Abstract
Vaccine adjuvants activate the innate immune system and thus influence subsequent adaptive T-cell responses. However, little is known about the initial immune mechanisms preceding the adjuvant-induced differentiation of T-helper (Th) cells. The effect of a T-helper 1 (Th1) adjuvant, dimethyldioctadecylammonium liposomes with monophosphoryl lipid-A (DDA/MPL), and a T-helper 2 adjuvant, aluminium hydroxide [Al(OH)3], on early, innate chemotactic signals and inflammatory cell influx at the site of injection was therefore investigated. Injection of the adjuvants into the peritoneal cavity of mice demonstrated distinct differences in the magnitude, quality and kinetics of the response. The inflammatory response to DDA/MPL was prominent, inducing high local levels of pro-inflammatory cytokines, chemokines and a pronounced inflammatory exudate consisting of neutrophils, monocytes/macrophages and activated natural killer cells. This was in contrast to the response induced by Al(OH)3, which, although sharing some of the early chemokine signals, was more moderate and consisted almost exclusively of neutrophils and eosinophils. Notably, Al(OH)3 specifically induced the release of a significant amount of interleukin (IL)-5, whereas DDA/MPL induced high amounts of tumour necrosis factor-α (TNF-α), IL-1α and IL-6. Finally, a microarray analysis confirmed that the effect of DDA/MPL was broader with more than five times as many genes being specifically up-regulated after injection of DDA/MPL compared with Al(OH)3. Thus, the adjuvants induced qualitatively distinct local inflammatory signals early after injection.
Keywords: adjuvants, cytokines, inflammation, T-helper 1/T-helper 2
Introduction
With the vast amount of information on T-cell differentiation that has been obtained in recent years, and in particular that on the role of pathogen-associated molecular patterns (PAMPs) in the induction of immune responses, it should in theory be possible to tailor vaccines and adjuvants to selectively induce cell-mediated/humoral and T-helper 1/T-helper 2 (Th1/Th2) responses. This would obviously have tremendous impact on the ongoing attempts to develop vaccines against some of the remaining challenges, such as human immunodeficiency virus (HIV), tuberculosis, chlamydia and hepatitis C. The common denominator for these infectious diseases is the fact that immunity resides largely within the cellular arm of the immune response, in contrast to infections for which efficient vaccines have already been developed, such as polio, diphtheria and measles, where immunity primarily depends on antibodies.
In practical vaccine development it has, however, proven difficult to translate the results obtained from defined in vitro observations to the complexity of the integrated immune system of living organisms, and limited information is currently available on the activity of aluminium salts, the only adjuvant in general use for human vaccines. In this regard, the early, innate inflammatory response induced by different adjuvants is a parameter that immunologists have only recently started to dissect. The inflammatory response is rapid and antigen-independent and it precedes the initiation of the antigen-specific response. It is characterized by the local release of pro-inflammatory cytokines, chemokines and the immediate influx of polymorphonuclear granulocytes from the bloodstream. Initiation of inflammation can occur through the activation of pattern-recognition receptors (PRRs), which are expressed in different forms and compositions by a variety of cells, including lymphocytes, granulocytes and endothelial cells.1,2 Ligation of PAMPs to PRRs expressed by dendritic cells (DCs), the type of PRR and the surrounding cytokine milieu are all factors that contribute to DC maturation and hence influence the cytokines and costimulatory molecules expressed by the mature DCs.3,4 As T-cell differentiation is influenced by the different costimulatory signals received from the DCs,5 the early stimulation of this subset and the degree of inflammation at the site of injection are involved in shaping the ensuing immune response and thus have a major impact on the profile of the subsequent T-cell response.6
One adjuvant that induces strong Th1 responses in various animal models is monophosphoryl lipid-A (MPL) formulated with cationic dimethyldioctadecylammonium (DDA) liposomes (DDA/MPL).7–9 Much is already known about the ability of this adjuvant to enhance antigen uptake, antigen presentation to T cells10 and stimulate DCs through Toll-like receptors (TLRs),11,12 but limited information exists on the mechanism of this adjuvant in vivo. By comparison, the mechanism operating when aluminium salts induce an immune response has until recently remained elusive and even in defined in vitro models its activity is less clear than that of DDA/MPL. Initially, aluminium salts were believed to work primarily through the formation of a depot of antigen at the site of injection. However, although aluminium compounds are able to adsorb a variety of vaccine antigens, and some aluminium compounds do remain at the site of injection for prolonged periods of time,13 the actual importance of this mechanism has become unclear since it was shown that removing the injection site did not compromise the efficacy of an aluminium-adjuvanted vaccine.14 In a recent study, the aluminium-containing Imject® (Pierce Biochemicals) adjuvant recruited and activated inflammatory monocytes to the site of injection through the induction of uric acid,15 and in the presence of TLR agonists aluminium induces inflammasome activation.16–18 Aluminium salts induce Th2 differentiation and humoral immunity,19,20 but the exact mechanism, as well as the dependence on the classical Th2-inducing cytokine, interleukin (IL)-4, remains unclear both in vitro21–25 and in vivo.26,27 In this respect it is a problem that negative results are rarely published because this may lead to an erroneous bias in the general view of how aluminium salts function as adjuvants.
Here, we have investigated the early, innate chemotactic signals and inflammatory influx induced by Th1- and Th2-polarizing adjuvants. Using DDA/MPL as our model Th1 adjuvant and aluminium hydroxide [Al(OH)3] as our model Th2 adjuvant, we have found that whereas the Th1 adjuvant induced high amounts of a large range of pro-inflammatory mediators and the influx of various cell types, the Th2 adjuvant induced a more discrete response with the involvement of only few mediators and a different inflammatory cell recruitment. The only Th2-specific signals found at the site of injection were an influx of eosinophilic granulocytes and the local release of IL-5.
