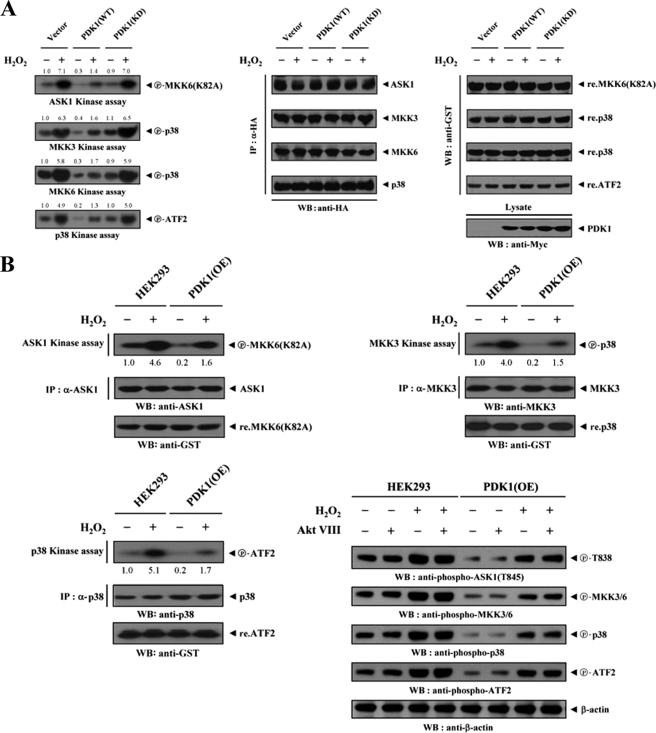
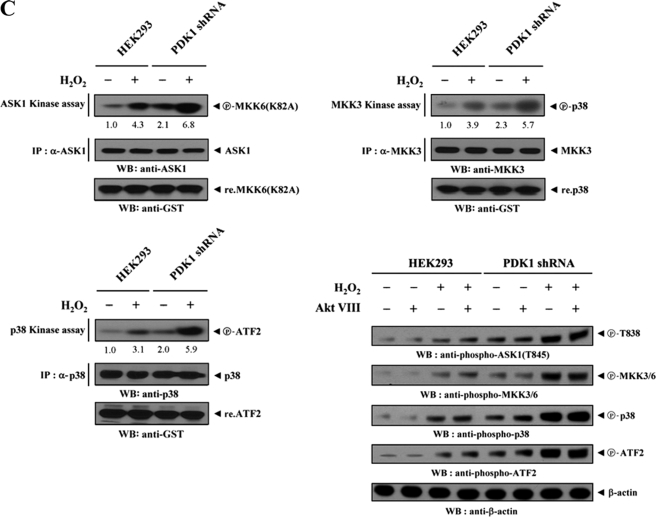
To assess whether PDK1, an interacting partner of ASK1, could influence signaling downstream of ASK1, we assayed the activation of ASK1 and its downstream targets MKK3/6 and p38. HEK293 cells were transfected with combinations of HA-ASK1, HA-MKK3, HA-MKK6, HA-p38, empty Myc vector, and Myc-tagged PDK1 (WT and KD), and then treated with H2O2. ASK1, MKK3, MKK6, and p38 kinase activities were then evaluated in HA immunoprecipitates by in vitro kinase assays. The results showed that wild-type PDK1 significantly inhibited the kinase activities of ASK1, MKK3, MKK6, and p38, whereas these inhibitory effects were not detectable in cells expressing kinase-dead PDK1 (Fig. 4A). This result provides evidence that, in addition to the binding of PDK1 to ASK1, the kinase activity of PDK1 is required for the regulation of ASK1-mediated signaling. To confirm further this effect, parental HEK293 cells or HEK293 cells (PDK1(OE)) stably overexpressing PDK1 were either untreated or treated with H2O2. Anti-ASK1, anti-MKK3, and anti-p38 antibodies were then used for immunoprecipitation, and endogenous ASK1, MKK3, and p38 kinase activities were assessed by in vitro kinase assays and immunoblottings. As expected, H2O2 stimulated the kinase activities of ASK1, MKK3, and p38 in parental HEK293 cells sufficiently, but such effects were significantly decreased in PDK1(OE) cells (Fig. 4B). Consistent with this result, the knockdown of endogenous PDK1 using HEK293 cells (PDK1 shRNA) stably expressing PDK1-specific shRNA showed an opposite trend in the modulation of ASK1, MKK3, and p38 kinase activities (Fig. 4C). Similar results were also observed in the immunoblot analyses using anti-phospho-specific antibodies for ASK1 Thr845, MKK3/6, p38, and ATF2, regardless of the presence of a specific Akt inhibitor VIII (Fig. 4, B and C, lower right). These findings pointed to the inhibition of ASK1-mediated signaling to p38 kinase upon PDK1 binding.
FIGURE 4.
PDK1 inhibits ASK1 downstream signaling. Transiently transfected HEK293 cells expressing an empty vector (Vector), PDK1(WT), or PDK1(KD) (A); parental HEK293 cells (B and C); and HEK293 cells (PDK1(OE)) stably overexpressing PDK1 or HEK293 cells (PDK1 shRNA) stably expressing PDK1-specific shRNA (B and C) were incubated with or without 2 mm H2O2 for 30 min. The activities of ASK1, MKK3, MKK6, or p38 were determined by in vitro kinase assays or immunoblot analyses using the immunoprecipitates indicated or total cell lysates treated (+) or untreated (−) with a specific Akt inhibitor VIII (Akt VIII). The overexpression or knockdown level of endogenous PDK1 was determined by anti-PDK1 immunoblotting (see supplemental Fig. 3). The relative level of kinase activity was quantitated by densitometric analyses, and the -fold increase relative to untreated samples in control cells containing an empty vector alone or parental HEK293 cells was calculated. The circled letters P indicate phosphorylation. WB, Western blot; IP, immunoprecipitation.
