Abstract
Adenovirus expressing ClC-3 (Ad-ClC-3) induces Cl−/H+ antiport current (IClC-3) in HEK293 cells. The outward rectification and time dependence of IClC-3 closely resemble an endogenous HEK293 cell acid-activated Cl− current (IClacid) seen at extracellular pH ≤ 5.5. IClacid was present in smooth muscle cells from wild-type but not ClC-3 null mice. We therefore sought to determine whether these currents were related. IClacid was larger in cells expressing Ad-ClC-3. Protons shifted the reversal potential (Erev) of IClC-3 between pH 8.2 and 6.2, but not pH 6.2 and 5.2, suggesting that Cl− and H+ transport become uncoupled at low pH. At pH 4.0 Erev was completely Cl− dependent (55.8 ± 2.3 mV/decade). Several findings linked ClC-3 with native IClacid; 1) RNA interference directed at ClC-3 message reduced native IClacid; 2) removal of the extracellular “fast gate” (E224A) produced large currents that were pH-insensitive; and 3) wild-type IClC-3 and IClacid were both inhibited by (2-sulfonatoethyl)methanethiosulfonate (MTSES; 10–500 μm)-induced alkanethiolation at exposed cysteine residues. However, a ClC-3 mutant lacking four extracellular cysteine residues (C103_P130del) was completely resistant to MTSES. C103_P130del currents were still acid-activated, but could be distinguished from wild-type IClC-3 and from native IClacid by a much slower response to low pH. Thus, ClC-3 currents are activated by protons and ClC-3 protein may account for native IClacid. Low pH uncouples Cl−/H+ transport so that at pH 4.0 ClC-3 behaves as an anion-selective channel. These findings have important implications for the biology of Cl−/H+ antiporters and perhaps for pH regulation in highly acidic intracellular compartments.
Keywords: Biophysics, Cell/Endocytosis, Channels/Chloride, Exchange, Membrane/Biophysics, Membrane/Channels, Transport/Chloride, Transport/Proton
Introduction
ClC-3, -4, and -5 are Cl−/H+ antiporters that are expressed primarily in intracellular organelles. They have been proposed to provide shunt conductances for proton current generated by the vacuolar (V-type) H+-ATPase (V-ATPase)2 (1). Sufficient ClC-4 and -5 protein localizes to the plasma membrane to readily allow recording of ion currents when these proteins are heterologously expressed in Xenopus oocytes (2). Unfortunately, recombinant ClC-3 currents have been much more difficult to express (2–4) and interpretation of the currents observed (5–8) has been controversial (for review see Ref. 9). ClC-3 localizes to endosomes (10) and lysosomes (11) as well as secretory vesicles of various types (12–14) suggesting that the protein cycles through the plasma membrane. Membrane localization of ClC-3 was quantified in cultured fibroblasts using recombinant protein with both extracellular and intracellular epitope tags. It had a half-life in the membrane of ∼9 min and about 6% of total ClC-3 protein localized to the membrane at a given time (15). A proportion of ClC-3-eGFP fusion protein clearly appears to localize to the plasma membrane of ClC-3 expressing HEK293 cells (8).
We recently demonstrated that adenoviral-mediated overexpression of ClC-3 produced novel currents in HEK293 cells at neutral pH (8). These currents exhibited very steep outward rectification and time-dependent activation that was reminiscent of ClC-4 and ClC-5 (2, 16) except that the kinetics of activation were slower. Activation was also significantly slower than observed when much smaller currents were elicited by overexpression of ClC-3 plasmids in Chinese hamster ovary-K1 cells (11), although rectification properties and the effect of a specific mutation (E224A) were very similar. Changes in current amplitude and reversal potential observed in response to alterations in extracellular Cl− and H+ concentration were consistent with ClC-3 acting as a Cl−/H+ antiporter and deviated significantly from the behavior of an anion channel. These results supported predictions, based upon sequence homology, that like ClC-4 and ClC-5, ClC-3 would be a Cl−/H+ antiporter (17, 18).
ClC-3 is required for proper acidification of synaptic vesicles (12), insulin granules (19), lysosomes (11), and endosomes (20) by the V-ATPase. This acidification process leads to intra-organellar pH values ranging from 5.9 to 6.2 for early endosomes to 5.0–6.0 for late endosomes, and 4.6–5.5 for lysosomes (21, 22). A Cl− channel is well suited to provide charge neutralization for a proton pump. However, the realization that the intracellular ClCs are Cl−/H+ antiporters (8, 17, 18) yielded the surprising requirement that protons move out of the endosome in exchange for Cl− to provide countercurrent for the V-ATPase. The 2Cl− to 1H+ stoichiometry of this process (23, 24) makes this mechanism feasible, but has led to speculation that the primary goal of this coupled transport system may be to concentrate Cl− in the compartment rather than to facilitate acidification (18, 22). This adds an unanticipated layer of complexity to understanding the contribution of CLC proteins to endosomal biology (25).
Following endocytosis, the extracellular surface of plasma membrane proteins faces the vesicular lumen. Lowering extracellular pH to a degree commonly encountered in intracellular vesicles activates an outwardly rectifying chloride current (IClacid) in HEK293 cells (26, 27), astrocytes (26), Sertoli cells (28), ventricular myocytes (29), and endothelial cells (30). Following endocytosis, this conductance could theoretically provide shunt current for the V-ATPase. The protein responsible for IClacid has not been identified. It was proposed that it might represent an altered manifestation of the volume-activated Cl current (swelling induced anion current, IClswell) (27), however, a detailed analysis of IClacid in HEK293 cells revealed that these two currents are clearly distinct. They differ with respect to anion selectivity, pharmacology, and kinetics (26).
The time dependence and rectification properties of the ClC-3 current are highly reminiscent of native IClacid in HEK293 cells (26, 27). This raises the possibility that ClC-3 overexpression somehow activates this endogenous current. At first glance it is difficult to imagine that ClC-3 itself produces IClacid because enhancement of the current at positive holding potentials in response to an increase in the extracellular proton concentration is inconsistent with the behavior of a Cl−/H+ antiporter. ClC-3 would have to switch its behavior from that of a Cl−/H+ antiporter to an anion channel. Whereas this may seem odd, there is ample evidence that the degree of coupling between Cl− and H+ transport by ClC proteins is not fixed, and is less tight in the presence of non-halide anions (23, 31, 32). Furthermore, Cl− flux through the bacterial protein CLCec-1 varies according to the protonation state of the extracellular glutamate side chain “fast gate” (Glu148 of CLC-ec1, Glu224 of ClC-3). Neutralization or removal of this negatively charged side chain, which competes with Cl− for an extracellular anion binding site, uncouples Cl−/H+ exchange and yields a protein that only transports Cl− (33–35). Therefore extracellular H+ might alter the protonation state and hence the function of this gate. Given the longstanding controversy regarding the nature of ClC-3 currents, we felt it was important to define the relationship between IClC-3 and IClacid. Our findings demonstrate that ClC-3 expression produces an acid-activated anion current that is indistinguishable from native IClacid. These results have important implications for the mechanism of ion transport by ClC-3 and potentially for the control of pH in intracellular compartments.
