Abstract
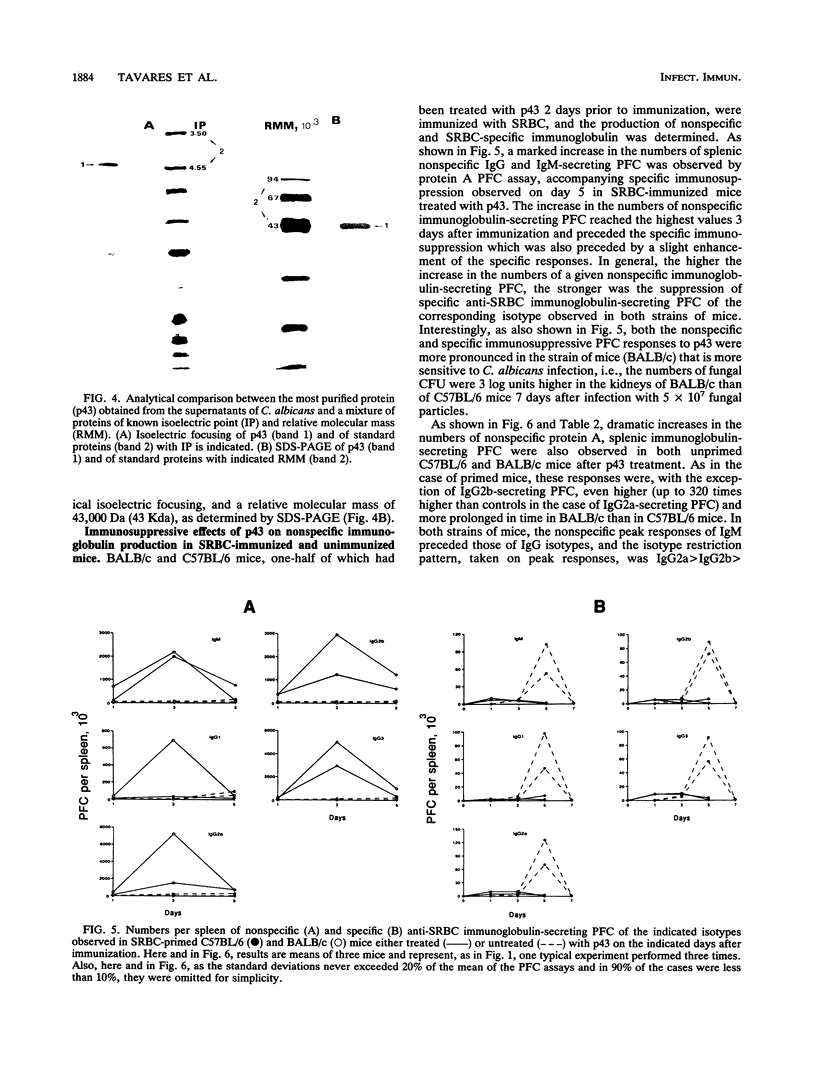
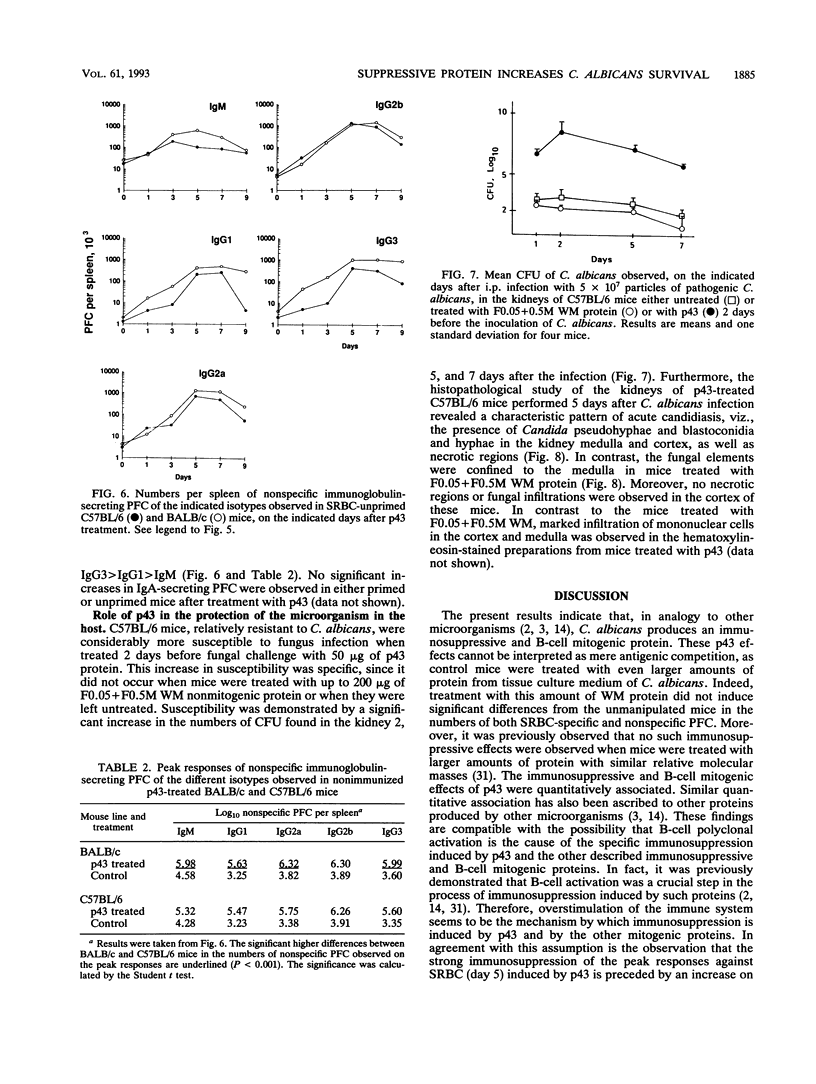
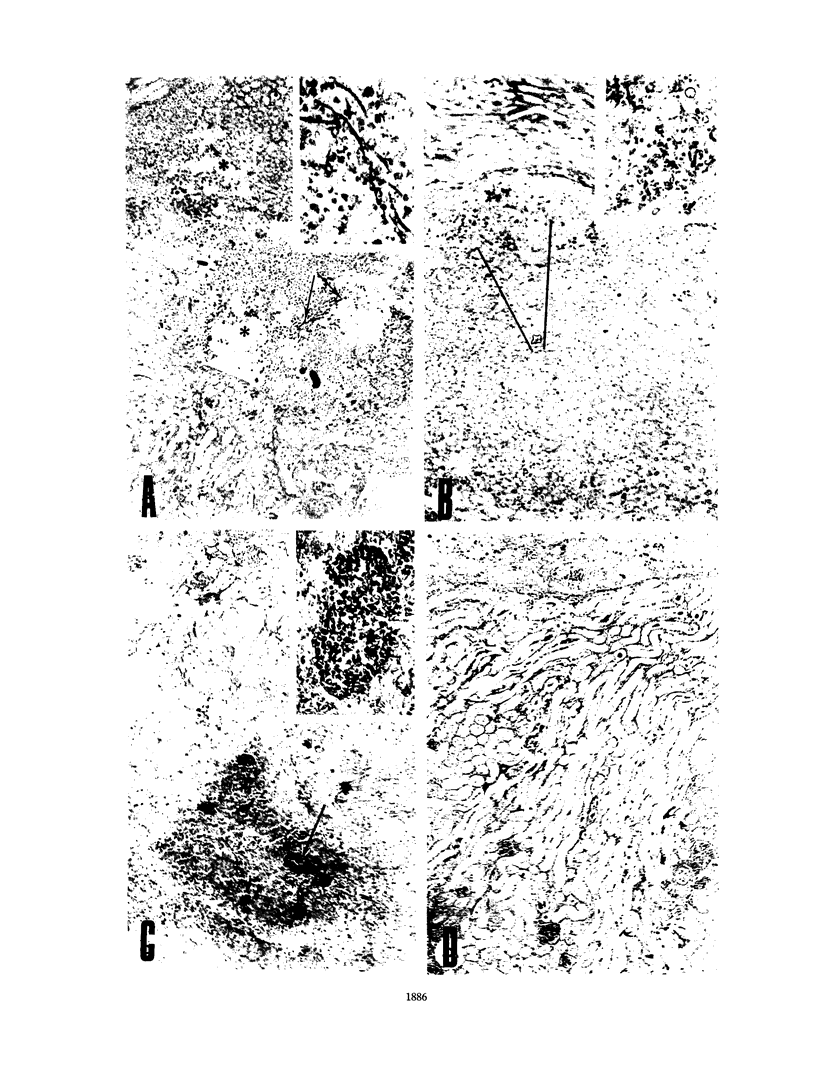
A protein with an isoelectric point of 4.3 and a relative molecular mass of 43 kDa (p43) was purified from the supernatants of the cultures of pathogenic Candida albicans but could not be detected in the supernatants of cultures of this fungus with pathogenicity previously attenuated after being repeatedly subcultured in vitro. Treatment of BALB/c and C57BL/6 mice with p43 resulted in (i) marked increases in the numbers of splenic immunoglobulin-secreting plaque-forming cells (PFC) with peak responses of immunoglobulin M (IgM) PFC preceding those of IgG PFC, with an isotype restriction pattern of IgG2a > IgG2b > IgG3 > IgG1 > IgM, and (ii) specific immunosuppression of the murine primary immune response against sheep erythrocytes. Immunosuppressive and B-cell mitogenic properties of p43 were quantitatively associated and inversely correlated with susceptibility to C. albicans infection. C57BL/6 mice treated with p43 2 days before inoculation with C. albicans were considerably more susceptible to the fungal infection than untreated mice. The immunobiological and chemical properties of p43 are compared with previously described immunosuppressive and B-cell mitogenic proteins produced by bacteria and viruses, and strategies for immunointervention are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arala-Chaves M. P., Porto M. T., Arnaud P., Saraiva M. J., Geada H., Patrick C. C., Fudenberg H. H. Fractionation and characterization of the immunosuppressive substance in crude extracellular products released by Streptococcus intermedius. J Clin Invest. 1981 Jul;68(1):294–302. doi: 10.1172/JCI110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arala-Chaves M. P., Ribeiro A. S., Santarém M. M., Coutinho A. Strong mitogenic effect for murine B lymphocytes of an immunosuppressor substance released by Streptococcus intermedius. Infect Immun. 1986 Nov;54(2):543–548. doi: 10.1128/iai.54.2.543-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arala-Chaves M. P., Ribeiro A. dos S., Vilanova M., Porto M. T., Santarem M. G., Lima M. Correlation between B-cell mitogenicity and immunosuppressor effects of a protein released by porcine monocytes infected with African swine fever virus. Am J Vet Res. 1988 Nov;49(11):1955–1961. [PubMed] [Google Scholar]
