Abstract
Peritoneal fibrosis, a major complication of peritoneal dialysis, limits the effectiveness of peritoneal dialysis as a treatment of end-stage renal disease. Preventing this complication by identifying targets for therapy has recently received much attention. In the present study, we showed that Notch signaling was highly activated in rats in peritoneal dialysis fluid-induced fibrotic peritoneum, as indicated by increased expression of Jagged-1, Notch-1, and HES-1. Blocking Notch signaling activation by intraperitoneal injection of a γ-secretase inhibitor, DAPT, significantly attenuated peritoneal fibrosis as indicated by the decreased expression of α-smooth muscle actin, collagen I, and vascular endothelial growth factor as well as increased expression of E-cadherin. Moreover, compared with control rats, DAPT-treated rats had a thinner peritoneum with less extracellular matrix accumulation, a lower mass transfer of glucose, and a higher ultrafiltration rate. In addition, transforming growth factor (TGF)-β1 induced Notch signaling activation in primary rat peritoneal mesothelial cells. DAPT blocked this TGF-β1–induced Notch signaling activation and therefore significantly inhibited TGF-β1–induced expression of α-smooth muscle actin, collagen I, and vascular endothelial growth factor. Thus, a γ-secretase inhibitor that interferes with Notch signaling prevents biochemical, histological, and functional consequences of peritoneal fibrosis through inhibiting epithelial to mesenchymal transition of rat peritoneal mesothelial cells. These results support the use of γ-secretase inhibitors as a novel therapeutic approach for peritoneal fibrosis.
Peritoneal dialysis (PD) is a convenient and inexpensive therapy for patients with end-stage renal disease. In long-term PD, the effectiveness is markedly limited mainly by the fibrotic changes in the peritoneal membrane.1,2 Thus, there is a pressing need for the understanding of the molecular pathogenesis of peritoneal fibrosis and the development of effective therapy for preventing peritoneal fibrosis.
The monolayer of peritoneal mesothelial cells is the key structure of the biological and physical barrier that are involved in regulating permeability and ultrafiltration in PD.3 In patients chronically exposed to the peritoneal dialysis fluid (PDF), there is a loss of mesothelial cells and the replacement of the peritoneal membrane by fibrous tissue.4,5 Recent studies revealed an important role of mesothelial cells in peritoneal injury through the epithelial-to-mesenchymal transition (EMT) induced by PDF. Submesothelial myofibroblasts, which participate in extracellular matrix accumulation (ECM) and angiogenesis, can originate from mesothelial cells through EMT.6,7 Therefore, EMT is an early event in peritoneal membrane fibrogenesis and is likely mediated by transforming growth factor (TGF)-β both in mesothelial cell culture and in vivo.8,9
Notch signaling is an ancient cell signaling system that regulates cell fate specification, stem cell maintenance, and initiation of differentiation in embryonic and postnatal tissues.10,11,12 Four Notch receptors isoforms, namely Notch-1,13 Notch-2,14 Notch-3,15 and Notch-4,16 and five ligands, Jagged-117 and Jagged-218 belonging to the Serrate family, and Delta-1,19 Delta-3,20 and Delta-like 421 belonging to the Delta family, have been identified in mammals. The pathway is activated through an interaction of a Notch receptor with a Jagged or Delta-like ligand leading to proteolytic cleavages of Notch receptor at two distinct sites. The cleavage releases the Notch intracellular domain (NICD) such that it can enter the nucleus and function as a transcription activator. Importantly, the second cleavage is mediated by the γ-secretase complex, and effective inhibition of Notch activation can be achieved by pharmacological inhibition of this proteolytic activity. Once within the nucleus, NICD interacts with CSL (RBP-Jk/CBF1) and Mastermind to generate a large transcriptional activator complex and activates transcription of downstream target genes.22 In mammals, primary target genes of the Notch-intracellular domain/RBP-J complex include the HES (Hairy/Enhancer of Split)23,24 and HEY (HES-related with YRPW motif, also named HERP, HES-related repressor protein)25,26,27 family of genes, which act as transcription factors.
Notch has recently been shown to promote EMT during cardiac valve formation.28 Moreover, an upregulation of Notch ligand Jagged-1 expression was detected in the kidney of a model of progressive interstitial fibrosis induced by ureteral obstruction.29 In epithelial cells from mammary gland, kidney tubules, and epidermis, TGF-β induces the Notch target gene Hey1 at the onset of EMT in a Smad3-dependent process.30 However, despite a most recent report showing expression of Jagged-1 in peritoneal mesothelial cells,31 little is known about the expression pattern and functional role of the Notch signaling pathway in normal and injured peritoneum induced by long term PD.
In the present study, we investigated the role of Notch signaling in the progression of peritoneal fibrosis induced by PDF. Our results demonstrated that the components of Notch signaling are expressed and activated in fibrotic peritoneum induced by PDF. Moreover, TGF-β induced the expression of Notch signaling components during the process of EMT of primary rat mesothelial cells (RPMCs). Because γ-secretase inhibitor (GSI) has been extensively used for inhibiting Notch signaling both in vitro32 and in vivo33 and also been clinically tested for T cell acute lymphoblastic leukemia,34,35 we used GSI to treat RPMCs and found that GSI dramatically inhibited TGF-β–induced EMT of RPMCs. Most importantly, we demonstrated that GSI could significantly attenuate peritoneum fibrosis and prevent a loss of peritoneal function in vivo. These results support the use of GSI as a novel therapeutic approach for PD-related peritoneal fibrosis.
Materials and Methods
Reagents and Antibodies
Antibodies against α–smooth muscle actin (α-SMA) and GAPDH were from Sigma-Aldrich (St. Louis, MO). Notch-1(sc-6015) and HES-1 antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Antibody specific for NICD (ab52301) was from Abcam (Cambridge, MA). Vascular endothelial growth factor (VEGF) antibody was purchased from Thermo Fisher Scientific Inc (Fremont, CA). Goat anti-type collagen antibody was obtained from Southern Biotech (Birmingham, AL). Jagged-1 antibody and secondary HRP-conjugated antibodies were from Cell Signaling (Danvers, MA). FITC-conjugated donkey anti-goat IgG antibody was purchased from Jackson Immuno Research Laboratories Inc (West Grove, PA). Fluorescent phallotoxin-labeled secondary antibodies were from Invitrogen (Carlsbad, CA). γ-secretase inhibitor DAPT was purchased from Calbiochem (San Diego, CA). Recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, MN).