PDK1 Inhibits ASK1 Activity by Modulating Complex Formation between ASK1 and 14-3-3 Protein or Its Substrate MKK3
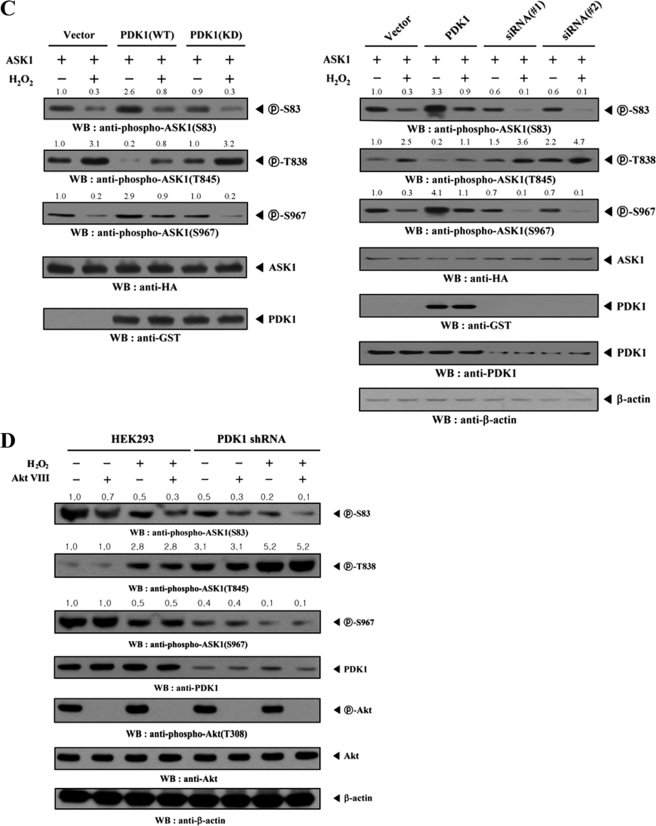
It has been shown previously that 14-3-3 protein interacts with ASK1 and suppresses the ASK1 proapoptotic function (16). As illustrated in Fig. 4, which shows the effect of PDK1 on ASK1 activity, PDK1 inhibited ASK1-mediated signaling. We therefore investigated whether PDK1 could modulate 14-3-3 protein binding to ASK1 in HEK293 cells. Compared with control cells expressing ASK1 and 14-3-3 protein in the absence of PDK1, the expression of wild-type PDK1 significantly increased complex formation between ASK1 and 14-3-3 protein in a dose-dependent manner. In contrast, kinase-dead PDK1 had no effect on complex formation (Fig. 5A, left). As expected, an opposite result was also obtained from the immunoblot analysis of the association between endogenous ASK1 and 14-3-3 protein in the presence of PDK1-specific siRNA (Fig. 5A, right). These results suggested that PDK1 inhibits ASK1 activity by stabilizing the ASK1–14-3-3 protein complex. Consistent with this observation, we also noted a similar effect of PDK1 on the association between ASK1 and thioredoxin, another negative regulator of ASK1 (12) (data not shown). We next evaluated the influence of PDK1 on ASK1-MKK3 complex formation, because ASK1-interacting proteins, such as HSP72 and MPK38, have been previously reported to regulate ASK1 activity by modulating the association between ASK1 and its substrate MKK3 (14, 17). In contrast to the results illustrated in Fig. 5A, a significant decrease in the association between ASK1 and MKK3 was detectable in cells expressing wild-type PDK1 but not in cells expressing kinase-dead PDK1 (Fig. 5B, left). The knockdown of endogenous PDK1 consistently showed an opposite trend in modulating the association between ASK1 and MKK3 (Fig. 5B, right).
FIGURE 5.
PDK1-mediated inhibition of ASK1 activity is dependent on the modulation of the association between ASK1 and its regulators, 14-3-3 and MKK3, and ASK1 phosphorylation. A and B, modulation of the endogenous association of ASK1 with 14-3-3 protein and MKK3. HEK293 cells were transfected with the indicated combinations of FLAG-14-3-3, FLAG-ASK1, HA-MKK3, HA-ASK1, Myc-tagged PDK1 (WT and KD), GST-PDK1, and PDK1-specific siRNA. Cell lysates were then subjected to immunoprecipitation with the antibodies indicated, and the resulting immunoprecipitates were analyzed by immunoblot analysis using the appropriate antibodies to determine the association of 14-3-3 protein (A) and MKK3 (B) with ASK1. The knockdown effect of endogenous PDK1 on the association between endogenous ASK1 and 14-3-3 protein or MKK3 was determined by immunoblot analysis with the antibodies indicated using HEK293 cells transiently transfected with PDK1-specific siRNA 1 (A and B, right panels). C, HEK293 cells were transiently transfected with the following expression vectors: empty vector (Vector), PDK1 (WT and KD), or PDK1 siRNAs (#1 and #2, each 200 nm) in the presence of ASK1. The cells were then treated with or without 2 mm H2O2 for 30 min. ASK1 phosphorylation was determined by immunoblot analysis using anti-phospho-ASK1(Ser83), anti-phospho-ASK1(Thr845), and anti-phospho-ASK1(Ser967) antibodies. D, parental HEK293 cells or HEK293 cells (PDK1 shRNA) stably expressing PDK1-specific shRNA were incubated for 30 min in the presence or absence of a specific Akt inhibitor VIII (20 μm). Cell lysates were examined for ASK1 phosphorylation (Ser83, Thr838, and Ser967) by immunoblot analysis using the antibodies indicated. The knockdown level of endogenous PDK1 was determined by anti-PDK1 immunoblotting (fourth panel). The relative level of phosphorylation was quantitated by densitometric analyses, and the -fold increase relative to untreated control cells expressing an empty vector alone in the presence of ASK1 or parental cells was calculated. The circled letters P indicate phosphorylation. WB, Western blot; IP, immunoprecipitation.