- Aronson I. K., Rieger C. H., Soltani K., Tkalcevic V., Chan W. C., Lorincz A. L., Matz G. Late onset chronic mucocutaneous candidiasis with lymphoma and specific serum inhibitory factor. Cancer. 1979 Jan;43(1):101–108. doi: 10.1002/1097-0142(197901)43:1<101::aid-cncr2820430116>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ashman R. B., Papadimitriou J. M. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol Cell Biol. 1987 Apr;65(Pt 2):163–171. doi: 10.1038/icb.1987.18. [DOI] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Meima F., van der Meulen G. M., van Muiswinkel W. B. Antibody formation in mouse bone marrow. I. Evidence for the development of plaque-forming cells in situ. Immunology. 1974 Feb;26(2):247–255. [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Marconi P., Puccetti P., Garaci E. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect Immun. 1983 Apr;40(1):46–55. doi: 10.1128/iai.40.1.46-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Marconi P., Frati L., Bonmassar E., Garaci E. Increase of mouse resistance to Candida albicans infection by thymosin alpha 1. Infect Immun. 1982 May;36(2):609–614. doi: 10.1128/iai.36.2.609-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow E. W., Domer J. E. Immunoregulation in experimental murine candidiasis: specific suppression induced by Candida albicans cell wall glycoprotein. Infect Immun. 1985 Jul;49(1):172–181. doi: 10.1128/iai.49.1.172-181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutelier J. P., Van Snick J. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol. 1985 Mar;15(3):250–255. doi: 10.1002/eji.1830150308. [DOI] [PubMed] [Google Scholar]
- Cutler J. E., Lloyd R. K. Enhanced antibody responses induced by Candida albicans in mice. Infect Immun. 1982 Dec;38(3):1102–1108. doi: 10.1128/iai.38.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ferreira P., Soares R., Ribeiro A., Arala-Chaves M. Correlation between specific immunosuppression and polyclonal B cell activation induced by a protein secreted by Streptococcus mutans. Scand J Immunol. 1988 May;27(5):549–554. doi: 10.1111/j.1365-3083.1988.tb02382.x. [DOI] [PubMed] [Google Scholar]
- GRIDLEY M. F. A stain for fungi in tissue sections. Am J Clin Pathol. 1953 Mar;23(3):303–307. doi: 10.1093/ajcp/23.3_ts.303. [DOI] [PubMed] [Google Scholar]