Animal Experiments
Male Sprague-Dawley (SD) rats weighing 150 to 180 g were obtained from the Experiment Animal Centre at the Northern Campus of Sun Yat-sen University. All animal experiments were approved by the Committee on Animal Experimentation of Sun Yat-sen University and performed in compliance with the university’s Guidelines for the Care and Use of Laboratory Animals. Thirty male SD rats were randomly allocated into five groups: rats in group A (n = 6) served as normal controls; rats in group B (n = 6) and group C (n = 6) received daily intraperitoneal injections of PDF named Dianeal® PD-2 Peritoneal Dialysis Solution with 4.25% Dextrose (4.25% Dianeal; Baxter HealthCare, Deerfield, IL) at 100 ml/kg of body weight36; rats in group D (n = 6) were intraperitoneally injected with 10 μmol/L DAPT together with 4.25% Dianeal; rats in group E (n = 6) received the same amount of DMSO (the vehicle for DAPT) as group D together with 4.25% Dianeal. Rats of group B were sacrificed at 14 days and the rest of rats were sacrificed at 28 days after initial treatment.
Peritoneal Function Test
Peritoneal function tests were performed as previously described.37 Briefly, for the peritoneal ultrafiltration rate, 4.25% Dianeal was administered intraperitoneally to the rats at 90 ml/kg body weight before being euthanized. Four hours later, the peritoneal fluid was removed for ultrafiltration measurement. Net ultrafiltration was the volume of fluid removed after four hours minus the volume of fluid administered. For glucose transportation assay, glucose was measured by a standard enzymatic test on a Hitachi automated chemistry analyzer (Hitachi 7170, Japan). Mass transfer of glucose from the peritoneum was calculated using the formula: (initial dialysate glucose × initial volume) − (final dialysate glucose × final volume). These values were corrected for animal weight at the time of euthanasia.
Histopathological and Immunofluoresecence Analysis of Rat Peritoneum
Four-μm paraffin sections from the anterior abdominal wall were stained with hematoxylin and eosin and Masson trichrome. The thickness (μm) of the peritoneum was measured in each animal using a micrometer fitted into the eyepiece of the microscope and expressed as the means ± SD. Each section was measured at 10 random sites.
For immunofluoresence analysis, 10-μm paraffin-embedded sections from the visceral peritoneum were cut and dehydrated in xylene, followed by microwave antigen retrieval. Sections were then blocked with PBS containing 5% BSA for 30 minutes at 37°C. Sections were incubated overnight at 4°C with indicated primary antibodies diluted in 3% BSA in PBS and secondary antibodies for 50 minutes at 37°C. Samples were mounted in fixation medium (Biomeda, Foster City, CA). DAPI (Sigma-Aldrich, St. Louis, MO) was used to stain the nucleus. Images were analyzed and collected with Zeiss LSM 510 Confocal Imaging System (Zeiss, Germany).
RPMCs Isolation and Culture
The isolation and culture of RPMCs was performed according to our previously reported method.37 Briefly, RPMCs were prepared by infusing 30 ml of 0.25% trypsinase-0.2% EDTA-Na2 into the rat abdominal cavity. The fluid was removed from the peritoneal cavity one hour later under sterile conditions. To harvest RPMCs, cellular components were isolated by centrifugation and then washed with PBS and suspended in DMEM/F12 medium (Invitrogen, Carlsbad, CA) supplemented with 12% (v/v) FCS (Invitrogen, Carlsbad, CA). Cells were placed into 25-cm2 culture flasks and incubated overnight at 37°C. Nonadherent cells were removed the next day, and the adherent population was incubated at 37°C in 5% CO2 in fresh culture medium. RPMCs in this study were derived from two to four passages grown as a monolayer to subconfluency.
To induce EMT, RPMCs were seeded into 35-mm diameter tissue culture plates. When 60% confluent, cells were cultured in serum-free medium for 24 hours and then indicated amount of TGF-β1 was added for various time period. To examine the effect of γ-secretase inhibitor DAPT on TGF-β1–induced EMT, RPMCs were pre-incubated for 15 minutes with 10 μmol/L DAPT before TGF-β1 treatment.
RT-PCR
Total peritoneal RNA was isolated from visceral peritoneum using Trizol Reagent (Invitrogen, Carlsbad, CA) and was reverse-transcribed using RevertAid First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) as previously described.37 Amplified cDNA was used as the template DNA, and PCR was performed with TaqDNA polymerase and specific primers. The steps were as follows: denaturation at 95°C for 5 minutes followed by denaturation at 95°C for 30 seconds, hybridization at 60°C for 30 seconds, and elongation at 72°C for 45 seconds, for 25–32 cycles. The following PCR primers were used: Jagged-1: forward, 5′-GCCAAGTGGGATGACGACT-3′, reverse, 5′-GCAACAGCAGCGATAAGTGA-3′; Notch-1: forward, 5′-GCAAGAAGAAGCGGAGAG-3′, reverse, 5′-AGCTGGCACCCTGATAGATG-3′; HES-1: forward, 5′-CAGATGACCGCCGCTCTCA-3′, reverse, 5′-GCGACACTGCGTTAGGACCC-3′; GAPDH: forward, 5′-AGATCCACAACGGATACATT-3′, reverse, 5′-TCCCTCAAGATTGTCAGCAA-3′.
Western Blot Analysis
Tissue and cells were lysed in lysis buffer (50 mmol/L Hepes pH 7.5, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1.5 mmol/L MgCl2, 1 mmol/L EGTA, 10 mmol/L NaF, 10 mmol/L Na4P2O7, 1 mmol/L Na3VO5, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin and 20 μg/ml aprotinin). Protein was quantified by the Bradford assay (Bio-Rad, Hercules, CA), equal amount of protein were separated on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). After blocking in 5% skim milk for 1 hour at room temperature, membranes were incubated with indicated primary antibody at 4°C overnight followed by horseradish peroxidase-conjugated second antibody for 1 hour at room temperature and detected by chemiluminescence (Amersham Biosciences, Piscataway, NJ). Quantification of the Western blot data were performed by measuring the intensity of the hybridization signals using NIH Image analysis program.
Statistics
The results were expressed as mean ± SD. Statistical analysis was performed using SPSS13.0. Data were analyzed using one-way analysis of variance followed by post hoc test. A value of P < 0.05 was considered as statistically significant.