EXPERIMENTAL PROCEDURES
Cell Culture and Modification of ClC-3 Expression
Aortic vascular smooth muscle (VSM) cells were prepared from ClC-3 wild-type and null mice using previously established methods (10, 36). VSM cells were grown in a high-glucose Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 44 mm NaHCO3, 25 mm HEPES (pH 7.4), 1% minimal essential medium vitamins, and 1% non-essential amino acids (Invitrogen). Aortic VSM cells were allowed to reach no more than 70% confluence prior to use and were passaged less than 10 times. HEK293 cells (HEK293T, adenoviral propagation resistant) were obtained from the American Tissue Culture Collection and maintained in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Atlanta Biological).
Modification of ClC-3 expression was achieved as previously described (8). Briefly, expression of the short N-terminal isoform of human ClC-3 (Swiss-Prot Q9R279) was driven by the cytomegalovirus promoter in a bicistronic adenoviral construct that also expressed eGFP behind the Rous sarcoma virus promoter. Adenovirus expressing only eGFP was used as a control. The adenovirus shuttle plasmid pacAd5 cytomegalovirus was used for expression of ClC-3 mutants. Site-directed mutations in the pacAd5 clones were created using the QuikChange II kit from Stratagene (La Jolla, CA).
In all experiments in which plasmids were used to express wild-type or mutant ClC-3, RNAi directed against the 5′-untranslated region of endogenous ClC-3 (Dicer duplex system, IDT Inc., Coralville, IA) was used as described previously (8) to suppress endogenous ClC-3 protein levels. Targeted sequences were: 5′-CAUCUGUUUCAAACCUAGAACCUAGCU-3′ or 5′-GAGUAAAGUAGGAUGGCUUUCAACCCA-3′. These sequences do not appear in the ClC-3 clones. This approach allows plasmid expression in the setting of very reduced levels of native ClC-3. Control, scrambled RNAi duplex was provided by the manufacturer. Cells grown in 2% serum were exposed to RNAi at concentrations of 25–50 nm in the presence of Oligofectamine (5 μl/ml) and studied 72–96 h later.
Electrophysiology
Currents were measured at room temperature (22 °C) using either standard whole cell voltage-clamp methods (37) or perforated patch recording (38) performed with an Axopatch 200B amplifier driven by pClamp 9 software (Molecular Devices Corporation, Sunnyvale, CA). Pipette resistance was 3–5 megohms. Pipette and whole cell capacitance and series resistance compensations were done prior to recording. Currents for I/V relationships were elicited from a holding potential of −40 mV to test potentials from −100 to +100 mV in 20-mV increments. Test pulses were 1 s in duration delivered at 3-s intervals. Currents were sampled at 5 kHz and filtered at 1 kHz. Ramp protocols for determination of reversal potential (Erev) went from −40 to +40 mV over 900 ms. 10 ramp currents acquired 2 s apart were averaged for each data point.
HEPES-buffered bath solutions contained (mm): NaCl 120, MgCl2 2.5, CaCl2 2.5, HEPES 10, glucose 5.5, pH 4.0–7.35, with NaOH or HCl. 10 mm MES or MOPS were used as alternative buffers in place of HEPES for some experiments. Citrate-buffered solutions contained (mm): NaCl 120, MgCl2 5.0, CaCl2 5.0, sodium citrate 5, glucose 5.5, pH 4.0–7.35, with NaOH or HCl. NaCl was sometimes substituted with 120 mm NaSCN. Osmolality of all solutions was determined using a μ OSMETTE osmometer and all extracellular solutions were titrated to 300 mosmol using 1 m mannitol. The 435 mm Cl bath solution contained (in mm): NaCl 425, HEPES 10, MgCl2 2.5, CaCl2 2.5, glucose 5.5. The 42 mm Cl− bath solution contained (in mm): NaCl 32, CaCl2 2.5, MgCl2 2.5, HEPES 10, and glucose 240. Liquid junction potentials were minimized by using 3 m KCl agar bridges and calculated using pClamp 9.0 to be 8.4, 5.0, and 2.4 mV for 435, 130, and 42 mm Cl− buffers, respectively. Pipette solutions for standard whole cell recordings contained (mm): CsCl 120, triethanolamine-Cl 4, EGTA 5, CaCl2 1.187, MgCl2 2, Na-ATP 5, Na-GTP 0.5, HEPES 10, pH 7.2, with CsOH, osmolality at 290 mosmol, and free [Ca2+] = 55 nm (calculated using WEBMAXC). The pipette solution for perforated patch recording contained (mm): CsCl 125, MgCl2 2.5, HEPES 10, pH 7.2, with CsOH (liquid junction potential 4.4 mV using standard bath solution). Amphotericin was dissolved in dimethyl sulfoxide at a concentration of 60 mg/ml and then 20 μl of this solution was mixed with 5 ml of pipette solution by vortexing. Currents were normalized to cell membrane capacitance and expressed as current density (pA/pF). Identification of GFP positive cells was done immediately prior to cell selection using a fluorescence-equipped inverted microscope (Zeiss Axiovert 25). All chemicals were obtained from Sigma, except for sodium (2-sulfonatoethyl)methanethiosulfonate (MTSES), which was purchased from Anatrace (Maumee, OH). MTSES and dithiothreitol (DTT) were dissolved in dimethyl sulfoxide and diluted at least 1:1000 into experimental solutions.
Data Analysis and Statistics
Unless otherwise indicated, steady state currents (measured 5 ms prior to the end of the depolarizing pulse) were used to calculate current-voltage relationships using pClamp 9 software. Erev was estimated by either 1) analysis of I/V relationships by fitting a straight line (y = mx + b) between consecutive steady state current data points that were immediately negative to, and positive to, zero current density, and determining the voltage at which this line crossed 0 pA, or 2) direct measurement of the voltage at which ramp currents crossed 0 pA. Reversal potential comparisons were made after correcting for liquid junction potentials. Activation time courses were fit using one or two exponentials and time constants of activation were determined using the Clampfit module of pClamp 9. Results are expressed as mean ± S.E. Unpaired Student's t tests were used to determine statistical significance.
RESULTS
IClacid in Wild-type and ClC-3 Null VSM Cells
As an initial approach to determining if IClC-3 and IClacid are related, we measured currents activated by pH 4.0 in wild-type and ClC-3 null cultured vascular smooth muscle cells (10, 36). IClacid has not previously been characterized in this cell type. Minimal current was observed at pH 7.35 in all cells. Following exposure to pH 4.0, outwardly rectifying currents with time-dependent activation were observed in ClC-3 wild-type but not null VSM cells (Fig. 1). These results encouraged us to pursue a detailed investigation of these two currents in HEK293 cells where others have carefully characterized IClacid (26), and we have studied IClC-3 (8).
