Abstract
We present an adenoid cystic carcinoma of the base of tongue in a 48-year-old male with a restricted chromosomal alteration by cytogenetic and spectral karyotypic analysis (SKY). SKY and G-banding analyses identified the t(6;14)(q25;q13) as the sole structural aberration in all metaphases analyzed. This finding supports a critical role for this event in the development of this tumor. The implications of chromosome 6q translocation in this case and in previously reported adenoid cystic carcinomas are highlighted and discussed.
Keywords: Adenoid cystic carcinoma, Cytogenetic, Comparative genomic hybridizations, Spectral karyotyping, Translocations
Introduction
Adenoid cystic carcinoma (ACC) is an uncommon salivary gland malignancy that manifests a spectrum of cytomorphologic patterns and pursues a relentless progressive clinical course. Attempts to understand the development and progression of these tumors have been unrewarding due to the lack of neoplastic precursors, difficulty in cultivating them and the absence of an animal model [1, 2]. Identifying unique and consistent chromosomal abnormalities in this entity is necessary for the characterization of genetic events associated with its evolution and the development of novel biological targets for therapy [3–12].
Several cytogenetic and molecular studies of ACCs have reported recurrent and nonrandom alterations including translocation and deletion at certain regions on the long arm of chromosome 6 [5–11, 13–16]. The majority of the translocations consisted of balanced swapping between chromosome 6q sites and different chromosomal partners including 9q, 12q 14q, 15q and Xp regions [3–5, 8–11, 13]. Collectively, the data indicates that an important locus on chromosome 6q may house a critical gene(s) associated with the development and/or progression of ACC [17–21].
We report the cytogenetic analysis of an ACC of the base of tongue and discuss previous reports and the implication of these findings.
Materials and Methods
Case Report
A 48-year-old male presented with a bulky midline 4.3 cm base of tongue mass. A biopsy of the lesion revealed adenoid cystic carcinoma with perineural invasion. The patient underwent partial pharyngectomy and base of tongue resection. The lesion was a well-circumscribed, 5.0 cm in diameter, tan and firm mass. The patient’s postoperative course was uneventful, and he remained free of recurrence four months after resection.
Cytogenetics
Tumor tissue was finely minced into small pieces and cultured in DMEM medium (GIBCO Invitrogen, Carlsbad, CA) containing 15% fetal calf serum, 1% l-glutamine and 1% penicillin-streptomycin (Invitrogen, GIBCO Invitrogen, Carlsbad, CA), and ITS-X (Sigma, St. Louis, MO). The cultures were harvested and G-banded using standard protocols. Clonal chromosomal abnormalities identified by G-banding were described according to an ISCN, 2005. Clonality was described by detection of two cells with the same chromosome aberration and loss of the same chromosome in three cells. The metaphase spreads were aged at room temperature for a week for SKY analysis. The consensus karyotype was generated for each tumor based on G-banding and SKY analysis.
Sky
The human chromosome-painted cocktail was obtained from Applied Spectral Imaging (ASI, Carlsbad, CA). Hybridization and detection were carried out according to the manufacturer’s protocol, with slight modifications. Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Images were acquired with a SD200 Spectra cube (ASI) mounted on a Zeiss Axioplan II microscope using a custom designed optical filter (SKY-1), (Chroma Technology, Brattleboro, VT) and analyzed using SKY View 1.2 software (ASI, Carlsbad, CA). The breakpoints on the SKY-painted chromosomes were determined by comparison of corresponding inverted-DAPI banding of the same chromosome and by comparison with the G-banded karyotype for each case.
Results
Histopathology
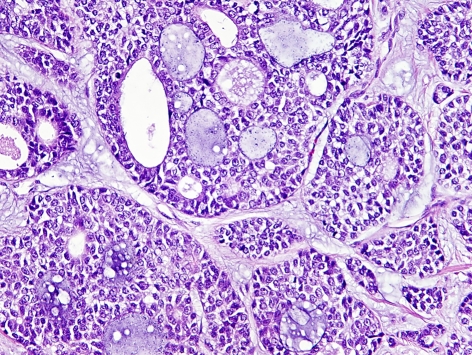
The tumor manifested the conventional cribriform and the ductal (tubular) patterns with the dual outer myoepithelial and inner ductal cell participation (Fig. 1). There was extensive perineural invasion identified by the tumor.
Fig. 1.