Materials and methods
Animals and antibodies
Female BALB/c or C57BL/6 mice were purchased from Harlan (Horst, the Netherlands). The mice were allowed free access to water and food, and they were under 6 months of age when used in the experiments. The protocol for the animal experiments was approved by the Danish Council for Animal Experiments. Unless stated otherwise, BALB/c mice were used in the experiments. All antibodies, cytometric bead array (CBA) kits and enzyme-linked immunosorbent assay (ELISA) sets were purchased from BD Biosciences/Pharmingen (Brøndby, Denmark) unless otherwise stated. The following antibodies were used for flow cytometry: rat α-mouse F4/80-conjugated phycoerythrin (PE) (AbD Serotec, Hamar, Norway), rat α-mouse F4/80-conjugated biotin (Caltag Laboratories, San Fransisco, CA), purified rat α-mouse CD16/CD32, rat α-mouse CD11c–PE–Cy7/allophycocyanin/PE, rat α-mouse CD19–allophycocyanin/peridinin chlorphyll protein (PerCP)–Cy5.5, rat α-mouse CD3–allophycocyanin/fluorescein isothiocyanate (FITC), rat α-mouse CD49b–allophycocyanin/PE, rat α-mouse CD4–PE, rat α-mouse Ly-6G–PE, or streptavidin–PerCP–Cy5.5.
Medium
The medium used in the experiments was complete RPMI, consisting of the following: RPMI-1640 (Gibco Invitrogen, Taastrup, Denmark) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 5 × 10−6 mβ-mercaptoethanol, 1% (v/v) penicillin–streptomycin, 1% sodium pyruvate, 1 mm l-glutamine, 10 mm HEPES and 100 μg/ml of gentamicin (Gibco Invitrogen).
Preparation of DDA liposomes and vaccines
DDA liposomes were prepared using the aqueous heat method as follows. DDA (as a bromide salt; Avanti Polar Lipids, Alabaster, AL) was suspended in sterile distilled water at 2·5 mg/ml and heated to 80° for 20 min. The suspension was vortexed regularly during the heating period. Before injection, vaccine and adjuvant formulations were mixed and left at 21° for 30 min with intermittent mixing.
Animal experiments
Antigen-specific responses: C57BL/6 or BALB/c mice were immunized subcutaneously three times, at 2-week intervals, with 200 μl of sterile saline containing 2 μg of recombinant Ag85B–ESAT-6 (Statens Serum Institut, Copenhagen, Denmark) adjuvanted in 25 μg of detoxified MPL (Avanti Polar Lipids) and 250 μg of DDA liposomes (DDA/MPL) or in 500 μg of Al(OH)3 (as 2% Alhydrogel; Brenntag Biosector, Frederikssund, Denmark). One week after the final immunization, six mice per group were bled. Single-cell suspensions of lymphocytes were washed and cultured in complete RPMI in round-bottom 96-well plates, containing 2 × 105 cells/well, and restimulated with 5 μg/ml of Ag85B–ESAT-6. After 3 days, the supernatants were collected and the cytokine release was analyzed by ELISAs. Four months after the final immunization, between three and six mice per group were bled and the serum was analyzed for Ag85B–ESAT-6-specific antibodies.
Adjuvant-specific responses: BALB/c mice were injected intraperitoneally (i.p.) with 200 μl of sterile saline containing either 25 μg of MPL and 250 μg of DDA liposomes or 500 of μg Al(OH)3. Mice were killed at the indicated time-points after injection and the peritoneal exudate cells (PECs) were harvested by washing the peritoneal cavity as follows: 2 ml of cold RPMI containing 10% FBS was injected, the abdomen was massaged gently for 30 seconds and 1 ml was extracted. The cells were put on ice immediately. A small volume of medium was used to prevent excessive dilution of the peritoneal exudate. The cells were spun down, washed and stained for flow cytometry. The peritoneal lavage fluid (i.e. the first supernatant) was analyzed for cytokines/chemokines by ELISA and CBA. For use in the microarray analysis, cells were centrifuged and resuspended in RNAlater RNA stabilizing Reagent (Qiagen, Copenhagen, Denmark) immediately after extraction from the peritoneal cavity. The observed effect of the adjuvants was not caused by the injection procedure and related tissue injury because injection of physiological saline did not induce cell recruitment (data not shown).
Flow cytometry
PECs were washed and Fc receptors were blocked with α-mouse CD16/CD32. The cells were kept at 4° during staining. TO-PRO-3 iodide (Invitrogen/Molecular Probes, Taastrup, Denmark) was added at 100 nm immediately before acquisition on a FACSCanto (BD Biosciences, Brøndby, Denmark) to exclude dead cells. All analyses were performed using the FCS express Software v3 (DeNovo Software, Thornhill, Canada) or FlowJo Flow Cytometry Analysis Software (Tree Star Inc., Ashland, OR). Cell types were identified according to the following markers: B cells (CD19+), DCs [CD11c+ major histocompatibility complex class II (MHC class II)+ F4/80− Ly-6G−/lo], eosinophils (Ly-6Gint CD49b+ CD3− [side-scatter (SSC)int/hi)], macrophages (F4/80+ MHC class II+ CD11c− Ly-6G−/lo), monocytes (F4/80+ MHC class II− CD11c− Ly-6G−/lo), neutrophils (Ly-6Ghi), natural killer (NK) cells [CD49b+ CD3− (SSClo)] and T cells (CD3+ CD49b−). For sorting of PECs from mice injected with Al(OH)3 according to their forward-scatter (FSC)-SSC profile, a BD FACSCalibur (BD Biosciences) was used. At least 10 000 cells of each population were collected.
Differential staining
Total PEC and sorted populations were spun onto microscopy slides using a Shandon Cytospin® (Thermo Fisher Scientific, Slangerup, Denmark). The cells were subsequently stained with Hemacolor (Merck, Hellerup, Denmark) according to the manufacturer’s protocol. The morphology of the cells was assessed using light microscopy.
Cytokine detection
Unless otherwise indicated, all cytokine analyses were performed using CBA. The peritoneal lavage fluid and supernatants were analyzed for C-C chemokine ligand (CCL)2 [monocyte-chemoattractant protein-1 (MCP-1)], IL-6, IL-10, tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and IL-12p70 using the CBA Inflammation kit according to the manufacturer’s protocol. IL-5 was measured by ELISA using the IL-5 minikit (Endogen; Thermo Fisher, Rockford, IL) following the manufacturer’s instructions. IL-1α was measured by ELISA using the IL-1α OptEia set, according to the protocol suggested by the manufacturer. CCL11 (Eotaxin-1) and CCL24 (Eotaxin-2) were measured by ELISA using the corresponding Mouse DuoSets (R&D Systems Europe Ltd., Oxon, UK), according to the manufacturer’s protocol. IFN-γ in restimulated cell culture supernatants was measured by ELISA using purified rat α-mouse IFN-γ as capture antibody and biotin-conjugated rat α-mouse IFN-γ as detection antibody followed by incubation with horseradish peroxidase-conjugated streptavidin (Zymed/Invitrogen, Taastrup, Denmark) and TMB Plus Ready-to-use substrate (Kem-En-Tec, Taastrup, Denmark). The reaction was stopped with 0·2 m H2SO4 and the absorbance was read at 450 nm.