FIGURE 1.
Activation of IClacid in wild-type and ClC-3 null VSM cells. Representative current tracings at pH 7.35 and 4.0 for wild-type (A) and null (B) cells (HP = −40 mV, TP = −100 to +100 mV in 20-mV increments). C, I/V relationships at pH 7.35 and pH 4.0. *, significantly different from the voltage-matched current at pH 7.35 (p < 0.05). No significant differences were noted between baseline wild-type and null currents at pH 7.35 (p < 0.05). Error bars indicate ±S.E.
Wild-type ClC-3 Currents Are pH-dependent
HEK293 cells expressing eGFP alone had no significant current at an extracellular pH of 7.35 (Fig. 2A). Reduction of pH to 4.0 elicited outwardly rectifying currents that underwent time-dependent activation (Fig. 2B). The current-voltage relationships for these currents are shown in Fig. 2E. These currents were very similar to the IClacid that was previously characterized under identical conditions (26, 27). Ad-ClC-3-infected HEK293 cells express more ClC-3 protein than either non-infected or Ad-eGFP-infected cells (8). Ad-ClC-3 elicited currents at pH 7.35 as previously described. These currents were smaller than native IClacid at pH 4.0 (Fig. 2, B compared with C and Ref. 8). ClC-3 overexpression greatly enhanced the magnitude of the currents elicited by pH 4.0 (Fig. 2, D and E). The activation time course of IClacid was well fit by 2 exponentials in both control and ClC-3 overexpressing cells. The two time constants at pH 4.0 (Table 1) did not differ significantly with ClC-3 expression and also were not different from the previously published time constants for overexpressed ClC-3 measured at pH 7.35 (8). To ensure that the effect of low pH was not an artifact associated with the internal dialysis process after membrane rupture, several cells were recorded using the amphotericin B-perforated patch technique. The currents elicited by pH 4.0 were indistinguishable from those obtained using dialyzed cells (n = 4, data not shown).
FIGURE 2.
IClacid in HEK293 cells. Representative tracings from Ad-eGFP (A and B) and Ad-ClC-3 expressing (C and D) cells at the indicated pH. HP = −40 mV, TP = −100 to +100 mV in 20-mV increments. E, I/V relationships for Ad-eGFP- and Ad-ClC-3-infected cells at the indicated pH. *, significantly different from current at pH 7.35 for a given TP (p < 0.05). Error bars indicate ±S.E.
TABLE 1.
Activation time constants
The previously published (8) activation time constants for ClC-3 currents (first row) are compared to those of the acid-activated whole cell currents from ClC-3 expressors (second row), eGFP controls (third row), E224A mutants at pH 7.35 (fourth row), and the C166_P188del mutants (fifth row). The E224A mutant current traces were best fit by 1 time constant; all other experimental groups were best fit by 2 time constants. HP = −40 mV, TP = +80 mV. Values are mean ± S.E. There were no significant differences between fast or slow time constants between groups (p < 0.05).
| Slow τ | Fast τ | n | |
|---|---|---|---|
| ms | |||
| Ad-ClC-3 (pH 7.35) | 228.2 ± 53.4 | 16.1 ± 3.3 | 14 |
| Ad-ClC-3 (pH 4.0) | 193.3 ± 25.5 | 19.3 ± 3.5 | 16 |
| Ad-eGFP (pH 4.0) | 248.9 ± 47.3 | 18.4 ± 4.4 | 14 |
| E224A (pH 7.35) | 218.8 ± 49.5 | 11 | |
| C166_P188del (pH 4.0) | 196.5 + 20.0 | 17.9 + 1.6 | 7 |
To make direct comparisons to IClacid currents recorded by other groups (26, 27), HEPES was initially used as the bath solution buffer. However, HEPES has virtually no buffering capacity at pH 4.0 (pKa = 7.55). We therefore evaluated the impact of buffer strength on IClacid by comparing currents induced by pH 4.0 in bath solutions containing 10 mm HEPES, 10 mm MOPS (pKa = 7.20), 10 mm MES (pKa = 6.15), or 5 mm citrate (triprotic acid with pKa values of 3.13, 4.76, and 6.40). Peak outward currents at pH 4.0 (TP = +100 mV) for ClC-3 expressing cells were 149.4 ± 27.4, 152.6 ± 27.5, 150.5 ± 27.9, and 151.4 ± 15.4 pA/pF for HEPES, MOPS, MES, and citrate containing solutions, respectively (n = 4–6). Supplemental Fig. S1 compares the magnitude of whole cell current at a test potential of +80 mV in control and ClC-3 expressing cells at pH values between 6.0 and 4.0 using either HEPES- or citrate-containing bath solution. No significant differences were observed at any pH. For all subsequent experiments in which there was potential for drug-induced changes in pH, citrate was used as the default buffer.
We previously reported that a modest increase in extracellular proton concentration, pH 6.35, reduced the magnitude of IClC-3 at positive intracellular potentials where Cl− is entering and H+ is exiting the cell (8). This is consistent with the behavior of a Cl−/H+ antiporter. However, further acidification to pH ≤ 5.5 activated outward current in a proton concentration-dependent manner in both Ad-GFP and Ad-ClC-3 expressing cells (Fig. 3, A and B). Representative raw current tracings from these experiments are shown in Fig. 3, C and D. These data are consistent with the reported pH sensitivity of IClacid in HEK293 cells (26, 27). For clarity, examples of the response of individual Ad-ClC-3-expressing cells to both modest acidification and very low pH are provided in supplemental Fig. S2, A and B, and C, which summarize the magnitude of current recorded in Ad-ClC-3 expressing cells exposed to a range of extracellular pH values from 4.0 to 7.35. Activation of current at very low pH cannot be attributed to enhanced Cl−/H+ antiport. Intracellular pH was held constant at 7.2 in these experiments, thus increases in extracellular proton concentration should resist proton efflux, which is required for the Cl−/H+ exchange current at positive voltages.
FIGURE 3.
The effect of lowering pH on whole cell currents. The I/V relationship for Ad-eGFP (A and C) and Ad-ClC-3 expressing cells (B and D) at the indicated pH values. Representative whole cell currents are shown in C and D. Error bars indicate ±S.E.
Low pH Uncouples Cl− and H+ Transport
Proton dependence of the ClC-3 current was demonstrated previously by shifts in reversal potential when the proton gradient was altered (8). IClC-3 activation at low pH would seem to require a transition from antiporter to “channel,” or at least to a much less tightly coupled antiporter. Changes in current magnitude are not a useful indicator of where this transition occurs, as protons may affect not only coupling but other parameters such as gating or conductance. We therefore compared the proton dependence of Erev across a wide pH range. We hypothesized that if Cl− and H+ transport became uncoupled, further elevations in extracellular proton concentration would no longer affect Erev.