A photomicrograph of the tumor illustrating the dual cellular composition forming cords and glandular structures characteristic of adenoid cystic carcinoma
G-banding and SKY Analysis
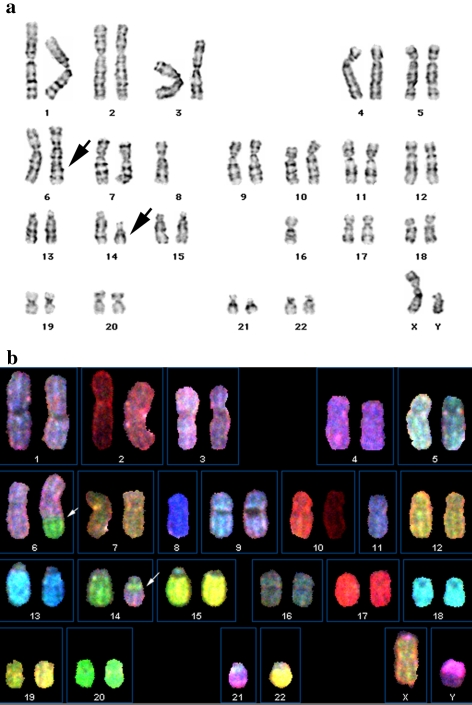
All the metaphase spreads displayed a balanced translocation involving the long arm of chromosome 6 at q25 and the long arm of chromosome 14 at q13 [46, XY, t(6;14) (q25;q13)] (Fig. 2a) [22]. Multicolor spectral karyotypic analysis from the same tumor confirmed the translocation in all 10 metaphases (Fig 2b). No other recurrent structural or numerical chromosomal changes were identified.
Fig. 2.
Karyotypic analysis of adenoid cystic carcinoma. (a) Conventional G-banded karyotype (arrow indicates the abnormal chromosomes). (b) A representative spectral classified karyotype showing t(6;14) translocation
Discussion
We report an adenoid cystic carcinoma of the tongue base with balanced translocation between chromosomes 6q25 and 14q13 regions as the only structural cytogenetic abnormality. The same translocation with a slightly different breakpoint [(6;14) (q22;q11)] has been identified among other structural and numerical abnormalities in a previous report of ACC [14]. In addition, the 6q25 breakpoint in our case had also been found in two different cytogenetic analyses of ACCs [8, 13] (Table 1). All together, 18 (48.6%) of the 37 ACCs cytogenetically analyzed to date manifested deletions and/or balanced translocations at the long arm of chromosome 6 [3–12]. Interestingly, the majority of these translocations involved the 6q regions in structural recombination with various chromosomal partners including 9q, 12q, 14q, 15q and Xp [23–25].
Table 1.
Chromosome 6q alterations in adenoid cystic carcinoma
| Reference #a | Tumor # | Deletion site | (#) | Translocation | (#) |
|---|---|---|---|---|---|
| [4] | 2 | 0 | (0) | t(6;9)(q21-22;p13-21), | (1) |
| (0) | t(X;6)(p22;q23) | (1) | |||
| [6] | 10 | 6q21 | (1) | t(6;9)(q23;p21) | (2) |
| [7] | 2 | 6q21 | (1) | t(6;9)(q21-22;p13-21) | (1) |
| [8] | 3 | 0 | (0) | t(6;9)(q23-25;p22-24) | (1) |
| [9] | 2 | 0 | (0) | 0 | (–) |
| [10] | 2 | 0 | (0) | t(6;9)(q23;p22) | (1) |
| [11] | 1 | 6q23 | (1) | 0 | (0) |
| [12] | 3 | 6q23 add9p22 | (0) | t(6;12)(p21;q13) | (2) |
| [13] | 1 | 0 | (0) | t(6;15)(q25-15) | (1) |
| [14] | 11 | 6q22-q24 | (5) | t(6;14)(q22;q11) | (1) |
| Present case | 1 | 0 | (0) | t(6;14) (q25;q13) | (1) |
a #: Number
Although, the significance of the multiple chromosomal partners (promiscuity) associated with the long arm of chromosome 6 translocation in ACCs is currently unknown, previous studies of lympho-proliferative disorders and sarcomas have reported similar balanced translocations are certain associated tumor phenotypes and biology [26–29]. These translocations are believed to result in activation of oncogenes or the formation of fusion genes that induce malignant transformation of a committed progenitor cell [27]. In that context, balanced promiscuous translocations may lead to the formation of phenotypically similar tumors of different clinical outcomes and therapeutic sensitivities [30]. Since the translocations in our case and in all previously reported ACCs involving chromosome 6q were exclusively balanced in nature and limited to a few breakpoints and chromosomal partners, we contend that an activated oncogene or a fusion gene is likely underlying the development of at least a subset of ACC [3, 4, 7, 8, 11]. Recently, several candidate genes on the 6q21–25 regions have been investigated in ACCs but none was implicated in their development [18, 21]. Therefore, additional attempts to clone these translocations are needed.