PDK1-mediated Inhibition of ASK1 Activity Involves Different Phosphorylation Events on ASK1
ASK1 is known to be phosphorylated at Ser83, Thr845, and Ser967 and is regulated in both a positive (at Thr845) and a negative (at Ser83 and Ser967) manner (33). From our studies, PDK1 appears to phosphorylate ASK1 at Ser967 through physical interaction (see Fig. 3C). To determine whether the suppressive effect of PDK1 on ASK1-p38/JNK signaling is dependent on the phosphorylation level of ASK1 at these sites, we examined ASK1 phosphorylation in HEK293 cells expressing wild-type or kinase-dead PDK1, in the presence of ASK1, using immunoblot analysis with phospho-specific antibodies against Ser(P)83, Thr(P)845, and Ser(P)967 on ASK1. Expression of wild-type PDK1 considerably alleviated H2O2-induced reduction of ASK1 phosphorylation at Ser83 and Ser967, whereas expression of kinase-dead PDK1 had no effect on ASK1 phosphorylation at Ser83 and Ser967 (Fig. 5C, left, top and third panels). In contrast, H2O2-induced stimulation of ASK1 phosphorylation at Thr845 (Thr838 in human ASK1) was significantly decreased by the expression of wild-type PDK1 compared with the control expressing an empty vector (Fig. 5C, left, second panel). However, such a change was not observed in cells expressing kinase-dead PDK1. Consistent with these results, the knockdown of endogenous PDK1, using either transfection of PDK1-specific siRNAs (Fig. 5C, right) or HEK293 cells (PDK1 shRNA) stably expressing PDK1-specific shRNA (Fig. 5D), resulted in a marked decrease in the phosphorylation levels of Ser83 and Ser967. PDK1 knockdown also caused an increase in the phosphorylation level of Thr838 when compared with parental cells or with the control expressing an empty vector. These findings indicated that PDK1-mediated inhibition of ASK1 function involves the modulation of the phosphorylation level of ASK1 at Ser83 and Thr838 in addition to its direct effect on the phosphorylation of Ser967.
PDK1 Suppresses ASK1-induced AP-1 Transcriptional Activity in a Kinase-dependent Manner
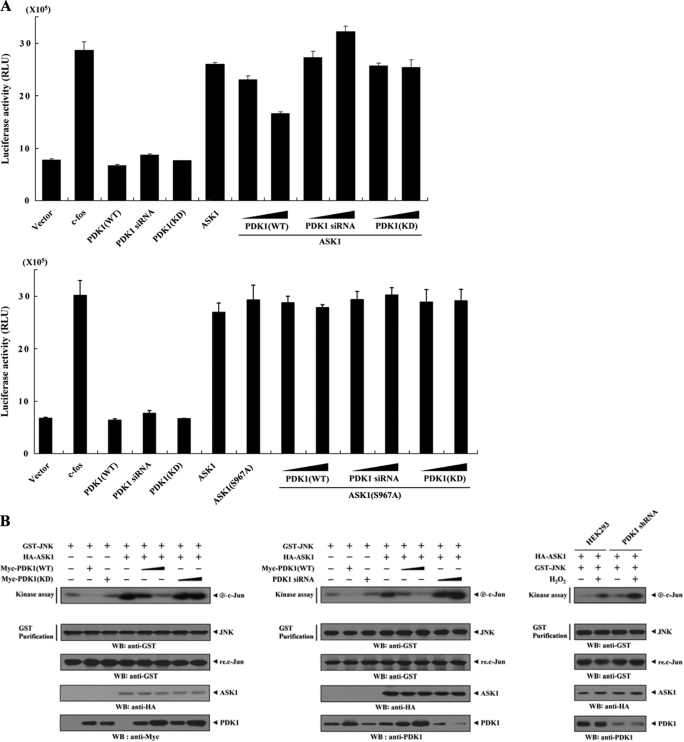
To determine the role of PDK1 in ASK1 function, we performed reporter assays to examine the effect of PDK1 on ASK1-induced activation of the AP-1 luciferase reporter gene. As shown in Fig. 6A, coexpression of wild-type PDK1 with ASK1 induced a marked decrease in AP-1-dependent luciferase activity in a dose-dependent manner (top, compare lane 6 with lanes 7 and 8). Consistent with this result, ASK1-induced AP-1 transcriptional activity was significantly increased in PDK1 knockdown cells compared with control cells expressing ASK1 alone (Fig. 6A, top, compare lane 6 with lanes 9 and 10). In order to investigate whether the kinase activity of PDK1 can influence the modulation of ASK1-mediated transactivation, we also analyzed the effect of kinase-dead PDK1 on ASK1-induced AP-1 transcriptional activity. No effect on ASK1-induced AP-1 transcriptional activity was detected in the presence of kinase-dead PDK1 (Fig. 6A, top, compare lane 6 with lanes 11 and 12), indicating that PDK1 kinase activity is essential for regulating ASK1-induced AP-1 transcriptional activity. To analyze further the cardinal role of PDK1 kinase activity in ASK1 function, we compared the effect of PDK1 on ASK1-mediated transactivation in the presence of wild-type ASK1 with its effect in the presence of ASK1 mutant S967A. Results showed that PDK1 had little effect on ASK1(S967A)-mediated transactivation (Fig. 6A, bottom, compare lane 7 with lanes 8 and 9). This result indicated an important role of ASK1 phosphorylation at Ser967 in the PDK1-mediated suppression of ASK1 function. Next, we examined the effect of PDK1 on ASK1-induced JNK activation using HEK293 cells expressing GST-JNK and HA-ASK1 in the presence or absence of wild-type and kinase-dead PDK1. ASK1-induced JNK activation was noticeably decreased by wild-type PDK1 in a dose-dependent manner, whereas kinase-dead PDK1 had no effect (Fig. 6B, left). Consistently, ASK1-induced JNK activation was greatly elevated by knockdown of endogenous PDK1 (Fig. 6B, middle and right). These data suggested that PDK1 might act as a negative regulator of ASK1-JNK/p38 signaling.
FIGURE 6.