We measured the currents elicited by ramp depolarizations from −40 to +40 mV in cells overexpressing ClC-3 over a pH range from 5.2 to 8.2 (Fig. 4). Only data from cells that were recorded at all pH values were included. As a control for nonspecific effects of pH we performed these experiments using either Cl− or SCN− as the extracellular anion. ClC antiporters become much less coupled in the presence of non-halide anions (23, 32, 35). Thus the Erev of SCN− currents would be expected to be much less sensitive to pH. We observed a shift in Erev of IClC-3 in NaCl buffers from −9.3 ± 1.25 to −20.0 ± 3.3 mV as pH increased from 7.2 to 8.2. The magnitude of this change is smaller than that predicted by the 2Cl−:1H+ stoichiometry (20 mV), but very similar to the magnitude of the shift previously observed upon alkalinization (8). Acidification from pH 7.2 to 6.2 produced a much smaller, but still significant shift in reversal potential to −6.7 ± 0.9 mV. This change is smaller than that previously observed (8) when pH was shifted from 7.35 to 6.35, suggesting that a reduction in coupling is already occurring at pH < 6.35. Interpretation of shifts in Erev over the pH range where the degree of coupling is changing (between pH 6 and 7) is difficult. However, it is very clear that further acidification from pH 6.2 to 5.2 did not cause a significant shift in Erev (−6.7 ± 0.9 to −5.2 ± 0.57 mV). This suggests that at pH 6.2 uncoupling is nearly complete. Of note, no significant differences in estimated reversal potential were observed when comparing ramp protocols with estimates based upon extrapolation of IV plots. For example, the average value obtained at pH 8.2 using ramps (20.0 ± 3.3) agreed well with that obtained previously by extrapolation (−18.9 ± 1.6 mV) (8). In addition, Erev values estimated by I-V analysis of data in this study at pH 4.0 (−6.0 ± 1.0 mV), 5.0 (−6.5 ± 1.0 mV), 5.5 (−5.0 ± 1.5 mV), and 6.0 (−5.8 ± 2.0 mV, n = 5 for all groups) did not differ from that estimated by ramps at pH 5.2 and 6.2.
FIGURE 4.
Shifts in the reversal potential of IClC-3 in response to changes in extracellular pH. A, substitution of extracellular Cl− with SCN− increases the size of the current. B, raw data tracings of ramp currents (−40 to +40 mV) at different pH values (5.2 to 8.2) with either Cl− (left) or SCN− (right) as the dominant extracellular anion. All recordings are from the same cell. C, comparison of average Erev of ramp currents for Cl− and SCN− at varying pH (n = 5 for Cl− and n = 4 for SCN−, two cells were assessed under both conditions). *, significantly different from pH 7.2 (p < 0.05).
SCN− not only reduces coupling of Cl− and H+ transport in ClC-4 and ClC-5, but also produces substantially larger currents than does Cl− under otherwise identical conditions. We observed a similar effect on IClC-3 (Fig. 4A). Currents were larger in the presence of extracellular SCN−, however, no significant changes in Erev were observed across the entire pH range tested. Ramp currents obtained from the same cell (Fig. 4B) clearly demonstrates the difference in the effect of pH on IClC-3 when the dominant extracellular anion is changed from Cl− to SCN−.
Native IClacid has previously been shown to be fully Cl−-dependent (26). We also assessed the Cl− dependence of IClC-3. Reversal potentials of currents recorded at pH 4.0 were shifted in response to changes in extracellular Cl− (−29.7 ± 1.9 at 435 mm, −7.2 ± 1.4 at 130 mm, and 26.1 ± 1.4 at 42 mm) in a manner consistent with a completely chloride-selective channel. The calculated slope for reversal potential/decade change in [Cl−]o was 55.2 ± 2.3 mV, very close to the 58.4 mV/decade Nernstian prediction for a chloride-selective channel at 22 °C.
RNAi Directed at ClC-3 Inhibits IClacid
We next attempted to reproduce the impact of knocking out ClC-3 in VSM cells by using RNAi in HEK293 cells. We have previously shown that RNAi targeted to the 5′-untranslated region of Clcn3 mRNA dramatically reduces the abundance of native ClC-3 protein in HEK293 cells (8). Clcn3-targeted RNAi treatment potently suppressed native IClacid, whereas scrambled control RNAi was without effect (Fig. 5). RNAi treatment also provided cells in which it was possible to express ClC-3 plasmids with no background of native ClC-3. These plasmids lacked the 5′-untranslated region sequence targeted by the RNAi, thus allowing reconstitution of ClC-3 protein expression as previously documented (8). The response to pH 4.0 was partially reconstituted by transfection with wild-type ClC-3 plasmid.
FIGURE 5.
RNAi knockdown of ClC-3 suppresses native IClacid. I/V relationships for HEK293 cells under the indicated conditions. Wild-type ClC-3 is expressed using plasmid. *, significantly different from pH 7.35 for a given condition. #, significantly different from scramble current at pH 4 (p < 0.05).
Neutralization of Glutamate 224 (E224A) Removes pH Sensitivity
Mutation of the extracellular glutamate fast gate in the exchanger-type ClCs, ClC-4, ClC-5 (17, 18), ClC-ec1 (39), and ClC-3 (8), removes sensitivity to extracellular protons and uncouples anion current from proton transport. In addition, this mutation dramatically alters rectification of ClC-3, -4, and -5 currents (8, 11, 17, 18). ClC-3 plasmid lacking the fast gate (E224A) was expressed in HEK293 cells that had been treated with RNAi as described above to remove native IClacid. E224A currents have previously been shown to be completely Cl−-dependent and uncoupled from proton transport (8). These currents also lack the sharp rectification of wild-type ClC-3. E224A mutant currents were present at pH 7.35 and were not further activated by extracellular acidification (Fig. 6). The I-V plot compares currents elicited at pH 4 and with previously published currents elicited in the same cells at pH 7.35 (see Fig. 8 of 8). Note that unlike Ad-ClC-3, plasmid-mediated overexpression of wild-type ClC-3 yielded virtually no current at pH 7.35 (2.3 ± 0.7 pA/pF at +100 mV, Fig. 5, open triangles). In contrast, the E224A plasmid yielded a significant current at neutral pH (48.3 ± 2.7 pA/pF, Fig. 6, open circles). The current density of the E224A mutant was unchanged at pH 4.0 (47.3 ± 1.1 pA/pF, Fig. 6, closed circles) and was still larger than the current induced by the wild-type plasmid at pH 4.0 (27.4 ± 7.5 pA/pF, Fig. 5, closed triangles). This suggests that either plasma membrane expression of the E224A mutant is higher than wild-type, or that acidity does not activate the current as potently as does mutation of the fast gate. The activation time constants for the E224A mutant currents are shown in Table 1. Unlike IClacid or wild-type IClC-3, these currents were well fit by a single time constant that was not significantly different from the slow time constant measured for IClacid and IClC-3.