Results
The Notch Signaling Cascade Was Activated in a Rat Model of Peritoneal Fibrosis Induced by PDF
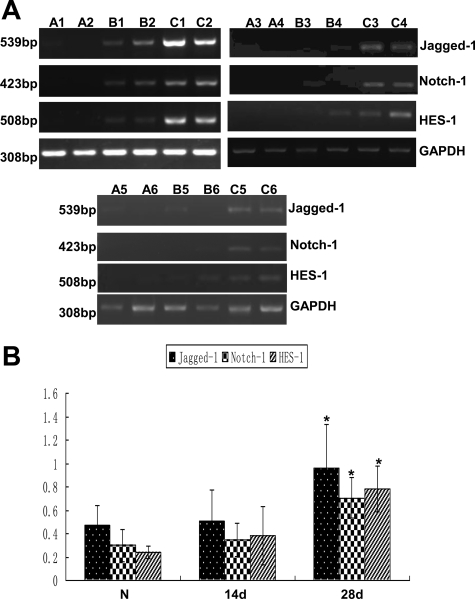
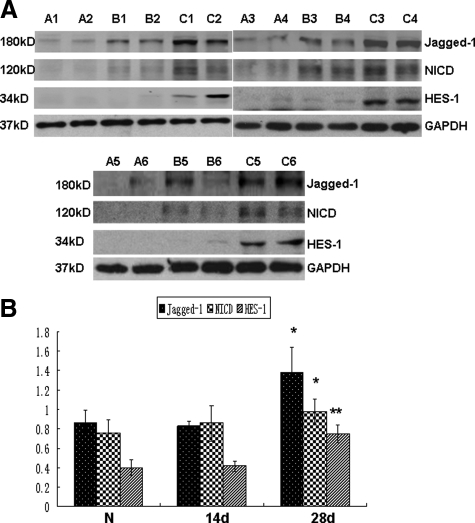
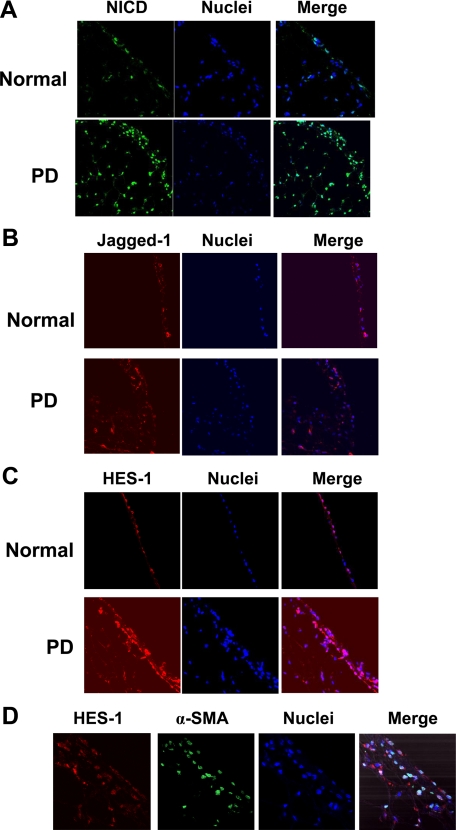
To investigate the expression of Notch signaling components in fibrotic peritoneum, we generated a rat model of peritoneal fibrosis induced by daily intraperitoneal injection of PDF containing 4.25% glucose at 100 ml/kg of body weight, and rats were sacrificed either 14 days or 28 days after the initial treatment. As shown in Figure 1, A and B, the mRNA levels of Jagged-1, Notch-1, and the Notch downstream target HES-1 was hardly detected in normal peritoneum by RT-PCR, but their expression was gradually increased in rats after 14 days of PDF treatment. The mRNA expression of Jagged-1, Notch-1, and HES-1 in 28 days group was readily detected and was significantly higher than that in control group. Similarly, the protein level of Jagged-1 and HES-1 was increased gradually after PDF treatment, and a significant increase was detected in 28 days group compared with that of control group (Figure 2, A and B). Because the cleavage of Notch-1 is an indicator of Notch signaling activation, we examined the protein level of NICD by Western blot and detected a significant increase of NICD in the peritoneum after 28 days of PDF treatment (Figure 2). The expression of NICD, Jagged-1, and HES-1 at the protein level was further documented by immunofluorescence. As shown in Figure 3, A–C, positive staining for NICD, Jagged-1, and HES-1 was readily detected in the mesothelial cells on the surface of the peritoneum and in cells in the submesothelial areas after 28 days of PDF treatment. In contrast, few cells with positive staining for NICD, Jagged-1, and HES-1 expression were detected in normal peritoneum. We next examined the localization of the upregulated HES-1 as a marker of activated Notch signaling by immunofluorescence. In the fibrotic peritoneum, the HES-1–positive staining was colocalized with α-SMA, a phenotypic marker of myofibroblast and a hallmark of EMT of mesothelial cells, in the cells on the surface of the peritoneum and in the submesothelial area (Figure 3D). These data indicated that the upregulation of Notch signaling occurs in myofibroblasts and transdifferentiated mesothelial cells. These data demonstrated that Notch signaling was highly activated in fibrotic peritoneum induced by PDF.
Figure 1.
Transcripts of Notch signaling components were increased in fibrotic peritoneum induced by PDF. A: Normal rats (A1 to A6) or rats that received daily intraperitoneal injections of 4.25% Dianeal (100 ml/kg) for 14 days (B1 to B6) or 28 days (C1 to C6) were sacrificed, mRNA was collected, and RT-PCR was performed to detect mRNA level of Notch-1, Jagged-1, and HES-1. GAPDH was used to verify equivalent loading. Each panel represents an independent experiment with two animals per group. B: Graphic representation of relative abundance of Notch-1, Jagged-1, and HES-1 normalized to GAPDH. Data are given as mean ± SD (n = 6). *P < 0.05 versus normal rats.
Figure 2.
The protein level of Notch signaling components was increased in fibrotic peritoneum induced by PDF. A: Normal rats (A1 to A6) or rats that received daily intraperitoneal injections of 4.25% Dianeal (100 ml/kg) for 14 days (B1 to B6) or 28 days (C1 to C6) were sacrificed. The protein levels of NICD, Jagged-1, and HES-1 were analyzed by Western blot. GAPDH was used to verify equivalent loading. Each panel represents an independent experiment with two animals per group. B: Graphic representation of relative abundance of NICD, Jagged-1, and HES-1 normalized to GAPDH. Data are given as mean ± SD (n = 6). *P < 0.05 versus normal rats. **P < 0.01 versus normal rats.
Figure 3.
Immunofluorescence evidence for the increased Notch signaling activation in fibrotic peritoneum. Peritoneal sections of normal rats or rats on 28-day PDF treatment (100 ml/kg) were paraffin-fixed and stained with antibodies against NICD (A), Jagged-1 (B), and HES-1 (C). D: Peritoneal sections of rats on 28-day PDF treatment (100 ml/kg) were paraffin-fixed and costained with antibodies against HES-1(red) and α-SMA (green). Nuclei were stained with DAPI (blue). Images (magnification ×400) were taken by confocal microscopy.