- Gatenby P., Basten A., Adams E. Thymoma and late onset mucocutaneous candidiasis associated with a plasma inhibitor of cell-mediated immune function. J Clin Lab Immunol. 1980 May;3(3):209–216. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. K., Hockey L. J., Vukalcic L. J., Edwards J. E., Jr Effect of immunosuppression on the development of experimental hematogenous Candida endophthalmitis. Infect Immun. 1980 Feb;27(2):628–631. doi: 10.1128/iai.27.2.628-631.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata K. Fungal toxins as a parasitic factor responsible for the establishment of fungal infections. Mycopathologia. 1978 Dec 18;65(1-3):141–154. doi: 10.1007/BF00447185. [DOI] [PubMed] [Google Scholar]
- Kuruganti U., Henderson L. A., Garner R. E., Asofsky R., Baker P. J., Domer J. E. Nonspecific and Candida-specific immune responses in mice suppressed by chronic administration of anti-mu. J Leukoc Biol. 1988 Nov;44(5):422–433. doi: 10.1002/jlb.44.5.422. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopes L. M., Pereira M. A., Gerken S. E., Vaz N. Polyclonal activation of B lymphocytes during experimental infection with Schistosoma mansoni. Parasitology. 1990 Feb;100(Pt 1):83–91. doi: 10.1017/s0031182000060145. [DOI] [PubMed] [Google Scholar]
- Minoprio P. M., Eisen H., Forni L., D'Imperio Lima M. R., Joskowicz M., Coutinho A. Polyclonal lymphocyte responses to murine Trypanosoma cruzi infection. I. Quantitation of both T- and B-cell responses. Scand J Immunol. 1986 Dec;24(6):661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Coutinho A., Spinella S., Hontebeyrie-Joskowicz M. Xid immunodeficiency imparts increased parasite clearance and resistance to pathology in experimental Chagas' disease. Int Immunol. 1991 May;3(5):427–433. doi: 10.1093/intimm/3.5.427. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Piccolella E., Lombardi G., Morelli R. Generation of suppressor cells in the response of human lymphocytes to a polysaccharide from Candida albicans. J Immunol. 1981 Jun;126(6):2151–2155. [PubMed] [Google Scholar]
- Ribeiro A. dos S., Arala-Chaves M. P., Vilanova M., Porto M. T., Coutinho A. Role of B and T lymphocytes in the specific immunosuppression induced by a protein released by porcine monocytes infected with African swine fever virus. Int Immunol. 1991 Feb;3(2):165–174. doi: 10.1093/intimm/3.2.165. [DOI] [PubMed] [Google Scholar]