Exclusive of these translocations, interstitial deletions and frequent loss of heterozygosity using microsatellite markers at the 6q regions have also been reported [17–21]. In some of these studies, deletions in this region have been associated with aggressive clinical features [21]. These data strongly implicate a suppressor gene at this region in the pathogenesis of at least some of these tumors [6, 14, 15]. Accordingly, various molecular events are manifested in ACCs. Some of these events involve activation of oncogenes that may play an early or etiologic role in their development, and others include alterations of tumor suppressor genes that may be linked to their progression. Further molecular analysis of these tumors should allow the identification of these events.
Acknowledgments
This work was supported in part by the Kenneth D. Müller Professorship (AEN), National Cancer Institute Specialized Program of Research Excellence (SPORE) grant in head and neck cancer and the National Cancer Institute grant CA-16672.
References
- 1.Batsakis JG, Regezi JA. The pathology of head and neck tumors: salivary glands, part 1. Head Neck Surg. 1978;1:59–68. doi: 10.1002/hed.2890010109. [DOI] [PubMed] [Google Scholar]
- 2.Chomette M, Auriol M, Tranbaloc P, et al. Adenoid cystic carcinoma of minor salivary glands. Analysis of 86 cases. Clinicopathological, histo-enzymological and ultra structural studies. Virch Arch (Pathol Anat) 1982;359:289–301. doi: 10.1007/BF00429355. [DOI] [PubMed] [Google Scholar]
- 3.Sandros J, Stenman G, Mark J. Cytogenetic and molecular observations in human and experimental salivary gland tumors. Cancer Genet Cytogenet. 1990;44:153–67. doi: 10.1016/0165-4608(90)90042-9. [DOI] [PubMed] [Google Scholar]
- 4.Higashi K, Jin Y, Johansson M, et al. Rearrangement of 9p13 as the primary chromosomal aberration in adenoid cystic carcinoma of the respiratory tract. Genes Chromosomes Cancer. 1991;3:21–3. doi: 10.1002/gcc.2870030105. [DOI] [PubMed] [Google Scholar]
- 5.Stenman G, Sandros J, Mark J, et al. Partial 6q deletion in a human salivary gland adenocarcinoma. Cancer Genet Cytogenet. 1989;39:151–6. doi: 10.1016/0165-4608(89)90180-5. [DOI] [PubMed] [Google Scholar]
- 6.Nordkvist A, Mark J, Gustaffson H, et al. Non-random chromosome rearrangement in adenoid cystic carcinoma of the salivary gland. Genes Chromosomes Cancer. 1994;10:115–31. doi: 10.1002/gcc.2870100206. [DOI] [PubMed] [Google Scholar]
- 7.Jin Y, Mertens F, Limon J, et al. Characteristic karyotypic features in lacrimal and salivary gland carcinomas. Br J Cancer. 1994;70:42–7. doi: 10.1038/bjc.1994.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins C, Fonesca I, Roque L, et al. Malignant salivary gland neoplasms: a cytogenetic study of 19 cases. Eur J Cancer B Oral Oncol. 1996;32:128–32. doi: 10.1016/0964-1955(95)00078-X. [DOI] [PubMed] [Google Scholar]
- 9.Mark HFL, Hann E, Gnepp DR. Cytogenetic analysis of salivary type tumors. Oral Surg Oral Med Oral Pathol Oral Radiol. 1996;82:184–92. doi: 10.1016/s1079-2104(96)80223-x. [DOI] [PubMed] [Google Scholar]
- 10.Hrynchak M, White V, Berean K, et al. Cytogenetic findings in seven lacrimal gland neoplasms. Cancer Genet Cytogenet. 1994;75:133–8. doi: 10.1016/0165-4608(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Gines C, Cerda-Nicolas M, Llombart-Bosch A. Cytogenetic findings in a new case of adenoid cystic carcinoma arising in sphenoidal sinus. Cancer Genet Cytogenet. 1994;75:150–2. doi: 10.1016/0165-4608(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 12.Martins C, Fonseca I, Roque L, et al. Cytogenetic similarities between two types of salivary gland carcinomas: adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma. Cancer Genet Cytogenet. 2001;128:130–6. doi: 10.1016/S0165-4608(01)00416-2. [DOI] [PubMed] [Google Scholar]
