Abstract
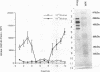
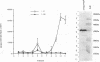
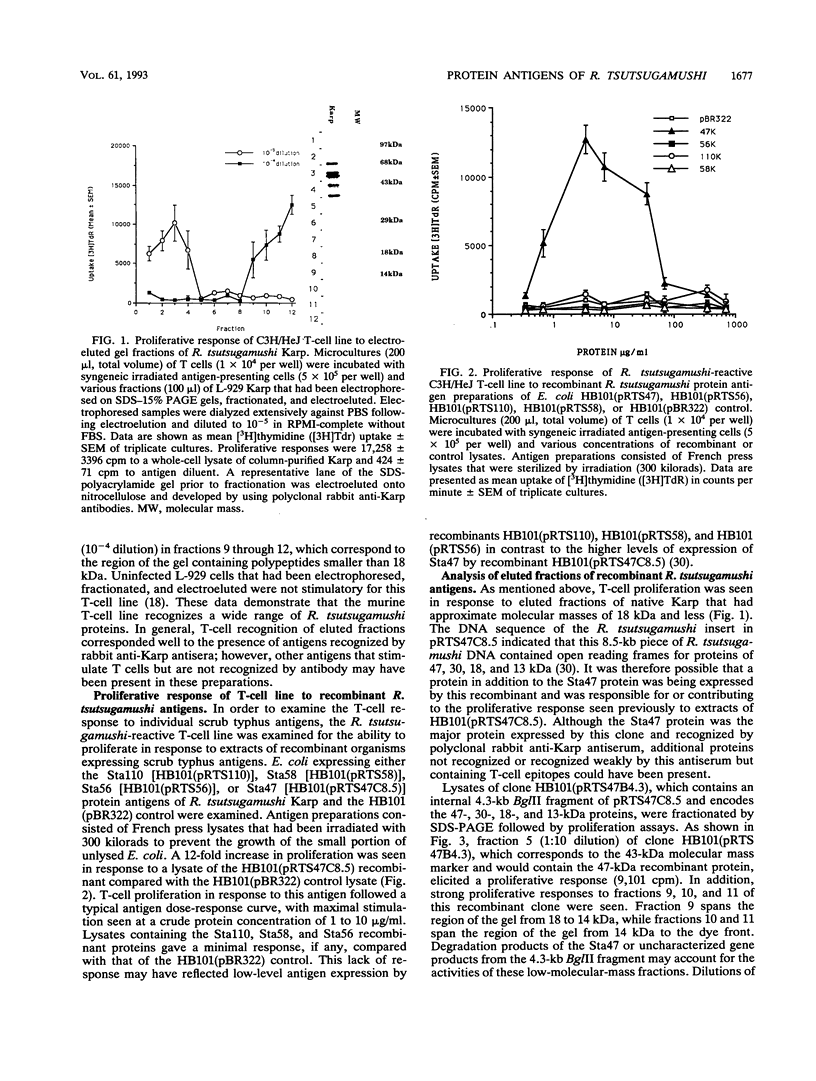
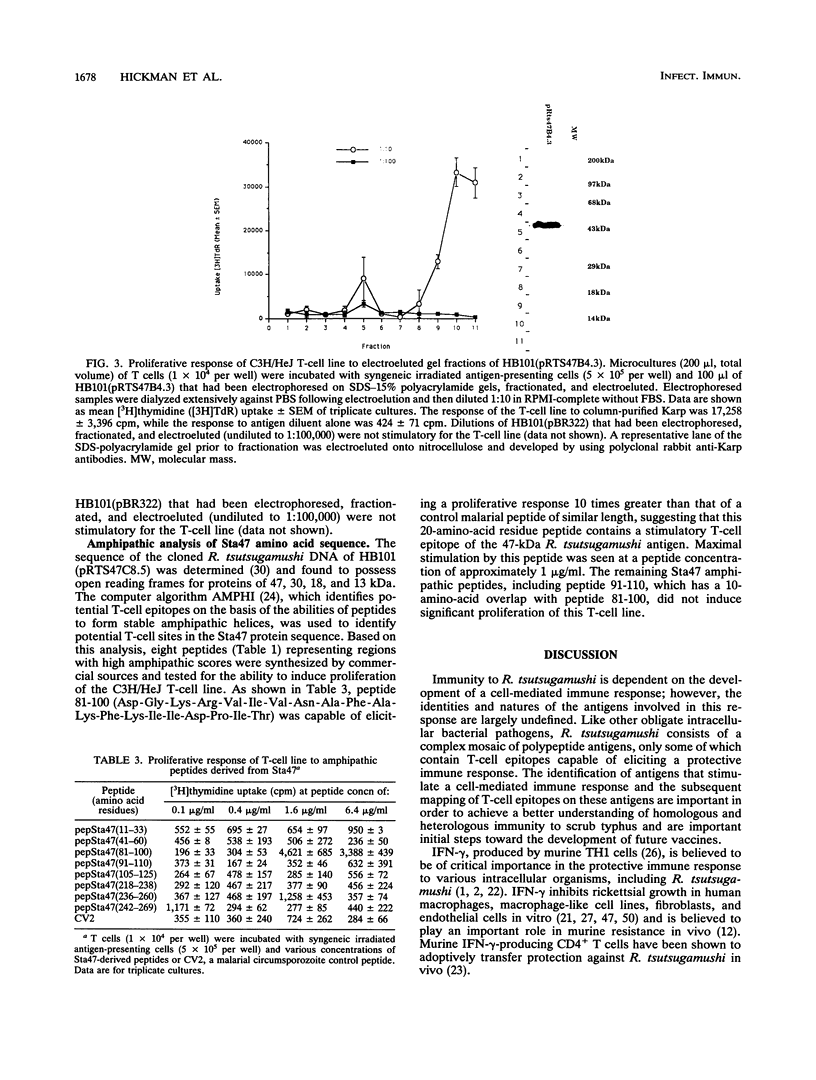
A polyclonal T-cell line with TH1 characteristics was used to assess the murine cellular immune response to native and recombinant Rickettsia tsutsugamushi antigens. Proliferation of this T-cell line was observed in response to numerous native antigen fractions, which indicates that the murine T-helper-cell response is directed at multiple scrub typhus antigens with no apparent antigenic immunodominance. Subsequent analysis of recombinant R. tsutsugamushi antigens made it possible to identify a 47-kDa scrub typhus antigen (Sta47) that was stimulatory for the polyclonal T-cell line. Recombinant clones encoding 56-, 58-, and 110-kDa antigens (Sta56, Sta58, and Sta110, respectively) were unable to induce proliferation of this T-cell line. DNA sequence analysis of the cloned rickettsial insert encoding the Sta47 protein revealed the presence of four open reading frames potentially encoding proteins of 47, 30, 18, and 13 kDa. Analysis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis-separated and eluted fractions of lysates from the recombinant HB101(pRTS47B4.3) demonstrated that the fractions containing the 47-kDa protein as well as those containing proteins less than 18 kDa were stimulatory. Selected synthetic amphipathic peptides derived from the Sta47 antigen sequence identified a 20-amino-acid peptide that gave a 10-fold increase in T-cell proliferation over a control malarial peptide of similar length. Recognition of the 47-kDa antigen by a T-cell line with TH1 characteristics implicates this protein as one of potential importance in protection studies and future vaccine development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee D. K., Sharp A. K., Lowrie D. B. The effect of gamma-interferon during Mycobacterium bovis (BCG) infection in athymic and euthymic mice. Microb Pathog. 1986 Apr;1(2):221–224. doi: 10.1016/0882-4010(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Identification of strain-specific and group-reactive antigenic determinants on the Karp, Gilliam and Kato strains of Rickettsia tsutsugamushi. Am J Trop Med Hyg. 1985 Nov;34(6):1173–1178. doi: 10.4269/ajtmh.1985.34.1173. [DOI] [PubMed] [Google Scholar]