PDK1 suppresses JNK-mediated transcription and H2O2-induced apoptosis. A, 293T cells were transiently transfected with the indicated combinations of expression vectors encoding c-fos (0.6 μg), PDK1 (WT and KD) (0.2 and 0.6 μg), wild-type and mutant (S967A) ASK1 (each 0.6 μg), and PDK1-specific siRNA 1 (50 and 200 nm) together with AP-1 luciferase plasmid (0.2 μg) and an expression plasmid (0.2 μg) for β-galactosidase as an internal control. Luciferase activity was measured 48 h after transfection and normalized to β-galactosidase activity. B, inhibition of JNK activity by PDK1. HEK293 cells were transfected with HA-ASK1 and GST-JNK in the presence or absence of increasing amounts of PDK1 (WT and KD) or PDK1-specific siRNA 1. Cell lysates were prepared and analyzed for JNK activity by an in vitro kinase assay using c-Jun as a substrate. JNK activity was also determined in PDK1 knockdown cells (PDK1 shRNA) transfected with HA-ASK1 and GST-JNK in the presence or absence of H2O2 (right). C and D, HEK293 cells transiently expressing the indicated combinations of PDK1 (WT and KD) (1 and 3 μg), wild-type ASK1 (1 and 3 μg), and PDK1- and ASK1-specific siRNAs (100 and 200 nm) were incubated with or without H2O2 (1 mm) for 9 h in the presence or absence of a specific Akt inhibitor VIII (20 μm). Apoptotic cell death was determined using the GFP expression system (GFP) or terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL), as described under “Materials and Methods.” E, HEK293 cells harboring stably integrated pSingle-tTS-shRNA empty vector (Vector) or pSingle-tTS-shRNA vector containing PDK1-specific shRNA (PDK1 shRNA (inducible)) were cultured in the presence or absence of 1 μg/ml doxycycline (Dox) for 72 h to determine the activities of ASK1, MKK3, MKK6, or p38. Inducible silencing of endogenous PDK1 expression by doxycycline was assessed by immunoblotting using an anti-PDK1 antibody. β-Actin was used as a loading control. The circled letters P indicate phosphorylation. WB, Western blot; RLU, relative luciferase units.
PDK1 Suppresses H2O2-induced Apoptosis by Inhibiting Caspase-3 Activity
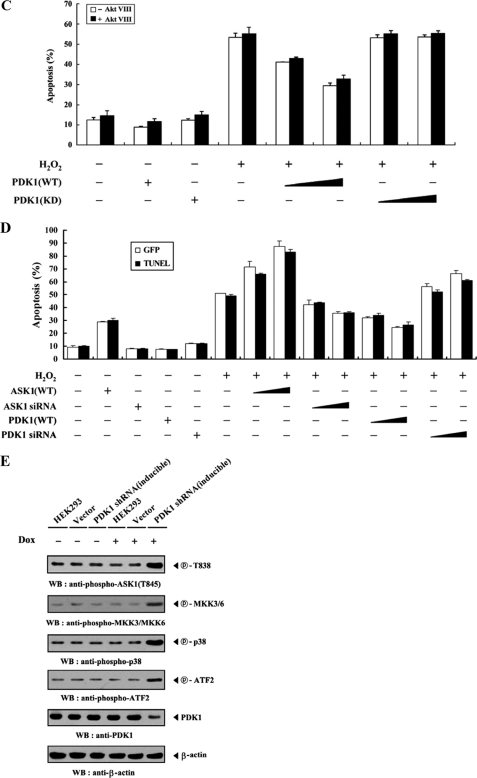
To characterize the roles of PDK1 in ASK1-JNK/p38 signaling further, we investigated the effect of PDK1 on H2O2-induced apoptosis using the GFP system and TUNEL staining (17). As expected, wild-type PDK1 resulted in a significant decrease of H2O2-induced apoptosis in a dose-dependent manner, whereas the expression of kinase-dead PDK1 had no effect, regardless of the presence of a specific Akt inhibitor VIII (Fig. 6C). These observations indicated that PDK1 kinase activity is also required for the regulation of H2O2-induced apoptosis. We then examined the effects of endogenous PDK1 knockdown on H2O2-induced apoptosis. Consistent with the result described above, transfection of HEK293 cells with siRNA duplexes targeting PDK1 resulted in a significant increase in H2O2-induced apoptosis, proportional to the amount of siRNA used in the transfection (Fig. 6D, compare lane 6 with lanes 13 and 14). To confirm physiologically the negative role of PDK1 in ASK1-JNK/p38 signaling, we also generated a stable system (inducible PDK1 shRNA) for the tetracycline-inducible expression of PDK1 shRNA in HEK293 cells. We used this system in conjunction with immunoblot analysis using anti-phospho-specific antibodies for ASK1 Thr845, MKK3/6, p38, and ATF2 to investigate the endogenous kinase activities of ASK1, MKK3/6, and p38. PDK1 knockdown cells showed a higher level of phosphorylation of ASK1 Thr838, MKK3/6, p38, and ATF2 in the presence of doxycycline, a tetracycline analogue, compared with either parental HEK293 cells (HEK293) or with HEK293 cells (Vector) stably expressing the empty vector alone (Fig. 6E). This result clearly implied that PDK1 physically associates with ASK1 and negatively regulates ASK1-mediated signaling. We also investigated whether PDK1-mediated suppression of H2O2-induced apoptosis affects caspase-3 activation, thereby facilitating the proteolytic cleavage of PARP, an in vivo substrate of capase-3, because caspase signaling was involved in ASK1-mediated apoptosis (34, 35). Expression of wild-type PDK1 significantly inhibited H2O2-induced capase-3 activity and PARP cleavage in a dose-dependent manner, whereas there was no effect in the presence of kinase-dead PDK1 (supplemental Fig. 4). These results suggested that caspase-3 inactivation is involved in PDK1-mediated suppression of H2O2-induced apoptosis.