FIGURE 6.
Effect of low pH on currents from HEK293 cells expressing E224A ClC-3 plasmid. RNAi was used to suppress endogenous ClC-3. A, whole cell currents from a representative cell at pH 7.35 and 4.0. The I/V relationships are shown in B. There were no significant differences between currents at 7.35 and pH 4.0 at any TP (p < 0.05). Currents at pH 7.35 were previously published (8) but are provided for comparison.
FIGURE 8.
MTSES inhibits native Iclacid. A, I/V relationships for currents recorded at pH 4.0 following incubation in varying concentrations of MTSES at pH 7.35. MTSES was washed off as the bath was changed to pH 4.0. B, reversal of the effect of MTSES (500 μm) by DTT (500 μm). Representative raw currents for these experiments are shown in C. The sequence of bath solution exchanges used to create the I/V relationships in B was as follows: 1) control, pH 7.35; 2) effect of reducing pH to 4.0; 3) return to pH 7.35 and incubation in MTSES; 4) change to pH 4 with wash-off of MTSES; 5) return to pH 7.35 and incubation in DTT; and 6) change to pH 4 after DTT. Error bars indicate ±S.E.
The Effect of MTSES on IClC-3 and IClacid
The experiments described above provide circumstantial evidence that ClC-3 is an IClacid. We felt that more direct evidence might be obtained by employing cysteine-scanning mutagenesis (40, 41). This method takes advantage of the fact that chemical modification of extracellular cysteine residues can alter protein function, including ion channel activity (42). The presence of a cysteine residue in a critical position that is accessible to alkanethiolation by MTS reagents can create a highly specific inhibitor of an ion current. As a prelude to the introduction of cysteine mutations in the pore region of ClC-3, we first studied the effect of the negatively charged reagent, MTSES, on wild-type Ad-ClC-3 expressing HEK293 cells. Surprisingly, a 5-min incubation in 100 μm MTSES completely inhibited IClC-3 (Fig. 7). This suggested that an endogenous cysteine in ClC-3 was positioned such that modification by MTS impaired ion transport. Alkanethiolation of cysteine by MTS reagents is reversed under highly reduced conditions, thus specific effects of these reagents should be reversed by DTT. A 5-min incubation in 500 μm DTT restored IClC-3.
FIGURE 7.
ClC-3 current induced by Ad-ClC-3 at pH 7.35 is inhibited by a 5-min incubation in 100 μm MTSES. This effect is reversed by a 5-min incubation in 500 μm DTT. *, significantly different from Ad-CIC-3. #, significantly different from MTSES (p < 0.05). Error bars indicate ±S.E.
We next determined if MTSES affected native IClacid by pre-incubating control HEK293 cells in MTSES for 5 min prior to exposure to pH 4.0. Fig. 8A demonstrates that MTSES caused a dose-dependent decrease in the amplitude of IClacid. At a test potential of +100 mV, MTSES inhibited IClacid by 95, 94, and 69% at 500, 100, and 10 μm concentrations, respectively. The effect of MTSES was not reversed by 10 min of washing with pH 7.35 buffer. Thus the effect of MTSES was virtually complete even though this reagent was no longer present when pH was lowered to 4.0. The effect of MTSES was reversed by a subsequent 5-min incubation in 500 μm DTT (Fig. 8, B and C). Incubation in DTT was performed at pH 7.35. This observation strongly suggests that the mechanism of IClacid activation is not related to recruitment of new protein to the plasma membrane.
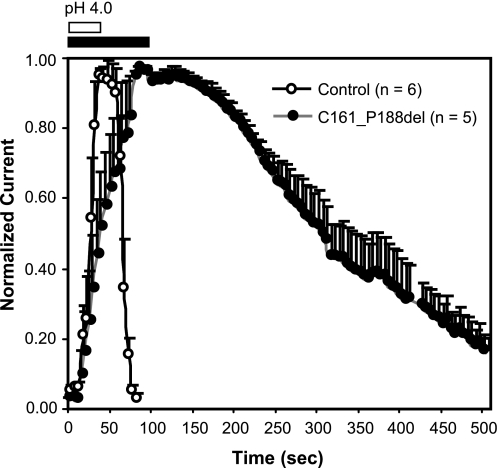
Because MSTES inhibited both IClacid and IClC-3, rather than inserting cysteine residues into ClC-3 we removed them. A ClC-3 mutant (C103_P130del) was created that lacks part of the extracellular loop that connects transmembrane domains B and C (43). This region contains 4 cysteine residues. Fig. 9 shows that even a high concentration of MTSES (500 μm) did not block the acid-induced current in cells expressing the mutant plasmid. The activation time constants of this current (Fig. 9B) were not significantly different from those of IClacid or IClC-3 (Table 1). However, the time course of the appearance of IClacid after exposure to pH 4.0, and the return of current amplitude to baseline after changing back to pH 7.35 were dramatically altered in the Cys103–Pro130 mutant. Fig. 10 compares the time course of IClacid activation and washout of this effect between control HEK293 cells and cells expressing the C103_P130del mutant upon brief exposure to pH 4.0 bath solutions. The time to maximal IClacid activation was 39.2 ± 3.3 s for controls and 98 ± 6.0 s for mutant expressing cells (n = 5, p < 0.05). The time to half-maximal current levels after pH 4.0 washout was 34.2 ± 1.5 s for control cells and 304 ± 24.3 s for cells expressing the mutant (n = 6, p < 0.05). The observed changes in MTSES sensitivity and acid activation time course induced by this deletion mutation unequivocally identify these currents as IClC-3. The ability of low pH to activate the mutant demonstrates that ClC-3 is indeed an IClacid.
FIGURE 9.
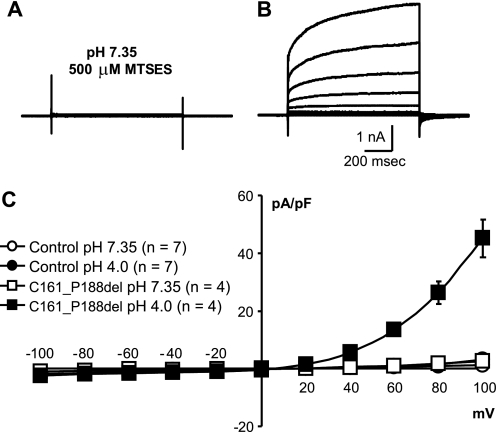
The effect of MTSES (500 μm) on cells expressing the C161_P188del plasmid. RNAi was used to suppress endogenous ClC-3. Raw current traces from a representative HEK293 cell expressing the C161_P188del mutant are shown at pH 7.35 during incubation in MTSES at pH 7.35 (A) and after changing to pH 4.0 (B). The I/V relationship for these experiments are shown in C. Error bars indicate ±S.E.