Antibody detection
Detection of antibodies in the sera from immunized mice was performed using ELISA. In short, 96-well MaxiSorp plates were coated with 0·5 μg/ml of Ag85B–ESAT-6 in 15 mm Na2CO3, 35 mm NaHCO3 (pH 9·7), overnight at 4°. The plates were blocked in PBS containing 2% (w/v) bovine serum albumin and sera were added in duplicate serial dilutions. Specific antibodies were detected following incubation with rabbit α-mouse IgG1 or IgG2a conjugated to horseradish peroxidase (Zymed/Invitrogen), and TMB Plus Ready-to-use was used as the substrate. The reaction was stopped with 0·2 m H2SO4 and the absorbance (optical density) was read at 450 nm.
Statistical analysis
Differences between the two adjuvant treatments were determined statistically using the t-test.
RNA isolation and microarray analysis
For the microarray analysis, PECs were stored at −20° in RNAlater (Qiagen) until required for isolation of RNA, which was performed using the RNeasy Plus Mini kit (Qiagen), according to the manufacturer’s instructions. The quality and quantity of RNA was evaluated using the RNA 6000 Nano LabChip kit and the Agilent 2100 bioanalyzer (Agilent Technologies, Nærum, Denmark). Messenger RNA (mRNA) amplification, biotin-labelling and purification of amplified RNA (aRNA) was performed using the MessageAmp II aRNA kit (Ambion, Applied Biosystems, Nærum, Denmark). Equal amounts of aRNA were applied to GeneChip® Mouse Genome 430A 2.0 Arrays (Affymetrix, High Wycombe, UK) using the protocol supplied by the manufacturer.
Bioinformatics
Scanned Affymetrix gene chip CEL files were analyzed using the statistical language R (version 2.4.1) with the use of the Bioconductor packages ‘affy’, ‘mouse430a2’ and ‘annotate’ (http://www.bioconductor.org). Normalization was performed using the non-linear Qspline method,28 expression indexes were calculated using the robust multi-array average method (RMA)29 and statistical testing was performed using probe-level t-tests in an approach similar to that taken by Lemon and co-workers.30 Volcano plots31 based on all possible combinations were used to set the level of statistical significance, which was subsequently used to determine which genes were differentially expressed. Hypergeometric testing based on gene ontologies defined by the GO Consortium was used to determine which biological processes were most significantly over-represented in the list of differentially expressed genes.32
Results
Al(OH)3 and DDA/MPL induce polarized immune responses
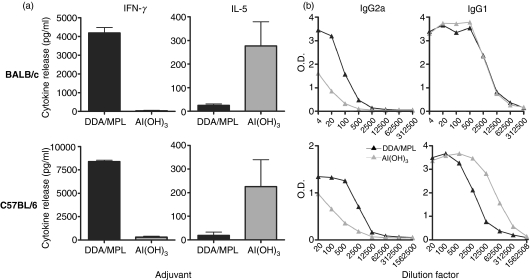
In the present study we investigated the innate signals preceding Th1/Th2 differentiation using the two model adjuvants Al(OH)3 and DDA/MPL. These two adjuvants were evaluated in vivo to confirm their respective capacities to induce Th1 or Th2 antigen-specific responses using a standard subcutaneous immunization protocol. Therefore, the mycobacterial fusion protein Ag85B–ESAT-6 was adjuvanted in either DDA/MPL or Al(OH)3 and injected three times, at 2-week intervals, into two different mouse strains. As expected, this gave rise to a polarized immune response with either a distinct Th1 (DDA/MPL) or Th2 [Al(OH)3] profile: the formulation with DDA/MPL led to the release of high levels of antigen-specific IFN-γ, whereas high amounts of IL-5 were found only in the Al(OH)3-vaccinated group (Fig. 1a). This difference in the overall immune profile was also reflected in the antibody subclass ratio where a high IgG2a : IgG1 ratio is commonly associated with Th1 responses and a lower ratio is associated with Th2 responses (Fig. 1b). The response to the two adjuvants was qualitatively very similar in BALB/c and C57BL/6 mice, with almost identical cytokine profiles and antibody responses observed.
Figure 1.
Dimethyldioctadecylammonium/monophosphoryl lipid-A (DDA/MPL) and aluminium hydroxide [Al(OH)3] adjuvants induce antigen-specific T-helper 1 (Th1) and T-helper 2 (Th2) responses, respectively. BALB/c or C57BL/6 mice were immunized three times with Ag85B–ESAT-6 in either DDA/MPL (black bars/symbols) or Al(OH)3 (grey bars/symbols). (a) One week after the final immunization the amount of interferon-γ (IFN-γ) and interleukin-5 (IL-5) in the supernatants of pooled blood lymphocytes from six mice restimulated with antigen was determined by enzyme-linked immunosorbent assay (ELISA). Bars represent the means of triplicate determinations and error bars represent the standard error of the means. (b) Four months after the final immunization, antigen-specific IgG2a and IgG1 antibody responses in pooled serum from four mice were determined by enzyme-linked immunosorbent assay (ELISA). The cytokine levels of naïve control mice were < 0·30 pg/ml and the antibody absorbance optical density (OD) levels were < 0·1. The results are representative of at least three individual experiments.