- 13.El-Naggar AK, Lovell M, Callender DL, et al. Limited nonrandom chromosomal aberrations in a recurrent adenoid cystic carcinoma of the parotid gland. Cancer Genet Cytogenet. 1999;109:66–9. doi: 10.1016/S0165-4608(98)00188-5. [DOI] [PubMed] [Google Scholar]
- 14.Sandros J, Mark J, Happonen RP, et al. Specificity of 6q markers and other recurrent deviations in human malignant salivary gland tumors. Anti Cancer Res. 1988;8:637–44. [PubMed] [Google Scholar]
- 15.Stenman G, Sandros J, Dahlenfors R, et al. 6q- and loss of the Y chromosome-two common deviations in malignant human salivary gland tumors. Cancer Genet Cytogenet. 1986;22:283–93. doi: 10.1016/0165-4608(86)90021-X. [DOI] [PubMed] [Google Scholar]
- 16.El-Naggar AK, Lovell M, Killary AM, et al. A mucoepidermoid carcinoma of minor salivary gland with t(11;19)(q21;p13.1) as the only karyotypic abnormality. Cancer Genet Cytogenet. 1996;87:29–33. doi: 10.1016/0165-4608(95)00266-9. [DOI] [PubMed] [Google Scholar]
- 17.El-Naggar AK, Hurr K, Kagan J, et al. Genotypic alterations in benign and malignant salivary gland tumors: histogenetic and clinical implications. Am J Surg Pathol. 1997;21:691–7. doi: 10.1097/00000478-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Rutherford S, Yu Y, Rumpel CA, et al. Chromosome 6 deletion and candidate tumor suppressor genes in adenoid cystic carcinoma. Cancer Lett. 2006;236:309–17. doi: 10.1016/j.canlet.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 19.Stallmach I, Zenklusen P, Komminoth P, et al. Loss of heterozygosity at chromosome 6q23–25 correlates with clinical and histologic parameters in salivary gland adenoid cystic carcinoma. Virchows Arch. 2002;440:77–84. doi: 10.1007/s004280100523. [DOI] [PubMed] [Google Scholar]
- 20.Johns MM, Westra WH, Califano JA, et al. Allelotype of salivary gland tumors. Cancer Res. 1996;56:1151–4. [PubMed] [Google Scholar]
- 21.Kishi M, Nakamura M, Nishimine M, et al. Loss of heterozygosity on chromosome 6q correlates with decreased thrombospondin-2 expression in human salivary gland carcinomas. Cancer Sci. 2003;94:530–5. doi: 10.1111/j.1349-7006.2003.tb01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger R, Bloomfield CD, Sutherland GR. Report of the committee on chromosome rearrangements in Neoplasia and on Fragile sites. Cytogenet Cell Genet. 1985;40:490–535. doi: 10.1159/000132181. [DOI] [PubMed] [Google Scholar]
- 23.Harper ME, Franchini G, Love J, et al. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983;304:169–71. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida MC, Satoh H, Sasaki M, et al. Regional location of a novel yes-related proto-oncogene, syn, on human chromosome 6 at band q21. Jpn J Cancer Res. 1986;77:1059–61. [PubMed] [Google Scholar]
- 25.Yoshida MC, Satoh H, Matsushime H, Shibuya M, Sasaki M. Two ras-related proto oncogenes, c-ros-1 and 8H, are regionally mapped on chromosomes 6q22 and 13q12, respectively. Ninth International Workshop on Human Gene Mapping 9. Cytogenet Cell Genet. 1987;46:724. [Google Scholar]
- 26.Wong KF. 11q13 is a cytogenetically promiscuous site in hematologic malignancies. Cancer Genet Cytogenet. 1999;113:93–5. doi: 10.1016/S0165-4608(98)00285-4. [DOI] [PubMed] [Google Scholar]
- 27.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–9. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 28.Lastowska M, Roberts P, Pearson AD, et al. Promiscuous translocations of chromosome arm 17q in human neuroblastomas. Genes Chromosomes Cancer. 1997;19:143–9. doi: 10.1002/(SICI)1098-2264(199707)19:3<143::AID-GCC2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Bergsagel PL, Chesi M, Nardini E, et al. Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA. 1996;93:13931–6. doi: 10.1073/pnas.93.24.13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins EC, Rabbitts TH. The promiscuous MLL gene links chromosomal translocations to cellular differentiation and tumour tropism. Trends Mol Med. 2002;8:436–42. doi: 10.1016/S1471-4914(02)02397-3. [DOI] [PubMed] [Google Scholar]