DAPT Attenuates PDF-Induced Peritoneal Fibrosis and Prevents a Loss of Peritoneal Function
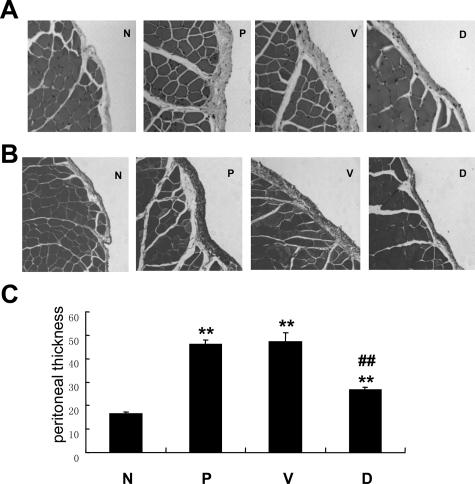
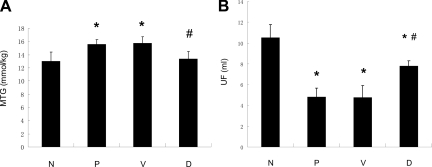
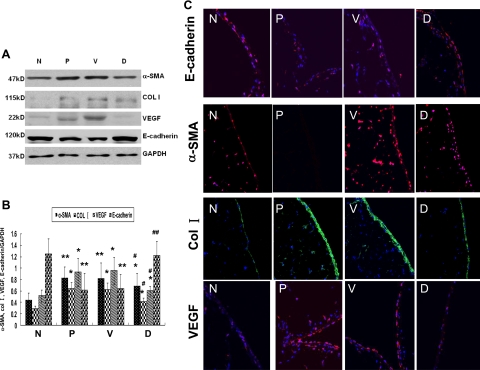
Given the potential clinical implication of GSI, we further tested the possibility that blocking Notch signaling activity by GSI could attenuate peritoneal fibrosis in vivo. Rats were administrated with 10 μmol/L N-[N-(3, 5-difluorophenacetyl)-l-alanyl]-S- phenylglycine t-butyl ester (DAPT), a highly active GSI, by intraperitoneal injection together with 4.25% Dianeal (100 ml/kg) for 28 days. Control rats received the same amount of DMSO (the vehicle for DAPT) together with 4.25% Dianeal (100 ml/kg). The efficiency of DAPT on inhibiting Notch signaling was demonstrated by decreased protein level of NICD and HES-1 (Figure 4, A and B). As expected, peritoneal fibrosis developed on day 28 after PDF treatment that was characterized by a marked peritoneal thickening, significant ECM accumulation, and a loss of linear mesothelial cells in rats that received PDF alone and in control rats receiving both DMSO and PDF. However, these features of peritoneal fibrosis remarkably attenuated in DAPT-treated rats. As shown in Figure 5, A–C, the thickness of peritoneum was significantly reduced in the DAPT-treated rats accompanied by less ECM accumulation. Moreover, the rates of mass transfer of glucose (MTG) and ultrafiltration rate, representative indexes of peritoneal function, were preserved in the DAPT-treated rats in comparison with those treated with PDF (Figure 6, A and B). Moreover, the preservation of peritoneal function by DAPT was accompanied by the attenuation in expression of several proteins characteristic of EMT and peritoneal fibrosis. By both Western blot analysis and immunofluorescence staining, the expression of α-SMA, collagen I, and VEGF was markedly attenuated in DAPT-treated rats compared with PDF treated rats, whereas the E-cadherin expression was increased significantly in DAPT-treated rats (Figure 7, A–C).
Figure 4.
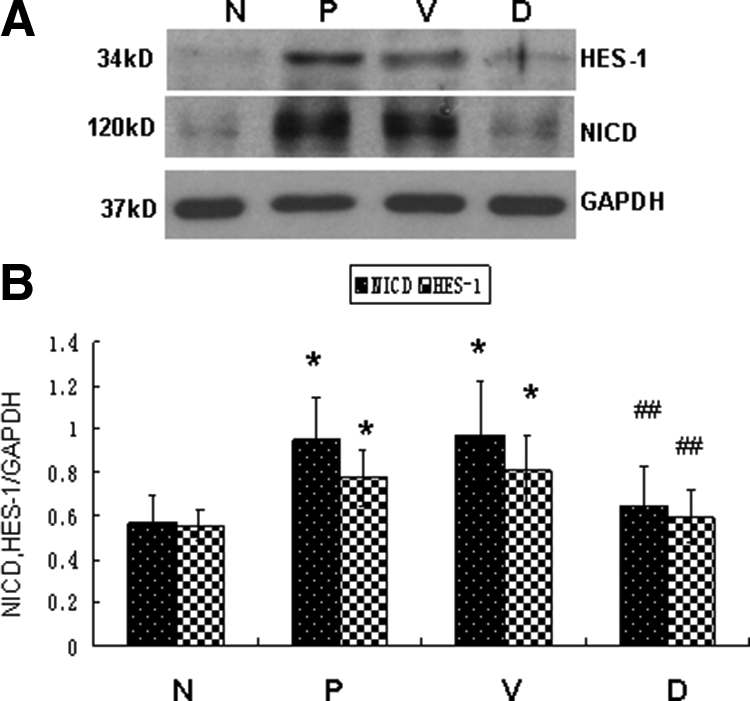
Effect of DAPT on inhibiting Notch signaling activation in fibrotic peritoneum. Peritoneal proteins were extracted, and the protein level of NICD and HES-1 was examined by Western blot. GAPDH was used to verify equivalent loading. B: Graphic representation of relative abundance of NICD and HES-1 normalized to GAPDH. Data are expressed as mean ± SD (n = 6). *P < 0.05 versus normal rats. ##P < 0.01 versus PDF-treated rats and vehicle control rats. N indicates normal rats; P, PDF-treated rats; V, vehicle control rats treated with DMSO together with PDF. D, Rats treated with DAPT together with PDF.
Figure 5.
DAPT treatment attenuated peritoneal fibrosis induced by PDF. A: Hematoxylin and eosin staining of paraffin-fixed parietal peritoneal sections. B: Masson Trichrome staining of paraffin-fixed parietal peritoneal sections. C: Semiquantification of the thickness of peritoneal membrane. Data are mean ± SD (n = 6). **P < 0.01 versus normal rats. ##P < 0.01 versus PDF-treated and vehicle control rats. N indicates normal rats; P, PDF-treated rats; V, vehicle control rats treated with DMSO together with PDF. D, Rats treated with DAPT together with PDF. Representative images (magnification ×200) were taken from groups of 6 rats.