Adjuvant-induced recruitment patterns
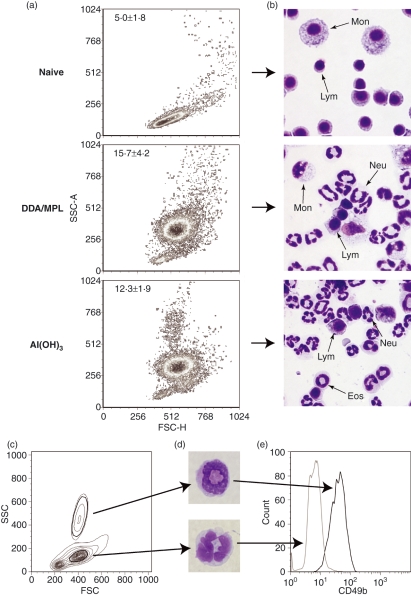
The adjuvant-induced innate signals preceding T-helper cell differentiation were subsequently investigated at the site of injection. As we had achieved similar Th1/Th2 profiles in BALB/c and C57BL/6 mice, we continued this more detailed analysis in the BALB/c strain only. We injected the adjuvants in the absence of antigen into the peritoneal cavities and isolated the cells recruited (the PECs). After 26 hr, we detected a major change in the FSC-SSC profiles of the two adjuvant groups compared with non-injected naïve mice (Fig. 2a). This was confirmed by differential staining, which showed increased numbers of polymorphonuclear granulocytes, mainly neutrophils, after injection of both adjuvants (Fig. 2b) and approximately three times more cells in total. After Al(OH)3 injection, a distinct population with an intermediate FSC (FSCint) profile, but a high SSC (SSChi) profile, was found in the inflammatory exudate (Fig. 2a). This correlated with the differential staining, which demonstrated a small, but clearly detectable, population of eosinophils in the exudate from these mice (Fig. 2b). As distinction of murine eosinophils by flow cytometry can be problematic, we sorted the FSCint SSChi cells, as well as the FSCint SSCint population as a control, by fluorescence-activated cell sorting (FACS) (Fig. 2c) and performed a differential staining, which confirmed distinct eosinophilic (SSChi) and neutrophilic (SSCint) morphologies of the two populations (Fig. 2d). A specific marker for eosinophils is lacking in mice and they can therefore be difficult to distinguish from peritoneal differentiating monocytes and neutrophils if using Gr-1 (Ly-6G/C, a commonly used marker for polymorphonuclear granulocytes), because their FSC-SSC profiles overlap when they differentiate or become activated. However, the α2-integrin, CD49b, which is commonly used as a marker for murine NK cells, is also expressed on eosinophils (Fig. 2e). As NK cells and eosinphils are easily distinguished on their SSC characteristics, and CD49b is not found on neutrophils or monocytes, CD49b was used as a marker for murine eosinophils.
Figure 2.
Adjuvant-induced inflammatory exudates. (a) Forward scatter (FSC)-side scatter (SSC) density plots of peritoneal exudate cells (PECs) from a naive mouse and from mice injected intraperitoneally (i.p.) with dimethyldioctadecylammonium/monophosphoryl lipid-A (DDA/MPL) or aluminium hydroxide [Al(OH)3] 26 hr previously. The average number (± standard deviation) of PECs (× 106) harvested from 3–10 mice is indicated in the top left corners of each plot. (b) As in (a) but PECs were stained with Hemacolor and analyzed by light microscopy for determination of monocytes/dendritic cells/macrophages (Mon), lymphocytes (Lym), neutrophils (Neu) and eosinophils (Eos). Images were recorded at 400 × magnification. (c) Contour plot of PECs from a mouse injected with Al(OH)3 26 hr previously. (d) The cells in (c) were sorted according to their FSC-SSC profile and stained with Hemacolor; one representative cell at 1000 × magnification is shown for each of the sorted populations. (e) The expression of CD49b on the two sorted populations, eosinophils (SSChi) and neutrophils (SSCint), was determined by flow cytometry.
Cells and chemokines at the site of injection
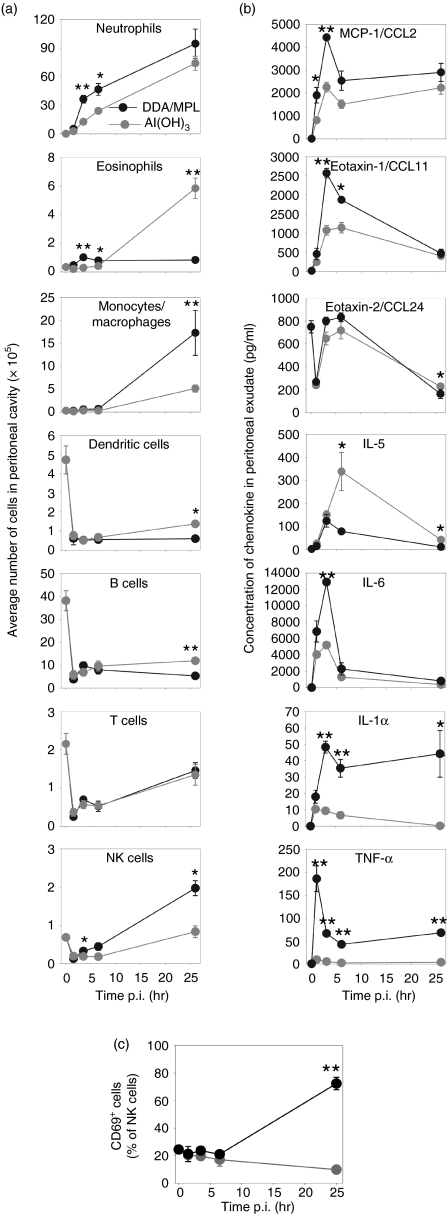
The distinct FSC-SSC profiles observed after injection of the two adjuvants were further characterized quantitatively over time by surface-staining for markers of different cell types (Fig. 3a). Confirming the differential staining, the predominant cells were, shortly after injection of either adjuvant, neutrophilic granulocytes. Furthermore, the Al(OH)3 group was characterized by the recruitment of high numbers of eosinophils at 26 hr after adjuvant injection. By contrast, DDA/MPL had recruited significantly more monocytes and mature macrophages at the same time-point. Another subset that was recruited preferentially by DDA/MPL was NK cells and at 26 hr their numbers were significantly higher than in the Al(OH)3 group. In addition, the NK cells from DDA/MPL-injected mice were activated to a higher extent, as assessed by the degree of CD69 expression (Fig. 3c). Before adjuvant injection (time = 0 corresponds to naïve mice), B cells and DCs were common in the peritoneal cavity but their numbers rapidly declined after injection of the adjuvants. However, a small, but significant, increase of B cells and DCs 26 hr after injection of Al(OH)3 was observed, although the levels were still lower than in naïve mice. In this antigen-free model, the number of T cells was also higher in naïve mice, in particular at the early time-points.
