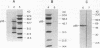Abstract
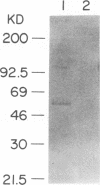
Previous studies have demonstrated that mycobacteria attach to fibronectin (FN). The attachment of mycobacteria to FN is considered to be biologically important in Mycobacterium bovis BCG therapy for superficial bladder cancer, initiation of delayed hypersensitivity to mycobacterial antigens, and the phagocytosis of mycobacteria by epithelial cells. Therefore, we purified the mycobacterial receptor for FN. Culture supernatants from 3-week cultures of Mycobacterium vaccae, which contained proteins that bound FN and inhibited the attachment of both M. vaccae and BCG to FN, were used as a source of receptor. Lyophilized M. vaccae supernatants were reconstituted in 0.02 M bis-Tris (pH 6.0) and applied sequentially to an ACA 54 gel filtration column and a DEAE-Sephacel anion-exchange column. A purified inhibitory protein of 55 kDa (p55) was obtained. The purified p55 protein was observed to bind to FN and to inhibit 125I-FN binding to viable BCG in a dose-dependent manner. Polyclonal and monoclonal antibodies to the protein were generated. The resulting polyclonal antiserum blotted a single protein band at 55 kDa in crude M. vaccae supernatants, cross-reacted with a 55-kDa BCG protein by Western blot (immunoblot), and recognized a 55-kDa band that was associated with the BCG cell wall, which is consistent with its function as a FN receptor. A monoclonal immunoglobulin M(lambda) was isolated from mice immunized with purified M. vaccae p55 protein that was not functional in Western blots but inhibited the attachment of viable BCG to FN. These studies demonstrate that a protein or antigenically related proteins with M(r)s of 55,000 function as FN receptors for at least two distinct mycobacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Garbe T., Lathigra R., Wiker H. G., Harboe M., Rook G. A., Young D. B. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect Immun. 1991 Aug;59(8):2712–2718. doi: 10.1128/iai.59.8.2712-2718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanzadeh J., Brown E. J., Quillin S. P., Ritchey J. K., Ratliff T. L. Characterization of soluble fibronectin binding to Bacille Calmette-Guérin. J Gen Microbiol. 1989 Oct;135(10):2735–2741. doi: 10.1099/00221287-135-10-2735. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Godfrey H. P., Feng Z., Mandy S., Mandy K., Huygen K., De Bruyn J., Abou-Zeid C., Wiker H. G., Nagai S., Tasaka H. Modulation of expression of delayed hypersensitivity by mycobacterial antigen 85 fibronectin-binding proteins. Infect Immun. 1992 Jun;60(6):2522–2528. doi: 10.1128/iai.60.6.2522-2528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr H. W., Pinsky C. M., Whitmore W. F., Jr, Oettgen H. F., Melamed M. R. Effect of intravesical Bacillus Calmette-Guerin (BCG) on carcinoma in situ of the bladder. Cancer. 1983 Apr 1;51(7):1323–1326. doi: 10.1002/1097-0142(19830401)51:7<1323::aid-cncr2820510724>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hudson M. A., Brown E. J., Ritchey J. K., Ratliff T. L. Modulation of fibronectin-mediated Bacillus Calmette-Guérin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Res. 1991 Jul 15;51(14):3726–3732. [PubMed] [Google Scholar]
- Hunter S. W., McNeil M., Modlin R. L., Mehra V., Bloom B. R., Brennan P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J Immunol. 1989 Apr 15;142(8):2864–2872. [PubMed] [Google Scholar]
- Kavoussi L. R., Brown E. J., Ritchey J. K., Ratliff T. L. Fibronectin-mediated Calmette-Guerin bacillus attachment to murine bladder mucosa. Requirement for the expression of an antitumor response. J Clin Invest. 1990 Jan;85(1):62–67. doi: 10.1172/JCI114434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen K., Baloda S. B., Larsen J. L., Wadström T. Toxins, putative cell adhesins and fibronectin binding properties of Salmonella dublin. Acta Pathol Microbiol Immunol Scand B. 1987 Feb;95(1):57–63. doi: 10.1111/j.1699-0463.1987.tb03087.x. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Brown E. J., Telle W. B., Russell D. G., Ratliff T. L. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J Clin Invest. 1993 Jan;91(1):69–76. doi: 10.1172/JCI116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm D. L., Blumenstein B. A., Crawford E. D., Montie J. E., Scardino P., Grossman H. B., Stanisic T. H., Smith J. A., Jr, Sullivan J., Sarosdy M. F. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991 Oct 24;325(17):1205–1209. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- Lamm D. L., Thor D. E., Stogdill V. D., Radwin H. M. Bladder cancer immunotherapy. J Urol. 1982 Nov;128(5):931–935. doi: 10.1016/s0022-5347(17)53283-8. [DOI] [PubMed] [Google Scholar]
- Pessolani M. C., Brennan P. J. Mycobacterium leprae produces extracellular homologs of the antigen 85 complex. Infect Immun. 1992 Nov;60(11):4452–4459. doi: 10.1128/iai.60.11.4452-4459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Ratliff T. L., Palmer J. O., McGarr J. A., Brown E. J. Intravesical Bacillus Calmette-Guérin therapy for murine bladder tumors: initiation of the response by fibronectin-mediated attachment of Bacillus Calmette-Guérin. Cancer Res. 1987 Apr 1;47(7):1762–1766. [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Pearson J., Chirigos M. Virulence of six strains of Mycobacterium bovis (BCG) in mice. Infect Immun. 1973 Nov;8(5):736–742. doi: 10.1128/iai.8.5.736-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziale P., Hök M., Switalski L. M., Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984 Feb;157(2):420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski L., Courtney H. S., Simpson W. A., Hasty D. L., Beachey E. H., Robert L., Ofek I. Hybridoma antibodies to the lipid-binding site(s) in the amino-terminal region of fibronectin inhibits binding of streptococcal lipoteichoic acid. J Infect Dis. 1987 Aug;156(2):344–349. doi: 10.1093/infdis/156.2.344. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Schöningh R., Janson A. A., Garbe T., Cornelisse Y. E., Clark-Curtiss J. E., Kolk A. H., Ottenhoff T. H., De Vries R. R., Abou-Zeid C. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae. Mol Microbiol. 1992 Jan;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]