FIGURE 10.
Time course of the increase in current elicited by pulsing from −40 to +80 mV following application of pH 4.0 buffer (bars at top) and washout of this effect after returning to pH 7.35. The cells all started in pH 7.35 buffer and returned to pH 7.35 immediately after the effect of pH 4.0 was stable. In control HEK293 cells (open circles) the response to pH changes is very brisk. In C161_P188del expressing cells the changes in current magnitude are much slower. Depolarizing pulses were applied every 5 s. Error bars indicate ±S.E.
DISCUSSION
ClC-3 Is an IClacid
This investigation was undertaken because significant similarity was noted between the ion currents induced by Ad-ClC-3 at neutral pH in HEK293 cells (IClC-3) and an endogenous acid-induced anion current that was previously characterized in these cells (IClacid) (26, 27). HEK293 cells express native ClC-3 protein (8). We therefore wished to determine whether ClC-3 expression is required for activation of endogenous IClacid, or alternatively, if ClC-3 protein is responsible for IClacid. The observation that IClacid is present in wild-type but not ClC-3 null murine VSM cells provided an initial hint that a relationship existed between the two currents. Additional results supporting this conclusion included: 1) IClacid is much larger in HEK293 cells overexpressing ClC-3; 2) the two time constants of activation for IClC-3 and IClacid are indistinguishable; and 3) IClacid is absent in cells treated with RNAi directed against ClC-3. Although these data seem to link IClC-3 and IClacid, they do not establish whether this relationship is direct or indirect. The first clue that ClC-3 is itself an IClacid was provided by the behavior of the E224A mutant. This mutant lacks the extracellular “gating” glutamate. Unlike with wild-type plasmid expression, currents were present at pH 7.35 and were easily identified by their markedly reduced rectification. Reduction of extracellular pH to 4.0 did not enhance the mutant current. E224A currents also lacked the faster of the two time constants of activation that characterize IClC-3 and IClacid. The strongest evidence that ClC-3 is an IClacid was provided via the use of the negatively charged methanethiosulfonate reagent MTSES, which reacts with thiol groups and modifies accessible cysteine residues. Both wild-type IClC-3 and native IClacid were inhibited by MTSES. A ClC-3 mutant lacking four extracellular cysteine residues (C103_P130del) was identified by its resistance to inhibition by MTSES and slower response to low pH. However, C103_P130del ClC-3 was still responsive to protons and retained the same activation time constants as wild-type IClC-3 and native IClacid. Thus, we conclude that ClC-3 currents are activated by low pH.
The historical difficulty of expressing recombinant IClC-3 (2–4) suggests that in Xenopus oocytes and in many other cell types, the percentage of ClC-3 that localizes to the plasma membrane is not sufficient to produce identifiable currents, at least not at neutral pH. This percentage may differ between resting and stimulated cells but this has not been routinely tested. We observed no significant current at pH 7.35 in HEK293 cells expressing wild-type ClC-3 plasmid (Fig. 5). However, robust currents are induced by Ad-ClC-3 at this pH (see Ref. 8 and Fig. 2C). This difference may be related to the increased abundance of recombinant protein, or the virus may affect the percentage of protein that localizes to the plasma membrane. In either case, the rapid provocation of current by low pH at room temperature, and the ability of prior exposure to MTSES to completely inhibit IClacid, makes rapid membrane insertion of ClC-3 in response to extracellular protons very unlikely.
Activation of ClC Current by Protons
Activation by extracellular protons is a well documented property of ClC-0, ClC-1, and ClC-2 channel-type ClC currents (44–46). Fast gating of all ClC pores has been proposed to be mediated by the same extracellular glutamate (Glu232 of ClC-1, Glu224 of ClC-3). It is thought that in the closed state the charged COO− side chain of this amino acid competes with Cl− for access to its extracellular binding site. Protonation to COOH causes this side chain to swing out of the way and allows Cl− to interact (43). If this COOH is accessible to the extracellular aqueous environment, extracellular pH might be anticipated to impact its protonation state. In exchanger-type ClC proteins, neutralization or deletion mutations of this glutamate render transport activity pH-insensitive and uncouples Cl− from H+ transport (2, 39, 47).
Mutation of the fast gate also alters rectification of both channel (48) and exchanger-type ClCs (2, 33, 39, 47) including E224A ClC-3 (8, 11). The mechanism of the prominent rectification of the mammalian ClC exchangers is not established. The loss of rectification in response to neutralization of the gating glutamate suggests that rectification may result from the voltage dependence of gating. It has also been proposed that protons may much more readily reach the fast gate glutamate from the intracellar side and must be moved there against an energy barrier by voltage (35).
Comparing wild-type ClC-3 plasmid-induced current density at pH 7.35 (Fig. 5, open triangles) to E224A current density at the same pH (Fig. 6, open circles) suggests that there is an increase in transport in the absence of the fast gate. The mutant current is much larger than wild-type and is very similar in magnitude to the wild-type current at pH 4.0 (Fig. 5, closed triangles). E224A currents are not further activated by low pH. Thus, both extracellular protons and the E224A mutation activate the ClC-3 current. Both IClC-3 at pH 4.0 and E224A currents are completely Cl− dependent, as is native IClacid (26). However, two properties clearly distinguish E224A currents from wild-type IClC-3 and IClacid. First, whereas both wild-type IClC-3 and IClacid are best fit by two time constants, E224A currents are well fit by a single constant that is not significantly different from the slower of the two wild-type constants (Table 1). This is consistent with the fast gate accounting for the shorter of the two time constants. Second, rectification is dramatically reduced in the E224A mutant, but is unaltered by extracellular protons. Thus, whereas low extracellular pH could impact the protonation state of Glu224, the effect of low pH on current is not readily explained solely by this mechanism. Extracellular protons may also exert acid-induced changes in the conformation of other regions of the extracellular face of ClC-3.
MTSES Identifies ClC-3 Current
Both IClC-3 and native IClacid are inhibited by MTSES. This effect is dose-dependent and reversed by DTT as expected for a specific action of the compound. MTSES resistance directly identifies C103_P130del ClC-3 mutant currents. These currents are still acid-activated, and the altered time course of the response to changes in pH also identifies the C103_P130del mutant. IClC-3 is therefore unequivocally activated by protons. This rules out tightly coupled Cl−/H+ antiport as a mechanism. Combined with the lack of acid-induced changes in reversal potential at pH ≤ 6.2 and the virtually complete Cl− dependence of IClC-3 at pH 4.0, we conclude that low pH must drastically reduce or eliminate the coupling of Cl− to H+ transport. The Cys103 to Pro130 segment provides an interesting candidate region for modulating responsiveness to protons. Future studies will focus on determining which of the four cysteine residues deleted by our mutation (Cys103, Cys114, Cys115, and Cys129) are important for MTSES sensitivity.
