Abstract
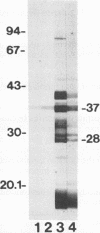
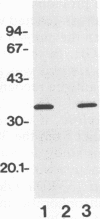
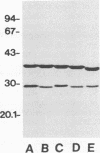
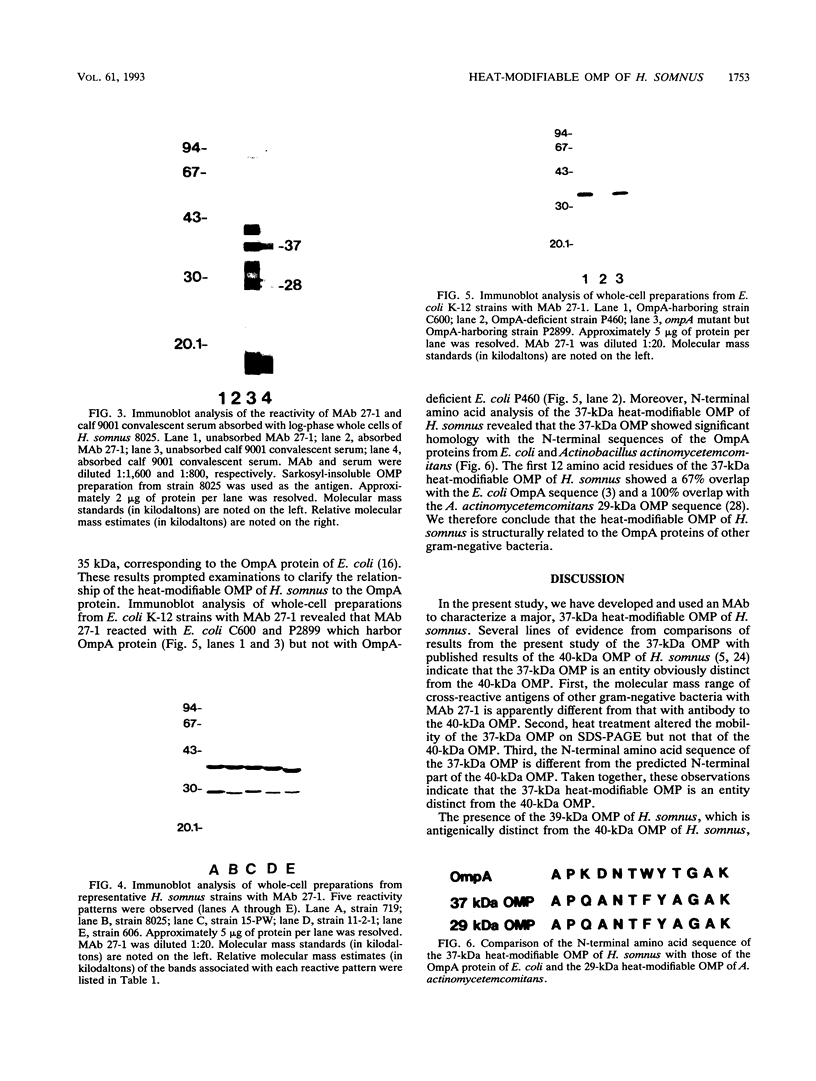
In immunoblot analysis, a murine monoclonal antibody (MAb), 27-1, which was produced to an outer membrane protein (OMP) of Haemophilus somnus, showed that a major OMP is heat modifiable, having a molecular mass of 28 kDa when the N-lauroylsarcosine-insoluble OMP preparation was solubilized at 60 degrees C and a mass of 37 kDa when the OMP preparation was solubilized at 100 degrees C. The heat-modifiable OMP reacted intensely with convalescent sera obtained from calves with experimental H. somnus pneumonia in immunoblot analysis. Immunoelectron microscopic and antibody absorption studies revealed that the MAb 27-1 epitope was not surface exposed on the intact bacterium. However, a decrease in antibody reactivity to the heat-modifiable OMP in immunoblot analysis after absorption of convalescent serum with intact bacterial cells of H. somnus suggests that a surface-exposed portion of the heat-modifiable OMP is expressed on the intact bacterium. MAb 27-1 reacted with 45 of 45 strains of H. somnus tested in immunoblot analysis. The apparent molecular mass of the antigen varied among strains, and five reactivity patterns demonstrated by MAb 27-1 were observed. MAb 27-1 also reacted with six species in the family Pasteurellaceae, Escherichia coli, and Salmonella dublin, but not with the other eight species of gram-negative bacteria. The heat-modifiable OMP of H. somnus showed immunological cross-reactivity with the OmpA protein of E. coli K-12 and significant N-terminal amino acid sequence homology with the OmpA proteins of gram-negative bacteria. We conclude that a major, 37-kDa heat-modifiable OMP of H. somnus, which elicits an antibody response in H. somnus-infected animals, is a common antigen among H. somnus strains tested and is structurally related to the OmpA protein of E. coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Kania S. A., Gogolewski R. P. Characterization of immunodominant surface antigens of Haemophilus somnus. Infect Immun. 1991 Dec;59(12):4295–4301. doi: 10.1128/iai.59.12.4295-4301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corboz L., Wild P. Epidemiologie der Haemophilus somnus-Infektion beim Rind: Vergleich von Stämmen in der Polyacrylamidgel-Elektrophorese (PAGE). Schweiz Arch Tierheilkd. 1981 Feb;123(2):79–88. [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolewski R. P., Kania S. A., Inzana T. J., Widders P. R., Liggitt H. D., Corbeil L. B. Protective ability and specificity of convalescent serum from calves with Haemophilus somnus pneumonia. Infect Immun. 1987 Jun;55(6):1403–1411. doi: 10.1128/iai.55.6.1403-1411.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolewski R. P., Kania S. A., Liggitt H. D., Corbeil L. B. Protective ability of antibodies against 78- and 40-kilodalton outer membrane antigens of Haemophilus somnus. Infect Immun. 1988 Sep;56(9):2307–2316. doi: 10.1128/iai.56.9.2307-2316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Jansen H. M., Zanen H. C. Changes in outer membrane proteins of nontypable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Infect Dis. 1988 Aug;158(2):360–365. doi: 10.1093/infdis/158.2.360. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Visschers G., Jansen H. M., Zanen H. C. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic obstructive pulmonary disease: the effect of antibiotic treatment on persistence. J Infect Dis. 1990 Mar;161(3):512–517. doi: 10.1093/infdis/161.3.512. [DOI] [PubMed] [Google Scholar]