- Falla J. C., Parra C. A., Mendoza M., Franco L. C., Guzmán F., Forero J., Orozco O., Patarroyo M. E. Identification of B- and T-cell epitopes within the MTP40 protein of Mycobacterium tuberculosis and their correlation with the disease course. Infect Immun. 1991 Jul;59(7):2265–2273. doi: 10.1128/iai.59.7.2265-2273.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. Identification and partial characterization of Rickettsia tsutsugamushi major protein immunogens. Infect Immun. 1985 Dec;50(3):603–609. doi: 10.1128/iai.50.3.603-609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque S., Saizawa K., Rojo J., Janeway C. A., Jr The influence of valence on the functional activities of monoclonal anti-L3T4 antibodies. Discrimination of signaling from other effects. J Immunol. 1987 Nov 15;139(10):3207–3212. [PubMed] [Google Scholar]
- Harris D. P., Bäckström B. T., Booth R. J., Love S. G., Harding D. R., Watson J. D. The mapping of epitopes of the 18-kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J Immunol. 1989 Sep 15;143(6):2006–2012. [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hickman C. J., Stover C. K., Joseph S. W., Oaks E. V. Molecular cloning and sequence analysis of a Rickettsia tsutsugamushi 22 kDa antigen containing B- and T-cell epitopes. Microb Pathog. 1991 Jul;11(1):19–31. doi: 10.1016/0882-4010(91)90090-w. [DOI] [PubMed] [Google Scholar]
- Jarboe D. L., Eisemann C. S., Jerrells T. R. Production and characterization of cloned T-cell hybridomas that are responsive to Rickettsia conorii antigens. Infect Immun. 1986 Apr;52(1):326–330. doi: 10.1128/iai.52.1.326-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Turco J., Winkler H. H., Spitalny G. L. Neutralization of lymphokine-mediated antirickettsial activity of fibroblasts and macrophages with monoclonal antibody specific for murine interferon gamma. Infect Immun. 1986 Jan;51(1):355–359. doi: 10.1128/iai.51.1.355-359.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kodama K., Kawamura S., Yasukawa M., Kobayashi Y. Establishment and characterization of a T-cell line specific for Rickettsia tsutsugamushi. Infect Immun. 1987 Oct;55(10):2490–2495. doi: 10.1128/iai.55.10.2490-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Moree M. F., Hanson B. Growth characteristics and proteins of plaque-purified strains of Rickettsia tsutsugamushi. Infect Immun. 1992 Aug;60(8):3405–3415. doi: 10.1128/iai.60.8.3405-3415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Oaks E. V., Rice R. M., Kelly D. J., Stover C. K. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect Immun. 1989 Oct;57(10):3116–3122. doi: 10.1128/iai.57.10.3116-3122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Wingfield M. E., Formal S. B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985 Apr;48(1):124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidberry H., Kaufman B., Wright D. C., Sadoff J. Immunoenzymatic analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985 Feb 11;76(2):299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- Sjöstedt A., Sandström G., Tärnvik A., Jaurin B. Nucleotide sequence and T cell epitopes of a membrane protein of Francisella tularensis. J Immunol. 1990 Jul 1;145(1):311–317. [PubMed] [Google Scholar]