ASK1 Inhibits PDK1 Function by Phosphorylating PDK1 at Ser394 and Ser398
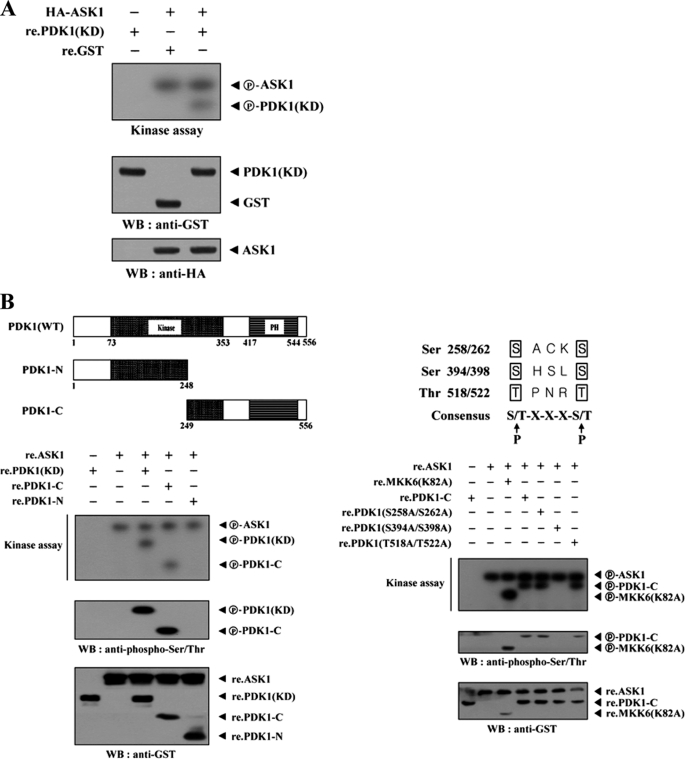
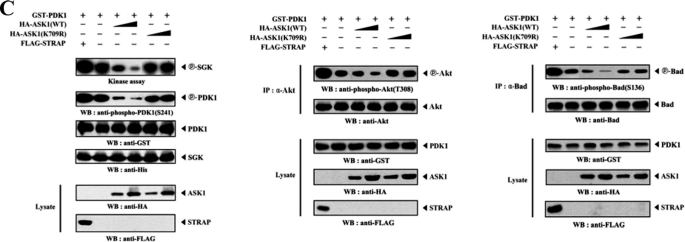
Using the same approach, we examined whether PDK1 could act as a substrate for ASK1. ASK1 was immunoprecipitated from HEK293 cells transfected with HA-ASK1, and its kinase activity was determined by an in vitro kinase assay using recombinant nonphosphorylated PDK1 as a substrate. The immunoprecipitated ASK1 was able to phosphorylate the recombinant PDK1, suggesting that PDK1 can function as an ASK1 substrate in HEK293 cells (Fig. 7A). An identical result was also obtained using recombinant ASK1 protein instead of the immunoprecipitated ASK1 (data not shown). To identify potential phosphorylation sites in PDK1, we first generated two recombinant PDK1 deletion constructs, PDK1-N (amino acids 1–248) and PDK1-C (amino acids 249–556) and assessed their phosphorylation status using an in vitro kinase assay. ASK1 induced the phosphorylation of PDK1-C but not PDK1-N (Fig. 7B, left). This result indicates that the C-terminal region of PDK1 is required for ASK1-mediated phosphorylation. To define the ASK1 phosphorylation sites of PDK-C more precisely, we searched for consensus ASK1 phosphorylation sites ((S/T)XXX(S/T)) and performed in vitro kinase assays using three amino acid substitution mutants, S258A/S262A, S394A/S398A, and T518A/T522A, of PDK1-C. The S394A/S398A mutant completely abolished ASK1-mediated phosphorylation, whereas ASK1-mediated phosphorylation was detected in the presence of the other two substitution mutants, S258A/S262A and T518A/T522A, indicating that Ser394 and Ser398 of PDK1 are potential phosphorylation sites for ASK1 (Fig. 7B, right). The next step was to investigate whether ASK1-mediated PDK1 phosphorylation can affect PDK1 activity as well as that of its downstream targets, such as PKB/Akt and Bad (36). We thus performed cotransfection experiments with plasmid vectors expressing GST-PDK1, HA-tagged ASK1 (WT and K709R), and a positive control FLAG-STRAP (28) to assess the kinase activity of PDK1 and the phosphorylation levels of Akt and Bad. As shown in Fig. 7C, ASK1 significantly decreased PDK1 kinase activity, Akt phosphorylation, and Bad phosphorylation in a kinase-dependent manner. These results suggested that Ser394 and Ser398 of PDK1, potential phosphorylation sites for ASK1, might play an important role in the negative regulation of PDK1 activity.
FIGURE 7.
ASK1 phosphorylates PDK1. A, for the in vitro kinase assay, ∼5 μg of recombinant PDK1(KD) proteins were mixed with 20 μm ATP, 0.3 μCi of [γ-32P]ATP, and 20 mm MgCl2 in 30 μl of kinase buffer and incubated with the HA immunoprecipitates for HA-ASK1 at 30 °C for 30 min with frequent gentle mixing. B, identification of ASK1 phosphorylation sites on PDK1. The recombinant ASK1 protein was analyzed for its kinase activity by an in vitro kinase assay using recombinant PDK1-N, PDK1-C, substitution mutants (S258A/S262A, S394A/S398A, or T518A/T522A) of PDK1-C, and the positive control MKK6(K82A) as substrates. C, effect of ASK1 on PI3K/PDK1-mediated signaling. HEK293 cells, transfected with the expression vectors indicated, were lysed, and the GST precipitates were then analyzed for PDK1 activity by an in vitro kinase assay using serum/glucocorticoid-regulated kinase as a substrate and by immunoblot analysis with an anti-phospho-PDK1(Ser241) antibody (left). The Akt and Bad immunoprecipitates were also analyzed for Akt and Bad activation by immunoblotting with anti-phospho-Akt(Thr308) and anti-phospho-Bad(Ser136) antibodies (middle and right). The circled letters P indicate phosphorylation. re., recombinant. WB, Western blot.