Figure 3.
Cell types and cytokines/chemokines induced by T-helper 1 (Th1)- and T-helper 2 (Th2)-polarizing adjuvants. (a) Peritoneal exudate cells (PECs) were harvested at the indicated time-points after intraperitoneal (i.p.) injection of either dimethyldioctadecylammonium/monophosphoryl lipid-A (DDA/MPL) (black) or aluminium hydroxide [Al(OH)3] (grey) and analyzed by flow cytometry. (b) Cytokines/chemokines expressed at the site of adjuvant injection. Expression of cytokines/chemokines in the peritoneal lavage fluid was investigated by cytometric bead array (CBA) or enzyme-linked immunosorbent assay (ELISA). CCL, C–C chemokine ligand; IL, interleukin; TNF-α, tumour necrosis factor-α. (c) The percentages of natural killer (NK) cells from (a) expressing CD69. Each data point represents the average of three individual mice and the error bars show the standard error of the mean. Statistically significant differences between the two groups have been indicated: *P< 0·05, **P< 0·01. The results are representative of three experiments. p.i., postinjection.
The peritoneal lavage was harvested in parallel to investigate the expression of selected chemokines and cytokines by CBA and ELISA. In general, DDA/MPL induced much higher quantities of the various cytokines and chemokines investigated and the only cytokine found in higher quantities in mice injected with Al(OH)3 was IL-5 (Fig. 3b). With the exception of IL-5 and CCL24 (Eotaxin-2), which remained at or below the relatively high background level, the amounts of all cytokines and chemokines investigated in the peritoneal lavage were 2–50-fold higher after injection of DDA/MPL compared with Al(OH)3 (Fig. 3b). The Th1/Th2-associated cytokines, IL-12p70 and IL-4, as well as IL-17, which is associated with inflammation and neutrophil homeostasis, were below the level of detection, whereas IFN-γ levels were repeatedly very low and only just detectable after injection with DDA/MPL (data not shown).
Gene expression profiles
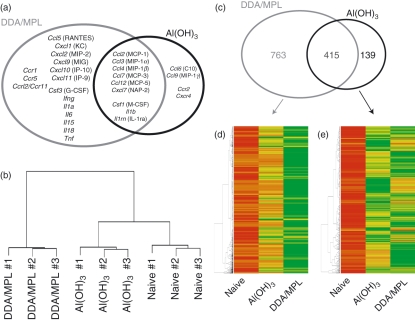
We also investigated the expression of genes for cytokines, chemokines and chemokine receptors in a microarray analysis on mRNA extracted from the PECs of mice injected with the adjuvants 26 hr previously (Fig. 4). This time-point was chosen to ascertain a sufficient number of cells to achieve a high enough amount of mRNA for the subsequent microarray analysis to be carried out on individual mice and thus strengthen the statistics of the experiment. The microarray data from the experiment have been submitted to ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae). Both adjuvants induced the expression of several granulocyte and monocyte chemoattractants and activators (Csf1, Ccl2, Ccl3, Ccl4, Ccl7, Ccl12 and Cxcl7) and also Il1b (Fig. 4a). However, in confirmation of its much stronger inflammatory potential, the injection of DDA/MPL induced the expression of genes for a range of C-X-C chemokines, including the NK- and T-cell-attracting C-X-C chemokine ligand (CXCL) 9 [monokine induced by IFN-γ (MIG)], CXCL10 [IFN-γ-inducible protein-10 (IP-10)], CXCL11 (IP-9), the C-C chemokine CCL5 [regulated on activation normal T-cell expressed and secreted (RANTES)], as well as the neutrophil-promoting granulocyte colony-stimulating factor (G-CSF) and several Th1-associated cytokines, including IFN-γ, IL-15 and TNF-α. In contrast to the large panel of genes expressed after injection of DDA/MPL, only a few genes were selectively expressed after injection of Al(OH)3. Among these were the genes for CCL9 [macrophage inflammatory protein-1γ (MIP-1γ)] and CCL6 (C10). A hierarchical cluster analysis of the overall microarray results (∼14 000 genes) illustrated that based on their entire gene-expression profiles, all nine individual mice clustered according to their treatment (Fig. 4b). In addition, this analysis demonstrated that the Al(OH)3-induced gene-expression profile more closely resembled that of naïve mice than that of DDA/MPL-injected mice. Considering the entire microarray, DDA/MPL up-regulated more than twice as many genes as Al(OH)3, and 75% of the genes up-regulated by Al(OH)3 were also up-regulated by DDA/MPL (Fig. 4c). In addition, most of the genes significantly up-regulated after injection of DDA/MPL were more highly expressed in the DDA/MPL group compared with the Al(OH)3 group (Fig. 4d). This was in contrast to the genes up-regulated by Al(OH)3 (Fig. 4e).
Figure 4.
Hierarchical clustering and specifically up-regulated genes. Mice were injected intraperitoneally (i.p.) with dimethyldioctadecylammonium/monophosphoryl lipid-A (DDA/MPL) or aluminium hydroxide [Al(OH)3], and the peritoneal exudate cells (PECs) were harvested 26 hr later and their gene-expression profiles were analyzed on Affymetrix microarrays. (a) Up-regulated genes for chemokines, chemokine receptors and cytokines after injection of the indicated adjuvants [grey circle: DDA/MPL; black circle: Al(OH)3]. Genes up-regulated after injection of both adjuvants are shown in the overlapping part of the circles. Common names of the chemokines are shown in parenthesis. (b) Comparison of the expression profile of each microarray chip (each chip representing one individual mouse, corresponding to the nine branches), showed that the mice clustered according to their treatment. The cluster dendrogram is based on the Euclidian distance. (c) The number of up-regulated genes after injection of the indicated adjuvants [grey circle: DDA/MPL; black circle: Al(OH)3]. The number of genes up-regulated after injection of both adjuvants is shown in the overlapping part of the circles. (d) The genes significantly up-regulated after injection of DDA/MPL are represented in a clustered heatmap based on the average gene-expression intensities of three individual mice per group. Red represents low expression, yellow represents intermediate expression and green represents high expression of the genes. (e) As in (d) but the heat map is based on the genes up-regulated after injection of Al(OH)3. Up-regulated genes were determined at the level of statistical significance (P < 0·05) as determined by Volcano plots.31 KC, keratinocyte-derived chemokine; NAP, neutrophil-activating peptide.