- Sjöstedt A., Tärnvik A., Sandström G. The T-cell-stimulating 17-kilodalton protein of Francisella tularensis LVS is a lipoprotein. Infect Immun. 1991 Sep;59(9):3163–3168. doi: 10.1128/iai.59.9.3163-3168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. D., Bottomly K. Antigen-specific activation of effector macrophages by IFN-gamma producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989 Feb 1;142(3):760–765. [PubMed] [Google Scholar]
- Stover C. K., Marana D. P., Carter J. M., Roe B. A., Mardis E., Oaks E. V. The 56-kilodalton major protein antigen of Rickettsia tsutsugamushi: molecular cloning and sequence analysis of the sta56 gene and precise identification of a strain-specific epitope. Infect Immun. 1990 Jul;58(7):2076–2084. doi: 10.1128/iai.58.7.2076-2084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Marana D. P., Dasch G. A., Oaks E. V. Molecular cloning and sequence analysis of the Sta58 major antigen gene of Rickettsia tsutsugamushi: sequence homology and antigenic comparison of Sta58 to the 60-kilodalton family of stress proteins. Infect Immun. 1990 May;58(5):1360–1368. doi: 10.1128/iai.58.5.1360-1368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Surcel H. M., Syrjälä H., Karttunen R., Tapaninaho S., Herva E. Development of Francisella tularensis antigen responses measured as T-lymphocyte proliferation and cytokine production (tumor necrosis factor alpha, gamma interferon, and interleukin-2 and -4) during human tularemia. Infect Immun. 1991 Jun;59(6):1948–1953. doi: 10.1128/iai.59.6.1948-1953.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Ohashi N., Urakami H., Takahashi K., Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985 Jun;48(3):671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tärnvik A., Eriksson M., Sandström G., Sjöstedt A. Francisella tularensis--a model for studies of the immune response to intracellular bacteria in man. Immunology. 1992 Jul;76(3):349–354. [PMC free article] [PubMed] [Google Scholar]
- Van Schooten W. C., Elferink D. G., Van Embden J., Anderson D. C., De Vries R. R. DR3-restricted T cells from different HLA-DR3-positive individuals recognize the same peptide (amino acids 2-12) of the mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1989 Nov;19(11):2075–2079. doi: 10.1002/eji.1830191116. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]