Although we can clearly conclude that by definition ClC-3 is an “IClacid,” our data do not definitively demonstrate that the native IClacid in HEK293 cells is endogenous ClC-3. Several lines of evidence strongly suggest this, including similarities in basic biophysical properties (voltage dependence, time constants of activation, rectification, and MTSES sensitivity) and the absence of native IClacid following anti-ClC-3 RNAi treatment. However, these observations do not completely rule out the possibility that native IClacid is a distinct protein that is in some way dependent upon ClC-3 for activation.
Coupling of Cl−/H+ Antiport
The stoichiometry of the best understood of the ClC Cl−/H+ antiporters, ClCec-1 is 2Cl−:1H+ (23). Based on data supporting the same ratio for ClC-5, this has been suggested to be a general property of CLC antiporters (24). The coupling ratio of ClC-3 at neutral pH has not been rigorously established. However, whereas 2:1 antiport may be the normal mode of function for CLC antiporters, it is clear that this ratio is not tightly fixed. Coupling is structure-dependent and can be altered by mutations in both the extracellular “gating glutamate” and the intracellular “proton glutamate” (24, 33). Surprisingly, channel type CLC proteins actually share a similar mechanism of ion transport with the antiporters. ClC-0 channel gating also requires the transmembrane movement of protons, suggesting that the channel-type CLCs may be modified antiporters in which one of the conformational states is leaky for chloride ions (49, 50). Coupling of Cl−/H+ antiport is also dependent upon the permeant anion with non-halide anions resulting in reduced H+ countertransport (23, 35). In the presence of NO3− the stoichiometry of exchange also becomes voltage-dependent (24). Our data suggest that pH affects the Cl−/H+ coupling of the ClC-3 current and markedly enhances Cl− transport at high extracellular proton concentrations. Although coupling is clearly reduced by low pH, our methods are not adequate to determine the degree to which this ratio changes or to say if transport is completely uncoupled.
Functional Significance
ClC-3, ClC-4, and ClC-5 have been hypothesized to provide shunt conductances in the membranes of intracellular organelles, permitting intraluminal acidification by the V-ATPase (51). This idea was supported by the finding of impaired endosomal acidification in ClC-3-deficient mice (20). However, the realization that ClC-3 through 7 are all Cl−/H+ antiporters rather than channels has raised questions regarding the role of these proteins in vesicular electrophysiology (25, 52, 53). The direction of charge flow required to neutralize V-ATPase activity would necessitate removal of protons from the vesicle that is being acidified. Neutralizing the V-ATPase with a transporter that removes one proton from the endosomal lumen for every 3 units of charge moved yields a system that is only 66% efficient. It has been hypothesized that this method of charge neutralization makes more sense if increasing the luminal Cl− concentration is also critical for vesicular biology (18, 22). However, the sharp outward rectification of these currents does not favor Cl− entry into endosomes.
It remains to be determined if pH-dependent changes in coupling of Cl−/H+ transport by ClC-3 is physiologically significant. Our ability to interpret the current studies is limited by an inability to directly quantify IClC-3 in endosomes. We are left to speculate based upon the assumption that current characteristics observed in the plasma membrane can be extrapolated to endosome electrophysiology. If so, the pH of the vesicular lumen of some compartments clearly becomes sufficiently acidic to alter coupling of ClC-3 (21, 22). It is important to note that reducing pH to 5.5, which clearly activates and uncouples ClC-3, potently inhibits both ClC-4 (55) and ClC-5 (18) currents. Thus the ability of ClC-3 to become uncoupled and activated at low pH may have important physiologic implications for vesicles where it is the primary ClC protein. Transition from exchanger to channel mode might create the pure anion shunt that the ClCs were previously hypothesized to be (11, 12). More efficient charge compensation of the V-ATPase could enhance the ability of energy-dependent proton pumping to maintain lower pH in vesicles containing ClC-3 compared with those expressing ClC-4 or ClC-5. Consistent with this, ClC-3 protein is specifically enriched in synaptic-like microvesicles of neuroendocrine tissues (13). This compartment is surprisingly acidic (pH 5.11 versus 5.14 in lysosomes) in rat pinealocytes (54). If, on the other hand, vesicular Cl− uptake is a primary function of endosomal ClCs (18, 22), uncoupling of ClC-3 would terminate proton-driven Cl− accumulation. As we learn more about the biology of ClC Cl−/H+ antiporters, the ability of ClC-3 to uncouple at physiologic proton concentrations may provide important insight into the functional role of the protein.
Supplementary Material
Acknowledgment
We thank Dr. Alessio Accardi for consistently helpful advice and careful reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL62483 (to F. S. L.) and T32 DK07690-15 (to J. J. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- V-ATPase
- vacuolar-type hydrogen-ATPase
- eGFP
- enhanced green florescent protein
- Erev
- reversal potential
- IClacid
- acid-induced chloride current
- IClC-3
- ClC-3 current
- VSM
- vascular smooth muscle
- HEK
- human embryonic kidney
- RNAi
- RNA interference
- DTT
- dithiothreitol
- MES
- 4-morpholineethanesulfonic acid
- MOPS
- 4-morpholinepropanesulfonic acid
- Ad
- adenovirus
- MTSES
- (2-sulfonatoethyl)methanethiosulfonate.