The genes that were up-regulated or differentially expressed after injection of the adjuvants were subjected to a hypergeometrical test in which it was tested if the genes belonging to known biological processes were over-represented compared with their distribution on the microarray (functional annotation). The five most statistically significant biological processes are listed in Table 1. We found that both adjuvants induced genes involved in immune and inflammatory responses, but DDA/MPL also induced genes involved in cytokine and chemokine signalling and apoptosis (‘DDA/MPL > Naïve’), whereas Al(OH)3 induced genes related to metabolism [‘Al(OH)3 > Naïve’]. However, when the two adjuvant groups were compared in a separate test, it became clear that DDA/MPL induced more genes involved in immune and inflammatory responses than Al(OH)3 [‘DDA/MPL > Al(OH)3’], whereas Al(OH)3 induced more genes involved in energy transport and synthesis of proteins [‘Al(OH)3 > DDA/MPL’].
Table 1.
Functional annotation
| Up-regulated processes |
Differentially expressed processes |
||
|---|---|---|---|
| DDA/MPL > Naïve | Al(OH)3 > Naïve | DDA/MPL > Al(OH)3 | Al(OH)3 > DDA/MPL |
| Immune response | Inflammatory response | Immune response | Protein biosynthesis |
| Inflammatory response | Glycolysis | Inflammatory response | Ribosome biogenesis |
| Cytokine and chemokine mediated signalling pathway | Pentose-phosphate shunt | Ag processing, endogenous Ag via MHCI | Tricarboxylic acid cycle |
| Actin cytoskeleton organization and biogenesis | Immune response | Ag presentation, endogenous Ag | Ag processing, exogenous Ag via MHCII |
| Regulation of apoptosis | ATP biosynthesis | Defense response | Ribosome biogenesis and assembly |
A hypergeometric test based on the microarray data was used to determine which biological processes were up-regulated (test comparing adjuvant group with naïve) or differentially expressed (test comparing the two adjuvant groups) in peritoneal exudate cells (PECs) 26 hr after injection of adjuvants. The five most significantly changed processes are listed according to descending significance. The biological processes were defined according to the Gene Ontology (GO) Consortium.
Ag, antigen; Al(OH)3, aluminium hydroxide; DDA/MPL, dimethyldioctadecylammonium/monophosphoryl lipid-A; MHCI, major histocompatibility complex class I; MHCII, major histocompatibility complex class II.
Discussion
The cytokine milieu that is responsible for the polarization of T cells into either a Th1 or a Th2 phenotype has been the subject of thorough in vitro studies and, although this distinction is less strict and more complex in vivo, the studies clearly demonstrate that the presence of IFN-γ and/or IL-12 during T-cell differentiation leads to a Th1 profile, whereas the presence of IL-4 leads to a Th2 profile.33–35 More recently, the mechanisms behind the reciprocal regulation of Th1 and Th2 responses have been unravelled at the genetic level: activation of the signal transducer and activator of transcription (STAT)1 and STAT4 signalling pathways results in IFN-γ and IL-12 production and suppression of the STAT6 pathway, which would have led to IL-4 production and vice versa.36–38 At the cellular level, several studies have attempted to clarify the activity of adjuvants by studying their effect on antigen-presenting cells (APCs) but this is an oversimplification that does not take into consideration the complexity of the intact organism where multiple inter-related mechanisms act in concert to induce, propagate and maintain the immune response.
In our study we wanted to investigate the initial effect of two polarizing adjuvants on the immune system in vivo. We found that DDA/MPL induced a complex inflammatory influx that was dominated by neutrophils, APCs and NK cells. This response was associated with high concentrations of pro-inflammatory and Th1-associated cytokines as well as with several monocyte and granulocyte chemoattractants detectable at both the protein and gene-expression level. An early signal for the strong inflammation induced by Th1 adjuvants has been suggested to be pro-inflammatory cytokines secreted by NK cells, which, when activated, are able to produce large amounts of IFN-γ and thus drive Th1 differentiation.39 Here, we found that the Th1-inducing adjuvant, DDA/MPL, recruited high numbers of NK cells to the site of injection. Furthermore, approximately 80% of these cells were activated, as assessed by their expression of CD69, whereas < 10% of the NK cells were activated after injection of Al(OH)3. The ability of DDA/MPL to recruit NK cells could be mediated by chemokines such as the C-X-C chemokine receptor 3 (CXCR3) ligands CXCL9 (MIG), CXCL10 (IP-10) and CXCL11 (IP-9),40 which were all significantly expressed after injection of this adjuvant. In addition to the recruitment of NK cells, DDA/MPL seems to be a general activator of the immune system, in particular of the residential APCs and of the mesothelial cells lining the peritoneal cavity, most probably mediated through TLR4.41,42 Activation through TLR4 induces TNF-α,41 CCL-2 (MCP-1) and CXCL2 (MIP-2) expression42 which, as observed, leads to recruitment of inflammatory monocytes causing a positive feedback that further accelerates the recruitment and results in macrophage differentiation. In addition, inflammatory monocytes can differentiate into DCs en route to the draining lymphoid organs,43 thus providing a source of APCs capable of initiating subsequent antigen-specific responses.