ASK1 Alleviates PDK1-mediated Suppression of Apoptosis in a Kinase-dependent Manner
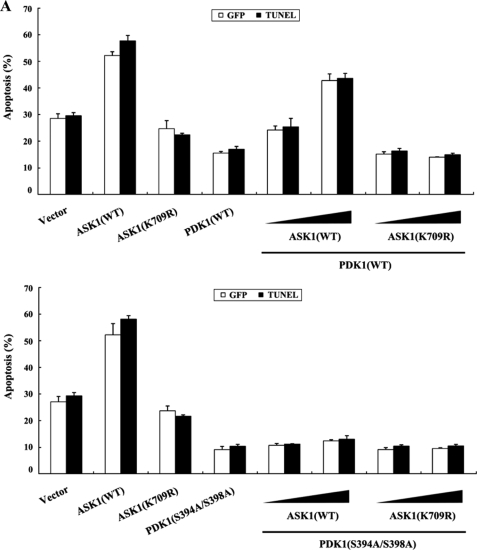
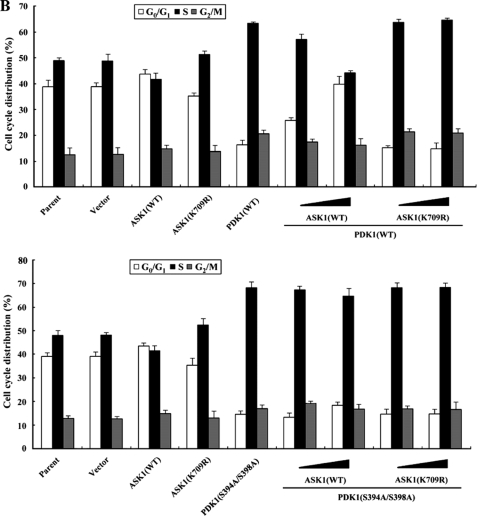
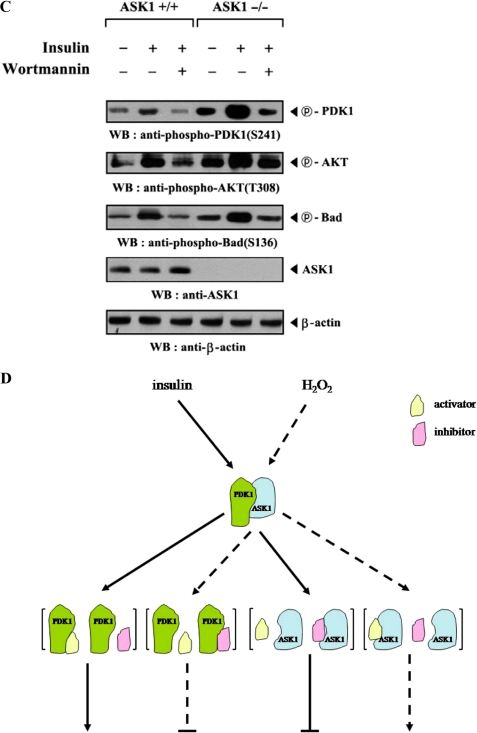
To investigate whether ASK1 can regulate the PDK1-mediated suppression of apoptosis, we analyzed the effect of ASK1 on PDK1-mediated suppression of TNF-α-induced apoptosis using the GFP system and TUNEL staining. In the presence of TNF-α, the expression of PDK1 resulted in a significant decrease in apoptotic cell death compared with control cells expressing an empty vector. However, wild-type ASK1, but not kinase-dead ASK1, alleviated this suppression in a dose-dependent manner (Fig. 8A, top, compare lane 4 with lanes 5 and 6). This result implied that ASK1 contributes to the modulation of the PDK1-mediated survival-signaling pathway through phosphorylation of PDK1. To verify the negative role of ASK1 in PI3K/PDK1 signaling, we also examined the ability of ASK1 to modulate serum-induced cell growth, one of the most important biological functions of PI3K/PDK1 signaling, in HaCaT cells using flow cytometry (30). As shown in Fig. 8B (top), coexpression of wild-type ASK1 with PDK1 significantly decreased the percentage of cells in S phase in a dose-dependent manner compared with control cells expressing PDK1 alone (63% versus 44%), whereas kinase-dead ASK1 had no effect in the presence of PDK1. These findings indicated that ASK1 also plays an important role in the regulation of PDK1-mediated stimulation of cell growth, probably through direct interaction and phosphorylation of PDK1 at Ser394 and Ser398. To investigate this hypothesis, we compared the effect of ASK1 on PDK1-mediated cell survival signaling in the presence of wild-type PDK1 with its effect in the presence of the PDK1 mutant S394A/S398A, because the S394A/S398A mutant was found to be defective in ASK1-mediated phosphorylation (see Fig. 7B). Results showed that ASK1 coexpression had little or no effect on PDK1(S394A/S398A)-mediated suppression of apoptosis or stimulation of cell growth when compared with the control expressing PDK1(S394A/S398A) alone (Fig. 8, A and B, lower panels). In addition, almost identical results were obtained when the comparison was made in a single experiment (supplemental Fig. 5). To confirm further the negative role of ASK1 in the regulation of PDK1 activity, we also compared the effect of ASK1 on PDK1 activation in ASK1-intact mouse embryonic fibroblasts (MEFs) with that in ASK1-deficient MEFs. PDK1 activity in the ASK1-deficient MEFs was much higher than in the ASK1-intact MEFs (Fig. 8C), again supporting the involvement of ASK1 as a negative regulator in PI3K/PDK1-mediated signaling.
FIGURE 8.
ASK1 negatively regulates PDK1-dependent apoptosis and cell growth. A, effect of ASK1 on PDK1-mediated apoptosis. 293T cells were transiently transfected with the expression vectors indicated. After 24 h, cells were treated with TNF-α (20 ng/ml) and cycloheximide (10 μg/ml) for 14 h to induce apoptosis. Apoptotic cell death was determined by the GFP system and TUNEL staining. B, effect of ASK1 on PDK1-mediated cell cycle progression. HaCaT cells (2 × 105/dish), transfected with the indicated combinations of plasmid vectors, were synchronized in G0/G1 by hydroxyurea treatment (30). Cells were collected after 10% serum treatment for 24 h and evaluated for determination of cell numbers in G0/G1, S, and G2/M phases by flow cytometry analysis. PDK1(S394A/S398A), the wild-type PDK1 mutant S394A/S398A. C, ASK1-intact MEFs or ASK1-deficient MEFs were incubated for 30 min with or without 100 nm wortmannin and then treated with 100 nm insulin for 20 min. The cell lysates were subjected to immunoblot analysis with the antibodies indicated. β-Actin was used as a loading control. D, a model for the regulation of PDK1-ASK1 complex formation in response to signals that act on PDK1 or ASK1. The dotted and solid lines represent ASK1 and PDK1 signals, respectively. Details of this model are given under “Discussion.” WB, Western blot.