REFERENCES
- 1.Jentsch T. J., Poët M., Fuhrmann J. C., Zdebik A. A. (2005) Annu. Rev. Physiol. 67, 779–807 [DOI] [PubMed] [Google Scholar]
- 2.Friedrich T., Breiderhoff T., Jentsch T. J. (1999) J. Biol. Chem. 274, 896–902 [DOI] [PubMed] [Google Scholar]
- 3.Jentsch T. J., Günther W., Pusch M., Schwappach B. (1995) J. Physiol. 482, 19S–25S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weylandt K. H., Valverde M. A., Nobles M., Raguz S., Amey J. S., Diaz M., Nastrucci C., Higgins C. F., Sardini A. (2001) J. Biol. Chem. 276, 17461–17467 [DOI] [PubMed] [Google Scholar]
- 5.Duan D., Winter C., Cowley S., Hume J. R., Horowitz B. (1997) Nature 390, 417–421 [DOI] [PubMed] [Google Scholar]
- 6.Li X., Shimada K., Showalter L. A., Weinman S. A. (2000) J. Biol. Chem. 275, 35994–35998 [DOI] [PubMed] [Google Scholar]
- 7.Huang P., Liu J., Di A., Robinson N. C., Musch M. W., Kaetzel M. A., Nelson D. J. (2001) J. Biol. Chem. 276, 20093–20100 [DOI] [PubMed] [Google Scholar]
- 8.Matsuda J. J., Filali M. S., Volk K. A., Collins M. M., Moreland J. G., Lamb F. S. (2008) Am. J. Physiol. Cell Physiol. 294, C251–C262 [DOI] [PubMed] [Google Scholar]
- 9.Jentsch T. J. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 3–36 [DOI] [PubMed] [Google Scholar]
- 10.Miller F. J., Jr., Filali M., Huss G. J., Stanic B., Chamseddine A., Barna T. J., Lamb F. S. (2007) Circ. Res. 101, 663–671 [DOI] [PubMed] [Google Scholar]
- 11.Li X., Wang T., Zhao Z., Weinman S. A. (2002) Am. J. Physiol. Cell Physiol. 282, C1483–C1491 [DOI] [PubMed] [Google Scholar]
- 12.Stobrawa S. M., Breiderhoff T., Takamori S., Engel D., Schweizer M., Zdebik A. A., Bösl M. R., Ruether K., Jahn H., Draguhn A., Jahn R., Jentsch T. J. (2001) Neuron 29, 185–196 [DOI] [PubMed] [Google Scholar]
- 13.Maritzen T., Keating D. J., Neagoe I., Zdebik A. A., Jentsch T. J. (2008) J. Neurosci. 28, 10587–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreland J. G., Davis A. P., Bailey G., Nauseef W. M., Lamb F. S. (2006) J. Biol. Chem. 281, 12277–12288 [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z., Li X., Hao J., Winston J. H., Weinman S. A. (2007) J. Biol. Chem. 282, 29022–29031 [DOI] [PubMed] [Google Scholar]
- 16.Weng T. X., Godley B. F., Jin G. F., Mangini N. J., Kennedy B. G., Yu A. S., Wills N. K. (2002) Am. J. Physiol. Cell Physiol. 283, C839–C849 [DOI] [PubMed] [Google Scholar]
- 17.Picollo A., Pusch M. (2005) Nature 436, 420–423 [DOI] [PubMed] [Google Scholar]
- 18.Scheel O., Zdebik A. A., Lourdel S., Jentsch T. J. (2005) Nature 436, 424–427 [DOI] [PubMed] [Google Scholar]
- 19.Barg S., Huang P., Eliasson L., Nelson D. J., Obermüller S., Rorsman P., Thévenod F., Renström E. (2001) J. Cell Sci. 114, 2145–2154 [DOI] [PubMed] [Google Scholar]
- 20.Hara-Chikuma M., Yang B., Sonawane N. D., Sasaki S., Uchida S., Verkman A. S. (2005) J. Biol. Chem. 280, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 21.Mellman I., Fuchs R., Helenius A. (1986) Annu. Rev. Biochem. 55, 663–700 [DOI] [PubMed] [Google Scholar]
- 22.Faundez V., Hartzell H. C. (2004) Sci. STKE 2004, re8. [DOI] [PubMed] [Google Scholar]
- 23.Nguitragool W., Miller C. (2006) J. Mol. Biol. 362, 682–690 [DOI] [PubMed] [Google Scholar]
- 24.Zifarelli G., Pusch M. (2009) EMBO J. 28, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb F. S., Moreland J. G., Miller F. J., Jr. (2009) Antioxid. Redox Signal. 11, 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert S., Oberwinkler J. (2005) J. Physiol. 567, 191–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobles M., Higgins C. F., Sardini A. (2004) Am. J. Physiol. Cell Physiol. 287, C1426–C1435 [DOI] [PubMed] [Google Scholar]
- 28.Auzanneau C., Thoreau V., Kitzis A., Becq F. (2003) J. Biol. Chem. 278, 19230–19236 [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S., Ehara T. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H1905–H1914 [DOI] [PubMed] [Google Scholar]
- 30.Ma Z. Y., Zhang W., Chen L., Wang R., Kan X. H., Sun G. Z., Liu C. X., Li L., Zhang Y. (2008) Biochem. Biophys. Res. Commun. 371, 437–440 [DOI] [PubMed] [Google Scholar]
- 31.Jayaram H., Accardi A., Wu F., Williams C., Miller C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11194–11199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alekov A. K., Fahlke C. (2009) J. Gen. Physiol. 133, 485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Accardi A., Walden M., Nguitragool W., Jayaram H., Williams C., Miller C. (2005) J. Gen. Physiol. 126, 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Accardi A., Miller C. (2004) Nature 427, 803–807 [DOI] [PubMed] [Google Scholar]
- 35.Zdebik A. A., Zifarelli G., Bergsdorf E. Y., Soliani P., Scheel O., Jentsch T. J., Pusch M. (2008) J. Biol. Chem. 283, 4219–4227 [DOI] [PubMed] [Google Scholar]
- 36.Robinson N. C., Huang P., Kaetzel M. A., Lamb F. S., Nelson D. J. (2004) J. Physiol. 556, 353–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. (1981) Pflugers Arch. 391, 85–100 [DOI] [PubMed] [Google Scholar]
- 38.Horn R., Marty A. (1988) J. Gen. Physiol. 92, 145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accardi A., Kolmakova-Partensky L., Williams C., Miller C. (2004) J. Gen. Physiol. 123, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S., Hartmann H. A., Kirsch G. E. (1997) J. Membr. Biol. 155, 11–25 [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y. Y., Sackin H., Palmer L. G. (2006) Biophys. J. 91, 2901–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan P. A., Gibbins J. M. (2006) Antioxid. Redox Signal. 8, 312–324 [DOI] [PubMed] [Google Scholar]
- 43.Dutzler R., Campbell E. B., Cadene M., Chait B. T., MacKinnon R. (2002) Nature 415, 287–294 [DOI] [PubMed] [Google Scholar]
- 44.Rychkov G. Y., Pusch M., Astill D. S., Roberts M. L., Jentsch T. J., Bretag A. H. (1996) J. Physiol. 497, 423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M. F., Chen T. Y. (2001) J Gen. Physiol. 118, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwiebert E. M., Cid-Soto L. P., Stafford D., Carter M., Blaisdell C. J., Zeitlin P. L., Guggino W. B., Cutting G. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 3879–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutzler R., Campbell E. B., MacKinnon R. (2003) Science 300, 108–112 [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Rose T., Jentsch T. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 7633–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lísal J., Maduke M. (2008) Nat. Struct. Mol. Biol. 15, 805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lísal J., Maduke M. (2009) Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jentsch T. J., Neagoe I., Scheel O. (2005) Curr. Opin. Neurobiol. 15, 319–325 [DOI] [PubMed] [Google Scholar]
- 52.Jentsch T. J. (2007) J. Physiol. 578, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sile S., Vanoye C. G., George A. L., Jr. (2006) Curr. Opin. Nephrol. Hypertens. 15, 511–516 [DOI] [PubMed] [Google Scholar]
- 54.Hayashi M., Yamamoto A., Moriyama Y. (2002) J. Neurochem. 82, 698–704 [DOI] [PubMed] [Google Scholar]
- 55.Vanoye C. G., George A. L., Jr. (2002) J. Physiol. 539, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.