- Haritani M., Nakazawa M., Oohashi S., Yamada Y., Haziroglu R., Narita M. Immunoperoxidase evaluation of pneumonic lesions induced by Pasteurella haemolytica in calves. Am J Vet Res. 1987 Sep;48(9):1358–1362. [PubMed] [Google Scholar]
- Kania S. A., Gogolewski R. P., Corbeil L. B. Characterization of a 78-kilodalton outer membrane protein of Haemophilus somnus. Infect Immun. 1990 Jan;58(1):237–244. doi: 10.1128/iai.58.1.237-244.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R. Immunoblot method for identifying surface components, determining their cross-reactivity, and investigating cell topology: results with Haemophilus influenzae type b. Anal Biochem. 1984 Nov 15;143(1):196–204. doi: 10.1016/0003-2697(84)90576-1. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Morona R., Klose M., Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984 Aug;159(2):570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunnariwo J. A., Cheng C., Ford J., Schryvers A. B. Response of Haemophilus somnus to iron limitation: expression and identification of a bovine-specific transferrin receptor. Microb Pathog. 1990 Dec;9(6):397–406. doi: 10.1016/0882-4010(90)90058-x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y., Ishikawa H., Yuasa N. Purification and partial characterization of the major outer membrane protein of Haemophilus somnus. Infect Immun. 1993 Jan;61(1):91–96. doi: 10.1128/iai.61.1.91-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen M., Rioux C. R., Potter A. A. Molecular cloning, nucleotide sequence, and characterization of a 40,000-molecular-weight lipoprotein of Haemophilus somnus. Infect Immun. 1992 Mar;60(3):826–831. doi: 10.1128/iai.60.3.826-831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Weiser J. N., Gotschlich E. C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991 Jul;59(7):2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widders P. R., Paisley L. G., Gogolewski R. P., Evermann J. F., Smith J. W., Corbeil L. B. Experimental abortion and the systemic immune response to "Haemophilus somnus" in cattle. Infect Immun. 1986 Nov;54(2):555–560. doi: 10.1128/iai.54.2.555-560.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect Immun. 1991 Jul;59(7):2505–2507. doi: 10.1128/iai.59.7.2505-2507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall M., Gogolewski R. P., Corbeil L. B. Characterization of two Haemophilus somnus Fc receptors. J Gen Microbiol. 1988 Jul;134(7):1993–1999. doi: 10.1099/00221287-134-7-1993. [DOI] [PubMed] [Google Scholar]
- Yarnall M., Widders P. R., Corbeil L. B. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol. 1988 Aug;28(2):129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]