Figure 6.
DAPT treatment prevented a loss of peritoneal function caused by PDF. Peritoneal function was assessed by (A) mass transfer of glucose (MTG) and (B) Ultrafiltration rate (UF). Data are mean ± SD (n = 6). *P < 0.05 versus normal rats. #P < 0.05 versus PDF treated rats and vehicle control rats. N indicates normal rats; P, PDF-treated rats; V, vehicle control rats treated with DMSO together with PDF. D, Rats treated with DAPT together with PDF.
Figure 7.
DAPT treatment attenuated the expression of several protein characteristics of EMT and peritoneal fibrosis. A: The protein expression of α-SMA, collagen I, VEGF, and E-cadherin in peritoneum was examined by Western blot. B: Graphic representation of relative abundance of α-SMA, collagen I, VEGF, and E-cadherin normalized to GAPDH. Data are expressed as mean ± SD (n = 6). *P < 0.05 versus normal rats. **P < 0.01 versus normal rats. #P < 0.05 versus PDF-treated rats and vehicle treated rats. ##P < 0.01 versus PDF-treated rats and vehicle control rats. C: Immunoflurescence staining of α-SMA, collagen I, VEGF, and E-cadherin in peritoneum. Paraffin-fixed peritoneum sections were stained with indicated antibodies. Nuclei were stained with DAPI (blue). Images (magnification ×400) were taken by confocal microscopy. N indicates normal rats; P, PDF-treated rats; V, vehicle control rats treated with DMSO together with PDF. D, Rats treated with DAPT together with PDF.
DAPT Attenuated TGF-β–Induced EMT of RPMCs
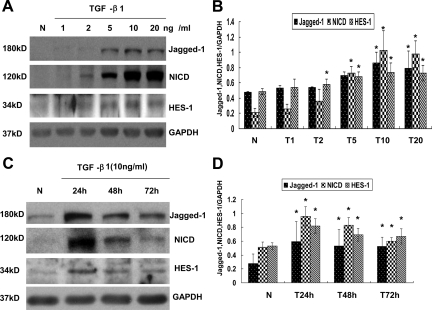
Prior studies demonstrated an important role of mesothelial cells in peritoneal fibrosis through EMT, and TGF-β could induce Notch downstream target Hey-1 expression in epithelial cells. Therefore, we hypothesized that TGF-β activates Notch signaling of RPMCs, and the mechanism by which DAPT prevents peritoneal fibrosis is by inhibiting EMT of RPMCs. To test this hypothesis, we isolated mesothelial cells from rat peritoneum and treated with incremental amount of TGF-β1 for 48 hours. As shown in Figure 8, A and B, the expression of NICD, Jagged-1, and HES-1 was gradually increased in a dose-dependent manner after TGF-β1 treatment, and the difference became significant after 10 ng/ml of TGF-β1 treatment compared with control cells. Because the expression of NICD, Jagged-1, and HES-1 reached a plateau at 10 ng/ml of TGF-β1 treatment, we thereof chose the dosage of 10 ng/ml TGF-β1 to study the kinetics of the Notch signaling activation. As shown in Figure 8, C and D, a significant increase of Jagged-1, NICD, and HES-1 was detected, and their levels peaked at 24 hours. The expression of Jagged-1 and HES-1 remained a plateau until 72 hours, whereas the expression of NICD was lower at 72 hours in comparison with that of the 24-hour time point. These results indicated that TGF-β1 could induce Notch signaling activation in a time- and dose-dependent manner in RPMCs.
Figure 8.
TGF-β1 induced Notch signaling cascade expression in RPMCs in a dose- and time-dependent manner. A: RPMCs were treated with the indicated amount of TGF-β1 for 48 hours, and cell lysates were harvested for Western blot analysis with antibodies against NICD, Jagged-1, and HES-1. The membrane was then stripped and blotted with anti-GAPDH to verify equal protein loading. B: Graphic representation of relative abundance of NICD, Jagged-1, and HES-1 normalized to GAPDH. Data are given as mean ± SD values of three independent experiments. *P < 0.05 versus normal cells. C: TGF-β1 induced Notch signaling cascade expression in RPMCs in a time dependent manner. RPMCs were treated with 10 ng/ml of TGF-β1 for the indicated time period, and cell lysates were harvested for Western blot analysis with antibody against NICD, Jagged-1, and HES-1. The membrane was then stripped and blotted with anti-GAPDH to verify equivalent protein loading. D: Graphic representation of the relative abundance of NICD, Jagged-1, and HES-1 normalized to GAPDH. Data are given as mean ± SD values of three independent experiments. *P < 0.05 versus normal cells.
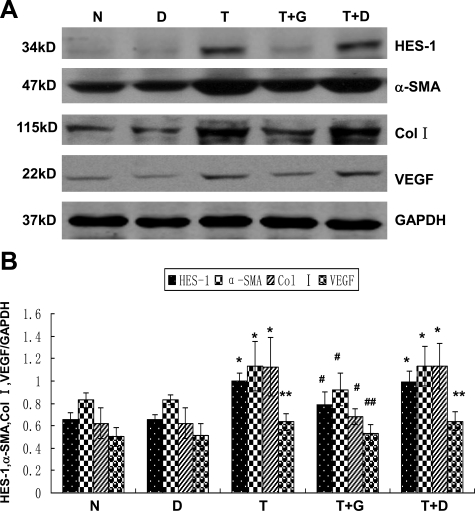
We next treated RPMCs with DAPT before the addition of TGF-β1. The expression of the Notch downstream target HES-1 was examined by Western blot analyses to monitor the efficiency of DAPT. As shown in Figure 9, A and B, 10-μmol/L DAPT treatment led to a significant decease of HES-1, indicating effective inhibition of TGF-β1–augmented Notch signaling activity. Similarly, TGF-β1–induced EMT of RPMCs was inhibited as indicated by the inhibition of TGF-β1–augmented α-SMA expression, a phenotypic marker of myofibroblasts and a hallmark of EMT at advanced stages. Similarly, DAPT was also effective in the inhibition of ECM accumulation attributable to increased synthesis of ECM proteins such as collagen I. It has been reported that transdifferentiated mesothelial cells synthesize VEGF6; therefore, we examined the expression of VEGF in RPMCs. Consistent with previous report, VEGF expression was significantly increased in TGF-β1–treated cells. DAPT treatment significantly attenuated TGF-β1–augmented VEGF expression. Taken together, these data demonstrated that Notch signaling is a downstream of TGF-β, and the key mechanism by which DAPT prevents peritoneal fibrosis is by inhibiting EMT of RPMCs.