DISCUSSION
Our results from this study have provided evidence that PDK1, a pivotal kinase controlling cell survival signaling, inhibits ASK1 and its downstream signaling pathways through direct interaction and phosphorylation. PDK1 inactivates ASK1 by phosphorylating ASK1 at Ser967, previously identified as a binding site for 14-3-3 protein (16). Given that 14-3-3 protein inhibits ASK1 function through binding to phosphorylated Ser967, PDK1 may be a key regulator for the 14-3-3-mediated suppression of ASK1 activity. On the other hand, our data showed that ASK1 phosphorylates PDK1 at Ser394 and Ser398, sites similar to the consensus sequences identified for ASK1 substrates, leading to the inhibition of PDK1 activity. This observation suggests that the phosphorylation of PDK1 at Ser394 and Ser398 is apparently involved in the negative regulation of PDK1 function.
In addition to protein-protein interactions by a number of ASK1-interacting proteins, it has also been found that reversible phosphorylation at specific sites, including Ser83, Thr845, Ser967, and Ser1034 of ASK1, can regulate ASK1 activity (33). Phosphorylation of Thr845 (corresponding to Thr838 in human ASK1) has been correlated with the activation of ASK1, whereas the phosphorylation levels of Ser83, Ser967, and Ser1034 were proportional to the inhibition of ASK1 activity. Therefore, it is possible that other intracellular survival or death signaling events, in which kinases/phosphatases act as essential mediators, are linked to the regulation of ASK1 function. For instance, Akt/PKB, a downstream kinase in the PI3K/PDK1 signaling pathway, binds to and phosphorylates Ser83 of ASK1, resulting in the inhibition of the ASK1 proapoptotic function (7). In addition, MPK38 and ASK2 phosphorylate the autophosphorylation site (Thr845) of ASK1, known to be critical for ASK1 activation, and stimulate ASK1-mediated apoptosis (17, 18). However, the kinases responsible for the phosphorylation of ASK1 at Ser967 and Ser1034 have not yet been identified. In this study, we investigated whether the four phosphorylation sites (Ser83, Thr845, Ser967, and Ser1034) on ASK1 are involved in PDK1-mediated phosphorylation. Our data demonstrated that, in the presence of PDK1, ASK1 is phosphorylated at Ser967 for 14-3-3 protein binding but not at Ser83, Thr838, and Ser1034 (Fig. 3, B and C). The knockdown and overexpression of endogenous PDK1 coincidentally decreases and increases the in vivo phosphorylation levels of Ser967, respectively (Fig. 5D) (data not shown). This result suggested that PDK1 is a putative Ser967 kinase, responsible for Ser967 phosphorylation for 14-3-3 binding in cells. It is equally possible that the Ser967 phosphorylation of ASK1 is mediated through the other protein kinases associated with PDK1 because PDK1 has been shown to interact with a variety of cellular protein kinases (20–23). However, our data showed that PDK1 directly phosphorylates ASK1 at Ser967 (Figs. 3–6 and supplemental Fig. 2), making this possibility unlikely.
Although an important role of PDK1 in cell survival signaling has been well characterized, the regulatory mechanism controlling PDK1 activity remains controversial. To date, there is no evidence that any particular protein kinase regulates the kinase activity of PDK1. In these studies, we have examined whether PDK1 functions as an ASK1 substrate because PDK1 and ASK1 interact with each other in vivo and in vitro (Fig. 1). ASK1 was able to phosphorylate PDK1 at Ser394 and Ser398 as determined by an in vitro kinase assay, leading to the suppression of PDK1 and its downstream targets (Figs. 7 and 8). This finding suggests that PDK1 may be a direct target for ASK1 and provides the first evidence that PDK1 activity can be regulated by a specific intracellular kinase known to be involved in cell death signaling. Indeed, ASK1-mediated phosphorylation of PDK1 at Ser394 and Ser398 suppresses PDK1 functions, such as apoptosis and cell growth, as illustrated in Fig. 8. However, the PDK1(S394A/S398A) mutant in itself exhibited a slightly enhanced activity of both PDK1-mediated suppression of apoptosis and PDK1-induced cell growth compared with the control wild-type PDK1 (Fig. 8, A and B, and supplemental Fig. 5), implying that the PDK1 phosphorylation at Ser394 and Ser398 is not enough for the inhibition of PDK1 function. Thus, it is tempting to speculate that, in addition to ASK1, other PDK1-associated kinases may be involved in the negative regulation of PDK1 function. Based on this, an investigation of the effect of MPK38, a serine/threonine kinase (37), on PDK1 function is now under way because MPK38 differentially phosphorylates, compared with ASK1, and inactivates PDK1 (data not shown). Moreover, the endogenous association between PDK1 and ASK1 was significantly decreased by ASK1 stimuli, including H2O2, TNF-α, thapsigargin, and ionomycin, as well as insulin treatment for PDK1 activation (Fig. 2, B and C). These results suggested that the physical association between endogenous PDK1 and ASK1, maintained during the resting state of the cell, contributes to the preservation of PDK1 and ASK1 in an inactive state until the cell is activated by PDK1 or ASK1 signals. We therefore propose the following model (Fig. 8D) as a potential mechanism for PDK1 or ASK1 activation in response to signals that induce the dissociation of a PDK1-ASK1 complex. In our model, the complex is inactive in resting cells but is induced to dissociate in response to stimuli, such as insulin or H2O2. This dissociation leads to the stimulation of the respective signaling pathways via modulation of the binding between PDK1 (or ASK1) and its positive or negative regulators, including STRAP, thioredoxin, MKK3, and 14-3-3 protein (supplemental Fig. 6).
In summary, we have demonstrated that PDK1 is a putative kinase for the phosphorylation of ASK1 at Ser967, which is required for 14-3-3 protein binding. Our studies have defined a novel mechanism wherein ASK1 directly interacts and phosphorylates Ser394 and Ser398 of PDK1, thereby suppressing PDK1 function, and provides evidence that Ser394 and Ser398 of PDK1 represent putative phosphorylation sites for the negative regulation of PDK1 activity. Furthermore, our current data strongly suggest that ASK1 and PDK1 may act as major points of integration for cell survival and death-inducing signals and that their relationship is important in deciding whether the cell is destined for survival or death.