In comparison, injection of the Th2 adjuvant, Al(OH)3, led to a more limited inflammatory response with a delayed cellular influx, restricted almost exclusively to neutrophils and eosinophils, and to a less pronounced release of pro-inflammatory cytokines and chemokines. In contrast to Th1 responses, the components that induce Th2 responses are few and the mechanisms less well understood. In general, Th2 responses are associated with allergic reactions and infections with extracellular parasites, such as helminths, as well as vaccines adjuvanted with aluminium salts. Understanding how Th2 responses are induced have therefore been investigated in detail using aluminium salts but despite these efforts some controversy still exists.19 Adsorption of antigen to the various aluminium compounds has long been accepted as important for their ability to induce good immune responses but this has recently been disputed.44,45 Similarly, the significance of a depot of antigen at the injection site is still unclear; as is the effect on APCs.21,22,24,25 In a recent study, aluminium, in the form of Imject® Alum, was shown to induce uric acid release at the injection site, leading to recruitment of inflammatory monocytes.15 Similarly, we observed increased numbers of monocytes and macrophages after injection of Al(OH)3 but we also found that the numbers were much lower than after injection of DDA/MPL and we did not observe increased numbers of mature DCs. The differences may be explained by the use of different sources of aluminium, because we used the licensed Alhydrogel, which is different from Imject® Alum that, besides aluminium, contains equal amounts of magnesium hydroxide.46In vitro, TLR ligands are required for aluminium compounds to induce IL-1β production by macrophages.17,18 Nevertheless, like Mosca et al.,47 we found that Al(OH)3 induced the expression of Il1b at the site of injection. This lack of dependence on TLR ligands in vivo is probably an effect of the inevitable local necrosis caused by injection of aluminium compounds and the subsequent release of uric acid and activation of the inflammasome.15,48
Characteristically, Al(OH)3 induced the accumulation of not only neutrophils but also of eosinophils at the injection site. This is in agreement with the work of Walls49 and with studies of aluminium-induced experimental allergy.50 Furthermore, IL-5 was the only cytokine or chemokine found to be selectively expressed early after injection of Al(OH)3. IL-5 is strongly involved in eosinophilopoiesis and has a known synergistic effect on the ability of eotaxins to attract eosinophils.51,52 That the observed eotaxin levels were lower after injection of Al(OH)3 than of DDA/MPL highlights the importance of IL-5 for optimal recruitment of eosinophils. That eosinophils may play a role in the ability of Al(OH)3 to induce optimal Th2 responses is suggested in a study where aluminium-induced B-cell responses in vivo required not only IL-4 but also the presence of eosinophils and/or an unidentified myeloid Gr-1+ cell population.53
Based on the microarray gene-expression profiles and the cluster analysis, we found that injection of Al(OH)3 resulted in an immunological profile which was more closely related to that of naïve mice than to that of DDA/MPL-injected mice. That Th2 responses are induced under more ‘immunologically silent’ conditions compared with Th1 responses is consistent with a default Th2 pathway, as suggested several years ago by Jankovic et al.,54 which states that during infectious disease a Th2 response will develop in the absence of Th1-promoting factors. More recently, the same group has found that parasite-induced Th2 responses are related to a dampening of Th1-stimulating immune reactions and a lack of MyD88-signalling.55 In addition, Sun et al.56,57 have found that Th2 responses arise when Th1 or TLR signals are lacking and, although it has been demonstrated that if IL-4 is blocked following immunization with aluminium a Th1-like response arises,58 Th2 responses can still be found in the absence of IL-4 or IL-4 signalling in vivo.26,27 Our microarray experiment, which was based on individual mice, indicates that the initial immune response to Al(OH)3 is more delayed compared with that of DDA/MPL because metabolic processes (e.g. glycolysis, ATP biosynthesis, protein biosynthesis, tricarboxylic acid cycle) precede the energy-consuming and protein-dependent immunological processes (e.g. immune response, inflammatory response, cytokine/chemokine signalling). As de novo synthesis of MHC class I is delayed compared with MHC class II in DCs infected with bacteria,59 it seems likely that the cells from DDA/MPL-injected mice were more advanced because they had a higher expression of genes related to endogenous antigen processing.
Our findings emphasize that adjuvants affect not just APCs but also the other cells at the injection site, leading to a rapid selective cellular recruitment. Early after injection, the Th2 adjuvant, Al(OH)3, selectively induced the release of IL-5 and a subsequent influx of eosinophils. Otherwise, Al(OH)3 induced a limited inflammatory response compared with the Th1 adjuvant, DDA/MPL, which was highly active, inducing a range of pro-inflammatory cytokines and chemokines and the consistent influx of inflammatory monocytes, macrophages and activated NK cells. Interestingly, at these early time-points, and in the absence of antigen, we were unable to detect the classical Th1- and Th2-associated cytokines IL-12 and IL-4 at the injection site, suggesting that these cytokines are expressed either later or, when antigen is present, in the draining lymphoid organs as part of the antigen-specific response. Understanding the precise molecular mechanisms that initiate these complex cascades and result in an immune response with the desired profile will allow the development of highly defined, tailored adjuvants that in the future can form the basis of new, successful vaccines.
Acknowledgments
We thank Anniezette Amentorp Lander, Maria Nørtoft Sørensen and Linda Christensen for their kind and excellent technical assistance. Furthermore, we greatly appreciate the opportunity to do the microarray analyses at the Center for Biological Sequence Analysis at the Technical University of Denmark. Thomas Lindenstrøm is acknowledged for critical revision of this manuscript and numerous, inspiring discussions. This work was supported by a grant from the Danish Council for Developmental Research.
Glossary
Abbreviations:
- Al(OH)3
aluminium hydroxide
- APC
antigen-presenting cell
- CBA
cytometric bead array
- CCL
C–C chemokine ligand
- CXCL
C-X-C chemokine ligand
- CXCR
C-X-C chemokine receptor
- DC
dendritic cell
- DDA
dimethyldioctadecylammonium
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- FSC
forward scatter
- G-CSF
granulocyte colony-stimulating factor
- IFN
interferon
- IL
interleukin
- IP
IFN-γ-inducible protein
- MCP
monocyte chemoattractant protein
- MIG
monokine induced by IFN-γ
- MIP
macrophage inflammatory protein
- MPL
monophosphoryl lipid-A
- NK
natural killer
- PAMP
pathogen-associated molecular pattern
- PE
phycoerythrin
- PEC
peritoneal exudate cell
- PRR
pattern-recognition receptor
- RANTES
regulated on activation normal T-cell expressed and secreted
- SSC
side scatter
- Th
T helper
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
Disclosures
None.