Figure 9.
DAPT inhibited TGF-β1–induced EMT of RPMCs. A: RPMCs were treated with 10 μmol/L DAPT together with 10 ng/ml TGF-β1 for 48 hours, and cell lysates were harvested for Western blot analysis with antibodies against HES-1, α-SMA, Collagen I, and VEGF. GAPDH was used to verify equivalent loading. B: Graphic representation of the relative abundance of α-SMA, Collagen I, VEGF, and HES-1 normalized to GAPDH. Data are given as mean ± SD values of three independent experiments. *P < 0.05 versus normal cells. **P < 0.01 versus normal cells. #P < 0.05 versus TGF-β1–treated cells. ##P < 0.01 versus TGF-β1–treated cells. N indicates normal RPMCs; D, RPMCs treated with DMSO; T, RPMCs treated with 10 ng/ml of TGF-β1; T+G, RPMCs treated with 10 μmol/L DAPT plus 10 ng/ml of TGF-β1; T+D, RPMCs treated with DMSO plus 10 ng/ml of TGF-β1.
Discussion
In this study, we showed that Notch signaling was markedly activated in fibrotic peritoneum induced by PDF. This is indicated by the increased expression of NICD, Jagged-1, and HES-1. Likewise, in RPMCs, we detected elevated level of expression for Notch signaling components after TGF-β treatment. Treatment of RPMCs with the Notch inhibitor DAPT led to a strong inhibition of TGF-β–induced EMT. Importantly, DAPT treatment attenuated PDF-induced peritoneum fibrosis in vivo as demonstrated by the decreased expression of α-SMA, collagen I, and VEGF. Histochemical analysis further revealed a thinner peritoneum membrane with less ECM accumulation in submesothelial zone after DAPT treatment compared with the PDF-treated group. Importantly, DAPT treatment prevented a loss of peritoneal functions induced by PDF.
Notch signaling is an evolutionarily conserved local cell-signaling mechanism that functions in all metazoa as a major pathway leading to the determination of cellular identity during developmental stages.38 It has also been reported that Notch signaling is activated in mature organs on injury.39 Niranjan et al reported that Notch pathway is activated de novo in glomeruli (mainly in podocytes) in humans with diabetic nephropathy and FSGS and in rodent models thereof.40 Kobayash showed an activation of Delta-1/Notch-2/HES-1 pathway in a rat ischemia-reperfusion injury model.41 In addition, upregulation of Notch-1 and Jagged-1 proteins was detected in the rat liver after partial hepatectomy,42 and elevated NICD levels was reported in the brain after cerebral ischemi-reperfusion.43 In this study, our data demonstrated, for the first time, that Notch signaling is elevated during PDF-induced peritoneal fibrosis.
Numerous reports have indicated a role for EMT in fibrosis. Besides TGF-β signaling, Notch signaling pathway was found to contribute to EMT. In endothelial cells, Notch signaling up-regulates Snail to promote mesenchymal transformation, which is critical for normal heart development.28,44 In human breast epithelial cells, Jagged-1–mediated activation of Notch signaling induces EMT through induction of Slug and subsequent repression of E-cadheirn.45 During development and tumor progression, Notch seems to be independent of TGF-β signaling. However, several recent studies revealed TGF-β–dependent Notch activation during the process of EMT. A microarray survey of transcriptional changes in human keratinocytes exposed to TGF-β identified increased expression of HES-1.46 In epithelial cells from mammary gland, kidney tubules, and epidermis, TGF-β induces the Notch target gene Hey1 at the onset of EMT in a Smad3-dependent manner, followed by delayed Notch-dependent activation of Hey1.30 It has also been reported that Jagged-1 is increased in a TGF-β1–dependent manner in fibrotic renal disease in mice.29 However, those studies only indicated the role of TGF-β1 on certain component of Notch pathway. In the present study, we observed elevated expression of NICD, Jagged-1, and HES-1 during TGF-β1–induced EMT of RPMCs indicating that Notch signaling pathway is the downstream of TGF-β1. Moreover, increased TGF-β1 expression was detected in rat peritoneum after PDF treatment (data not shown), suggesting that the activation of Notch signaling in fibrotic peritoneum is TGF-β dependent. The mechanism by which TGF-β regulates Notch signaling is currently under investigation. It has been reported that recruitment of Smad3 to CSL, an essential DNA-binding component of Notch signaling, by direct interaction with NICD is responsible for TGF-β–induced HES-1 expression in C2C12 cells.47 However, our observation that TGF-β induced expression of not only the target gene of Notch signaling, but also Notch receptor and ligand in RPMCs, argues against this scenario.
Besides fibrosis, an increased vascular surface area with a high peritoneal solute transport rate is another cause of ultrafiltration failure after long-term PD.48,49,50 VEGF is reported to play an important role in the process that leads to increased vascular permeability by inducing vasodilatation and stimulating angiogenesis.6,51,52,53 A number of studies have shown that mesothelial cells have the capacity to produce VEGF in response to a variety of stimuli, and the underlying mechanism of VEGF upregulation in mesothelial cells is the mesenchymal conversion of these cells.6,54 Consistent with previous studies, we demonstrated an increased expression of VEGF during TGF-β1–induced EMT of RPMCs. The increased VEGF production by mesothelial cells could contribute to the elevated VEGF protein level in whole peritoneum after PDF treatment. Therefore, the effect of DAPT on VEGF expression in peritoneum could be partially explained by its inhibitory effect on EMT of RPMCs. However, we could not exclude the effect of DAPT on the Notch signaling in vascular endothelial cells. Previous studies have demonstrated the expression of Notch-1 and Jagged-1 and Jagged-2 in vascular endothelial cells in situ.55 In addition, activation of Notch signaling with Jagged-1 peptide was reported to enhance vascular endothelial cell proliferation, migration, and tube formation, which are critical for angiogenesis.39 Based on the results presented herein, we speculate that global targeting of the Notch pathway in peritoneum is a particularly efficient therapeutic strategy because it may serve a dual purpose by inhibiting peritoneal fibrosis and, at the same time, impeding angiogenesis.