Supplementary Material
This work was supported by Korea Science and Engineering Foundation Grant R0A-2007-000-20006-0.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
- PKB
- protein kinase B
- MAPKKK
- mitogen-activated protein kinase kinase kinase
- MAPKK
- mitogen-activated protein kinase kinase
- PI3K
- phosphatidylinositol 3-kinase
- JNK
- c-Jun NH2-terminal kinase
- siRNA
- small interfering RNA
- shRNA
- short hairpin RNA
- GST
- glutathione S-transferase
- GFP
- green fluorescent protein
- WT
- wild type
- KD
- kinase-dead
- HA
- hemagglutinin
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick end labeling
- PH
- pleckstrin homology
- TNFα
- tumor necrosis factor α
- MEF
- mouse embryo fibroblast.
REFERENCES
- 1.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 2.Cardone M. H., Roy N., Stennicke H. R., Salvesen G. S., Franke T. F., Stanbridge E., Frisch S., Reed J. C. (1998) Science 282, 1318–1321 [DOI] [PubMed] [Google Scholar]
- 3.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 4.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. (1997) Cell 91, 231–241 [DOI] [PubMed] [Google Scholar]
- 5.Hetman M., Cavanaugh J. E., Kimelman D., Xia Z. (2000) J. Neurosci. 20, 2567–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kops G. J., de Ruiter N. D., De Vries-Smits A. M., Powell D. R., Bos J. L., Burgering B. M. (1999) Nature 398, 630–634 [DOI] [PubMed] [Google Scholar]
- 7.Kim A. H., Khursigara G., Sun X., Franke T. F., Chao M. V. (2001) Mol. Cell. Biol. 21, 893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J., Fujii K., Zhang L., Roberts T., Fu H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang H. Y., Nishitoh H., Yang X., Ichijo H., Baltimore D. (1998) Science 281, 1860–1863 [DOI] [PubMed] [Google Scholar]
- 10.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. (1997) Science 275, 90–94 [DOI] [PubMed] [Google Scholar]
- 11.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. (1998) EMBO J. 17, 2596–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H., Nishitoh H., Ichijo H., Kyriakis J. M. (2000) Mol. Cell. Biol. 20, 2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita K., Saitoh M., Tobiume K., Matsuura H., Enomoto S., Nishitoh H., Ichijo H. (2001) EMBO J. 20, 6028–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H. S., Cho S. G., Kim C. K., Hwang H. S., Noh K. T., Kim M. S., Huh S. H., Kim M. J., Ryoo K., Kim E. K., Kang W. J., Lee J. S., Seo J. S., Ko Y. G., Kim S., Choi E. J. (2002) Mol. Cell. Biol. 22, 7721–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song J. J., Rhee J. G., Suntharalingam M., Walsh S. A., Spitz D. R., Lee Y. J. (2002) J. Biol. Chem. 277, 46566–46575 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L., Chen J., Fu H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 8511–8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung H., Seong H. A., Ha H. (2008) J. Biol. Chem. 283, 34541–34553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K., Shimozono R., Noguchi T., Umeda T., Morimoto Y., Naguro I., Tobiume K., Saitoh M., Matsuzawa A., Ichijo H. (2007) J. Biol. Chem. 282, 7522–7531 [DOI] [PubMed] [Google Scholar]
- 19.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 20.Fayard E., Tintignac L. A., Baudry A., Hemmings B. A. (2005) J. Cell Sci. 118, 5675–5678 [DOI] [PubMed] [Google Scholar]
- 21.Hinton H. J., Alessi D. R., Cantrell D. A. (2004) Nat. Immunol. 5, 539–545 [DOI] [PubMed] [Google Scholar]
- 22.Kikani C. K., Dong L. Q., Liu F. (2005) J. Cell. Biochem. 96, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 23.Mora A., Komander D., van Aalten D. M., Alessi D. R. (2004) Semin. Cell Dev. Biol. 15, 161–170 [DOI] [PubMed] [Google Scholar]
- 24.Casamayor A., Morrice N. A., Alessi D. R. (1999) Biochem. J. 342, 287–292 [PMC free article] [PubMed] [Google Scholar]
- 25.Grillo S., Grémeaux T., Casamayor A., Alessi D. R., Le Marchand-Brustel Y., Tanti J. F. (2000) Eur. J. Biochem. 267, 6642–6649 [DOI] [PubMed] [Google Scholar]
- 26.Park J., Hill M. M., Hess D., Brazil D. P., Hofsteenge J., Hemmings B. A. (2001) J. Biol. Chem. 276, 37459–37471 [DOI] [PubMed] [Google Scholar]
- 27.Prasad N., Topping R. S., Zhou D., Decker S. J. (2000) Biochemistry 39, 6929–6935 [DOI] [PubMed] [Google Scholar]
- 28.Seong H. A., Jung H., Choi H. S., Kim K. T., Ha H. (2005) J. Biol. Chem. 280, 42897–42908 [DOI] [PubMed] [Google Scholar]
- 29.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. (2001) EMBO Rep. 2, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seong H. A., Jung H., Kim K. T., Ha H. (2007) J. Biol. Chem. 282, 12272–12289 [DOI] [PubMed] [Google Scholar]
- 31.Ouyang M., Shen X. (2006) J. Neurochem. 97, 234–244 [DOI] [PubMed] [Google Scholar]
- 32.Jung H., Seong H. A., Ha H. (2007) J. Biol. Chem. 282, 35293–35307 [DOI] [PubMed] [Google Scholar]
- 33.Fujii K., Goldman E. H., Park H. R., Zhang L., Chen J., Fu H. (2004) Oncogene 23, 5099–5104 [DOI] [PubMed] [Google Scholar]
- 34.Hatai T., Matsuzawa A., Inoshita S., Mochida Y., Kuroda T., Sakamaki K., Kuida K., Yonehara S., Ichijo H., Takeda K. (2000) J. Biol. Chem. 275, 26576–26581 [DOI] [PubMed] [Google Scholar]
- 35.Patel T., Gores G. J., Kaufmann S. H. (1996) FASEB J. 10, 587–597 [DOI] [PubMed] [Google Scholar]
- 36.del Peso L., González-García M., Page C., Herrera R., Nuñez G. (1997) Science 278, 687–689 [DOI] [PubMed] [Google Scholar]
- 37.Gil M., Yang Y., Lee Y., Choi I., Ha H. (1997) Gene 195, 295–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.