References
- 1.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 2.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–24. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.McLellan AD, Sorg RV, Williams LA, Hart DN. Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand- dependent pathway. Eur J Immunol. 1996;26:1204–10. doi: 10.1002/eji.1830260603. [DOI] [PubMed] [Google Scholar]
- 6.de Heusch M, Oldenhove G, Urbain J, Thielemans K, Maliszewski C, Leo O, Moser M. Depending on their maturation state, splenic dendritic cells induce the differentiation of CD4(+) T lymphocytes into memory and/or effector cells in vivo. Eur J Immunol. 2004;34:1861–9. doi: 10.1002/eji.200424878. [DOI] [PubMed] [Google Scholar]
- 7.Langermans JA, Doherty TM, Vervenne RA, et al. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23:2740–50. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 8.Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 2000;68:791–5. doi: 10.1128/iai.68.2.791-795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in Mycobacterium tuberculosis subunit vaccines; immunity, pathology and protection. Immunology. 2008;124:175–86. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121:216–26. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002;10:S32–7. doi: 10.1016/s0966-842x(02)02426-5. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine. 2005;23:1359–67. doi: 10.1016/j.vaccine.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Holt LB. Developments in Diptheria Profylaxis. London: Heinemann; 1950. pp. 67–99. [Google Scholar]
- 15.Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Willingham SB, Ting JP, Re F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–9. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewer JM. (How) do aluminium adjuvants work? Immunol Lett. 2006;102:10–5. doi: 10.1016/j.imlet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 20.HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20(Suppl. 3):S34–9. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- 21.Sun H, Pollock KG, Brewer JM. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine. 2003;10:849–55. doi: 10.1016/s0264-410x(02)00531-5. [DOI] [PubMed] [Google Scholar]
- 22.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson LA. The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69:1151–9. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimaniol AC, Gras G, Verdier F, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;24:3127–35. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- 24.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4+ T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25:4575–85. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–6. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 26.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 27.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to Freund’s complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–6. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 28.Workman C, Jensen LJ, Jarmer H, et al. A new non-linear normalization method for reducing variability in DNA microarray experiments. Genome Biol. 2002;3:research0048, 1–16. doi: 10.1186/gb-2002-3-9-research0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 30.Lemon WJ, Liyanarachchi S, You M. A high performance test of differential gene expression for oligonucleotide arrays. Genome Biol. 2003;4:R67, 1–11. doi: 10.1186/gb-2003-4-10-r67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolfinger RD, Gibson G, Wolfinger ED, Bennett L, Hamadeh H, Bushel P, Afshari C, Paules RS. Assessing gene significance from cDNA microarray expression data via mixed models. J Comput Biol. 2001;8:625–37. doi: 10.1089/106652701753307520. [DOI] [PubMed] [Google Scholar]
- 32.Wrobel G, Chalmel F, Primig M. goCluster integrates statistical analysis and functional interpretation of microarray expression data. Bioinformatics. 2005;21:3575–7. doi: 10.1093/bioinformatics/bti574. [DOI] [PubMed] [Google Scholar]
- 33.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 34.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 35.Abdi K, Singh N, Matzinger P. T-cell control of IL-12p75 production. Scand J Immunol. 2006;64:83–92. doi: 10.1111/j.1365-3083.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 36.Schindler H, Lutz MB, Rollinghoff M, Bogdan C. The production of IFN-gamma by IL-12/IL-18-activated macrophages requires STAT4 signaling and is inhibited by IL-4. J Immunol. 2001;166:3075–82. doi: 10.4049/jimmunol.166.5.3075. [DOI] [PubMed] [Google Scholar]
- 37.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 38.Heller NM, Matsukura S, Georas SN, Boothby MR, Rothman PB, Stellato C, Schleimer RP. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:573–82. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for TH1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 40.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73:2940–50. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato S, Yuzawa Y, Tsuboi N, Maruyama S, Morita Y, Matsuguchi T, Matsuo S. Endotoxin-induced chemokine expression in murine peritoneal mesothelial cells: the role of toll-like receptor 4. J Am Soc Nephrol. 2004;15:1289–99. [PubMed] [Google Scholar]
- 43.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 44.Romero Mendez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–33. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Hansen B, Sokolovska A, HogenEsch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine. 2007;25:6618–24. doi: 10.1016/j.vaccine.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 46.Hem SL, Johnston CT, HogenEsch H. Imject(R) Alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25:4985–6. doi: 10.1016/j.vaccine.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 47.Mosca F, Tritto E, Muzzi A, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–6. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Gregorio E, Tritto E, Rappuoli R. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol. 2008;38:2068–71. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 49.Walls RS. Eosinophil response to alum adjuvants: involvement of T cells in non-antigen-dependent mechanisms. Proc Soc Exp Biol Med. 1977;156:431–5. doi: 10.3181/00379727-156-39951. [DOI] [PubMed] [Google Scholar]
- 50.Hussain I, Randolph D, Brody SL, Song SK, Hsu A, Kahn AM, Chaplin DD, Hamilos DL. Induction, distribution and modulation of upper airway allergic inflammation in mice. Clin Exp Allergy. 2001;31:1048–59. doi: 10.1046/j.1365-2222.2001.01129.x. [DOI] [PubMed] [Google Scholar]
- 51.Shahabuddin S, Ponath P, Schleimer RP. Migration of eosinophils across endothelial cell monolayers: interactions among IL-5, endothelial-activating cytokines, and C-C chemokines. J Immunol. 2000;164:3847–54. doi: 10.4049/jimmunol.164.7.3847. [DOI] [PubMed] [Google Scholar]
- 52.Yang M, Hogan SP, Mahalingam S, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–43. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 53.Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–10. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- 54.Jankovic D, Liu Z, Gause WC. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 2001;22:450–7. doi: 10.1016/s1471-4906(01)01975-5. [DOI] [PubMed] [Google Scholar]
- 55.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–27. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Walsh M, Villarino AV, Cervi L, Hunter CA, Choi Y, Pearce EJ. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J Immunol. 2005;174:742–51. doi: 10.4049/jimmunol.174.2.742. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, Pearce EJ. Suppression of early IL-4 production underlies the failure of CD4 T cells activated by TLR-stimulated dendritic cells to differentiate into Th2 cells. J Immunol. 2007;178:1635–44. doi: 10.4049/jimmunol.178.3.1635. [DOI] [PubMed] [Google Scholar]
- 58.McDonald F, Mohrs M, Brewer J. Using bicistronic IL-4 reporter mice to identify IL-4 expressing cells following immunisation with aluminium adjuvant. Vaccine. 2006;24:5393–9. doi: 10.1016/j.vaccine.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 59.Rescigno M, Citterio S, Thery C, Rittig M, Medaglini D, Pozzi G, Amigorena S, Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc Natl Acad Sci USA. 1998;95:5229–34. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]