Increasing evidence suggests that peritoneal fibrosis is a major cause of failure of PD.2 Until recently, no efficient treatment for PD-related peritoneal fibrosis is available. To the best of our knowledge, this is the first study to indicate that targeting the Notch signaling, such as by the use of a GSI, may be a novel therapeutic intervention for PD-related peritoneal fibrosis. GSI has been shown to be beneficial in animal models of Alzheimer disease,56,57 colon cancer,58 and ischemic stroke.43 The clinical trial to test the effectiveness of blocking Notch signaling with GSI in T cell acute lymphoblastic leukemia was also initiated.59 However, the adverse effects of chronic treatment with GSI observed on thymus, spleen, and gastrointestinal hampered this trial.60 Thus far in our study, intraperitoneal injection of DAPT for 28 days appears to be well tolerated by rats because DAPT treatment did not affect the body weight of rats (data not shown). Jonas Sjölund et al showed that an intermittent administration regimen would allow partial recovery of the small intestine between the successive rounds of drug delivery and thus decreased the adverse effects on the intestine.61 Notably, Real et al demonstrated that combination therapy with GSI plus glucocortioids delivered by intraperitoneal injection could improve the antileukemic effects of GSI and reduce its gut toxicity in leukemia-bearing mice.62 These studies provide an effective method to limit the adverse effects of GSI and shed light on the clinical implication of GSI. A further comprehensive evaluation of the optimal administration regime and/or the combined therapy of GSI with glucocortioids may facilitate the clinical development of an optimal GSI-based therapy for PD-related peritoneal fibrosis.
Acknowledgments
We thank Dr. Shu-Man Fu (University of Virginia) for critical review for the manuscript.
Footnotes
Address reprint requests to Dr. Jing Nie, Department of Nephrology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, 510080, PR China. E-mail: niejing@mail.sysu.edu.cn.
Supported by the National Natural Science Foundation of China (30871033) and Natural Science Foundation of Guangdong province (8251008901000014).
References
- Williams JD, Craig KJ, Topley N, Williams GT. Peritoneal dialysis: changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int Suppl. 2003:S158–S161. doi: 10.1046/j.1523-1755.63.s84.46.x. [DOI] [PubMed] [Google Scholar]
- Krediet RT, Lindholm B, Rippe B. Pathophysiology of peritoneal membrane failure. Perit Dial Int. 2000;20 Suppl 4:S22–S42. [PubMed] [Google Scholar]
- Tamura M, Osajima A, Nakayamada S, Anai H, Kabashima N, Kanegae K, Ota T, Tanaka Y, Nakashima Y. High glucose levels inhibit focal adhesion kinase-mediated wound healing of rat peritoneal mesothelial cells. Kidney Int. 2003;63:722–731. doi: 10.1046/j.1523-1755.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, Mackenzie RK, Williams GT. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13:470–479. doi: 10.1681/ASN.V132470. [DOI] [PubMed] [Google Scholar]
- Mortier S, Faict D, Schalkwijk CG, Lameire NH, De Vriese AS. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int. 2004;66:1257–1265. doi: 10.1111/j.1523-1755.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- Aroeira LS, Aguilera A, Selgas R, Ramirez-Huesca M, Perez-Lozano ML, Cirugeda A, Bajo MA, del Peso G, Sanchez-Tomero JA, Jimenez-Heffernan JA, Lopez-Cabrera M. Mesenchymal conversion of mesothelial cells as a mechanism responsible for high solute transport rate in peritoneal dialysis: role of vascular endothelial growth factor. Am J Kidney Dis. 2005;46:938–948. doi: 10.1053/j.ajkd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeira LS, Aguilera A, Sanchez-Tomero JA, Bajo MA, del Peso G, Jimenez-Heffernan JA, Selgas R, Lopez-Cabrera M. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18:2004–2013. doi: 10.1681/ASN.2006111292. [DOI] [PubMed] [Google Scholar]
- Del Peso G, Jimenez-Heffernan JA, Bajo MA, Aroeira LS, Aguilera A, Fernandez-Perpen A, Cirugeda A, Castro MJ, de Gracia R, Sanchez-Villanueva R, Sanchez-Tomero JA, Lopez-Cabrera M, Selgas R. Epithelial-to-mesenchymal transition of mesothelial cells is an early event during peritoneal dialysis and is associated with high peritoneal transport. Kidney Int Suppl. 2008:S26–S33. doi: 10.1038/sj.ki.5002598. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD, Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, Roberts VJ, Lemke G. Notch2: a second mammalian Notch gene. Development. 1992;116:931–941. doi: 10.1242/dev.116.4.931. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46:123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Shawber CJ, Boulter J, Weinmaster G. Jagged: a mammalian ligand that activates Notch1. Cell. 1995;80:909–917. doi: 10.1016/0092-8674(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–3773. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- Bettenhausen B, Gossler A. Efficient isolation of novel mouse genes differentially expressed in early postimplantation embryos. Genomics. 1995;28:436–441. doi: 10.1006/geno.1995.1172. [DOI] [PubMed] [Google Scholar]
- Dunwoodie SL, Henrique D, Harrison SM, Beddington RS. Mouse Dll3: a novel divergent Delta gene, which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development. 1997;124:3065–3076. doi: 10.1242/dev.124.16.3065. [DOI] [PubMed] [Google Scholar]
- Rao PK, Dorsch M, Chickering T, Zheng G, Jiang C, Goodearl A, Kadesch T, McCarthy S. Isolation and characterization of the notch ligand delta4. Exp Cell Res. 2000;260:379–386. doi: 10.1006/excr.2000.5034. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Bailey AM, Posakony JW. Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 1995;9:2609–2622. doi: 10.1101/gad.9.21.2609. [DOI] [PubMed] [Google Scholar]
- Iso T, Sartorelli V, Poizat C, Iezzi S, Wu HY, Chung G, Kedes L, Hamamori Y. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol Cell Biol. 2001;21:6080–6089. doi: 10.1128/MCB.21.17.6080-6089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo H, Lun Y, Johnson RL. Identification and expression of a novel family of bHLH cDNAs related to Drosophila hairy and enhancer of split. Biochem Biophys Res Commun. 1999;260:459–465. doi: 10.1006/bbrc.1999.0880. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Schumacher N, Steidl C, Gessler M. Analysis of HeyL expression in wild-type and Notch pathway mutant mouse embryos. Mech Dev. 2000;98:175–178. doi: 10.1016/s0925-4773(00)00459-7. [DOI] [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial-mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J, Guo G, Moridaira K, Fitzgerald M, McCracken R, Tolley T, Klahr S. Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J Am Soc Nephrol. 2002;13:1499–1508. doi: 10.1097/01.asn.0000017905.77985.4a. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Park JT, Davidson B, Morin PJ, Shih Ie M, Wang TL. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–5723. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis HD, Perez Revuelta BI, Nadin A, Neduvelil JG, Harrison T, Pollack SJ, Shearman MS. Catalytic site-directed gamma-secretase complex inhibitors do not discriminate pharmacologically between Notch S3 and beta-APP cleavages. Biochemistry. 2003;42:7580–7586. doi: 10.1021/bi034310g. [DOI] [PubMed] [Google Scholar]
- Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- Lewis HD, Leveridge M, Strack PR, Haldon CD, O'Neil J, Kim H, Madin A, Hannam JC, Look AT, Kohl N, Draetta G, Harrison T, Kerby JA, Shearman MS, Beher D. Apoptosis in T cell acute lymphoblastic leukemia cells after cell cycle arrest induced by pharmacological inhibition of notch signaling. Chem Biol. 2007;14:209–219. doi: 10.1016/j.chembiol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Kogoshi H, Sato T, Koyama T, Nara N, Tohda S. Gamma-secretase inhibitors suppress the growth of leukemia and lymphoma cells. Oncol Rep. 2007;18:77–80. [PubMed] [Google Scholar]
- Nakav S, Kachko L, Vorobiov M, Rogachev B, Chaimovitz C, Zlotnik M, Douvdevani A. Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrol Dial Transplant. 2009;24:2392–2399. doi: 10.1093/ndt/gfp041. [DOI] [PubMed] [Google Scholar]
- Nie J, Dou X, Hao W, Wang X, Peng W, Jia Z, Chen W, Li X, Luo N, Lan HY, Yu XQ. Smad7 gene transfer inhibits peritoneal fibrosis. Kidney Int. 2007;72:1336–1344. doi: 10.1038/sj.ki.5002533. [DOI] [PubMed] [Google Scholar]
- Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Arumugam TV, Son TG, Lathia JD, Jameel S, Mughal MR, Tang SC, Jo DG, Camandola S, Giunta M, Rakova I, McDonnell N, Miele L, Mattson MP, Poosala S. Involvement of notch signaling in wound healing. PLoS ONE. 2007;2:e1167. doi: 10.1371/journal.pone.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, Tohda S, Sakano S, Sasaki S. Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int. 2008;73:1240–1250. doi: 10.1038/ki.2008.74. [DOI] [PubMed] [Google Scholar]
- Kohler C, Bell AW, Bowen WC, Monga SP, Fleig W, Michalopoulos GK. Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology. 2004;39:1056–1065. doi: 10.1002/hep.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Noseda M, McLean G, Niessen K, Chang L, Pollet I, Montpetit R, Shahidi R, Dorovini-Zis K, Li L, Beckstead B, Durand RE, Hoodless PA, Karsan A. Notch activation results in phenotypic and functional changes consistent with endothelial-to-mesenchymal transformation. Circ Res. 2004;94:910–917. doi: 10.1161/01.RES.0000124300.76171.C9. [DOI] [PubMed] [Google Scholar]
- Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204:2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci USA. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibanez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese AS, Mortier S, Lameire NH. Neoangiogenesis in the peritoneal membrane: does it play a role in ultrafiltration failure. Nephrol Dial Transplant. 2001;16:2143–2145. doi: 10.1093/ndt/16.11.2143. [DOI] [PubMed] [Google Scholar]
- Krediet RT, Zweers MM, van der Wal AC, Struijk DG. Neoangiogenesis in the peritoneal membrane. Perit Dial Int. 2000;20 Suppl 2:S19–S25. [PubMed] [Google Scholar]
- Sherif AM, Nakayama M, Maruyama Y, Yoshida H, Yamamoto H, Yokoyama K, Kawakami M. Quantitative assessment of the peritoneal vessel density and vasculopathy in CAPD patients. Nephrol Dial Transplant. 2006;21:1675–1681. doi: 10.1093/ndt/gfl054. [DOI] [PubMed] [Google Scholar]
- Lai KN, Lai KB, Lam CW, Chan TM, Li FK, Leung JC. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;35:644–652. doi: 10.1016/s0272-6386(00)70011-4. [DOI] [PubMed] [Google Scholar]
- Pecoits-Filho R, Araujo MR, Lindholm B, Stenvinkel P, Abensur H, Romao JE, Jr, Marcondes M, De Oliveira AH, Noronha IL. Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant. 2002;17:1480–1486. doi: 10.1093/ndt/17.8.1480. [DOI] [PubMed] [Google Scholar]
- Zweers MM, de Waart DR, Smit W, Struijk DG, Krediet RT. Growth factors VEGF and TGF-beta1 in peritoneal dialysis. J Lab Clin Med. 1999;134:124–132. doi: 10.1016/s0022-2143(99)90116-6. [DOI] [PubMed] [Google Scholar]
- Mandl-Weber S, Cohen CD, Haslinger B, Kretzler M, Sitter T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002;61:570–578. doi: 10.1046/j.1523-1755.2002.00143.x. [DOI] [PubMed] [Google Scholar]
- Lindner V, Booth C, Prudovsky I, Small D, Maciag T, Liaw L. Members of the Jagged/Notch gene families are expressed in injured arteries and regulate cell phenotype via alterations in cell matrix and cell-cell interaction. Am J Pathol. 2001;159:875–883. doi: 10.1016/S0002-9440(10)61763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asberom T, Zhao Z, Bara TA, Clader JW, Greenlee WJ, Hyde LA, Josien HB, Li W, McPhail AT, Nomeir AA, Parker EM, Rajagopalan M, Song L, Wong GT, Zhang L, Zhang Q, Pissarnitski DA. Discovery of gamma-secretase inhibitors efficacious in a transgenic animal model of Alzheimer’s disease. Bioorg Med Chem Lett. 2007;17:511–516. doi: 10.1016/j.bmcl.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Barten DM, Meredith JE, Jr, Zaczek R, Houston JG, Albright CF. Gamma-secretase inhibitors for Alzheimer’s disease: balancing efficacy and toxicity. Drugs RD. 2006;7:87–97. doi: 10.2165/00126839-200607020-00003. [DOI] [PubMed] [Google Scholar]
- Ghaleb AM, Aggarwal G, Bialkowska AB, Nandan MO, Yang VW. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6:1920–1927. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T, Ferrando A. Oncogenic NOTCH1 control of MYC and PI3K: challenges and opportunities for anti-NOTCH1 therapy in T-cell acute lymphoblastic leukemias and lymphomas. Clin Cancer Res. 2008;14:5314–5317. doi: 10.1158/1078-0432.CCR-07-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, Zhang L, Higgins GA, Parker EM. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Sjolund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, Nilsson E, Ljungberg B, Axelson H. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest. 2008;118:217–228. doi: 10.1172/JCI32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, Sulis ML, Barnes K, Sawai C, Homminga I, Meijerink J, Aifantis I, Basso G, Cordon-Cardo C, Ai W, Ferrando A. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]