- Rivas V., Rogers T. J. Studies on the cellular nature of Candida albicans-induced suppression. J Immunol. 1983 Jan;130(1):376–379. [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Effect of systemic candidiasis on blastogenesis of lymphocytes from germfree and conventional rats. Infect Immun. 1978 Apr;20(1):142–150. doi: 10.1128/iai.20.1.142-150.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Santarém M. M., Porto M. T., Ferreira P., Soares R., Arala-Chaves M. P. Semi-purification of an immunosuppressor substance secreted by Streptococcus mutans that plays a role in the protection of the bacteria in the host. Scand J Immunol. 1987 Dec;26(6):755–761. doi: 10.1111/j.1365-3083.1987.tb02313.x. [DOI] [PubMed] [Google Scholar]
- Scaringi L., Marconi P., Boccanera M., Tissi L., Bistoni F., Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988 May;134(5):1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- Soares R., Ferreira P., Santarem M. M., Teixeira da Silva M., Arala-Chaves M. Low T- and B-cell reactivity is an apparently paradoxical request for murine immunoprotection against Streptococcus mutans. Murine protection can be achieved by immunization against a B-cell mitogen produced by these bacteria. Scand J Immunol. 1990 Mar;31(3):361–366. doi: 10.1111/j.1365-3083.1990.tb02779.x. [DOI] [PubMed] [Google Scholar]
- Taylor C. E., Stashak P. W., Caldes G., Prescott B., Chused T. E., Brooks A., Baker P. J. Activation of antigen-specific suppressor T cells by B cells from mice immunized with type III pneumococcal polysaccharide. J Exp Med. 1983 Sep 1;158(3):703–717. doi: 10.1084/jem.158.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thang M. N., Dondon L., Godefroy-Colburn T. Degradation of Escherichia coli polynucleotide phosphorylase by E. coli endogenous proteases and by trypsin. Biochimie. 1971;53(3):291–302. doi: 10.1016/s0300-9084(71)80095-0. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Jacobs D. M. Modulation of immune response by bacterial lipopolysaccharide (LPS): multifocal effects of LPS-induced suppression of the primary antibody response to a T-dependent antigen. J Immunol. 1978 Dec;121(6):2340–2346. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]