Abstract
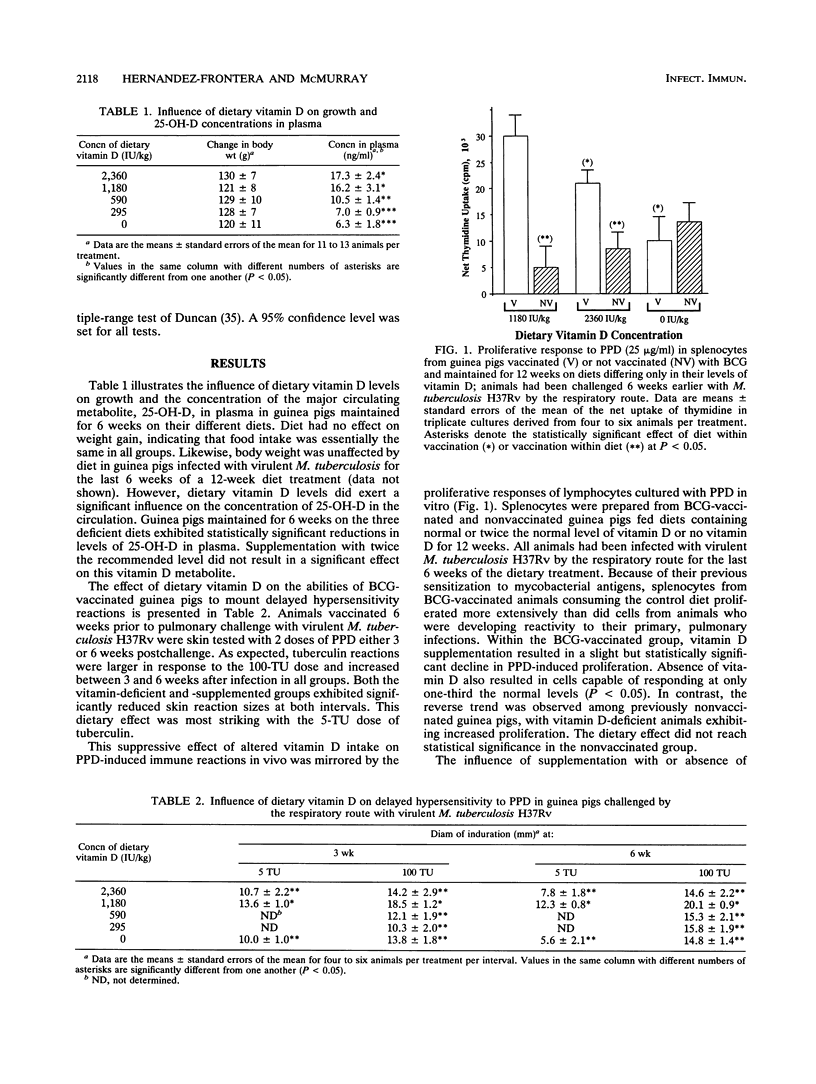
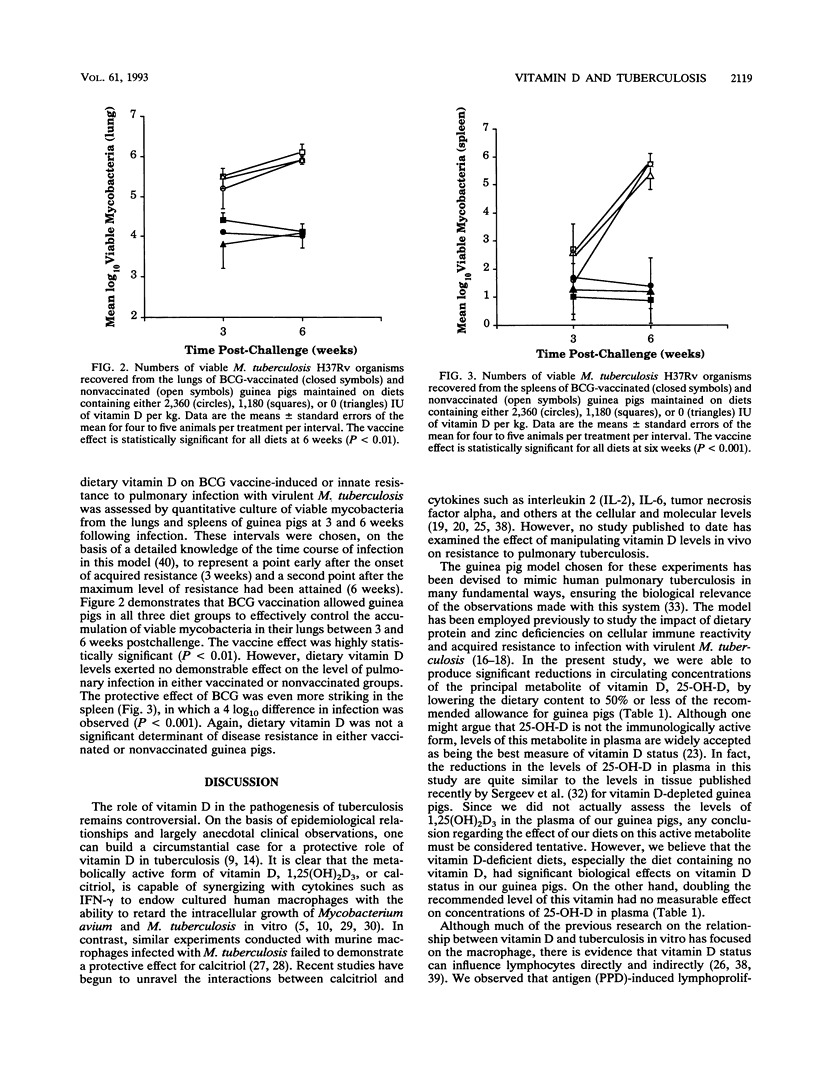
Outbred, Hartley strain guinea pigs were fed purified diets varying only in their levels of vitamin D. The amounts of vitamin D in the diets were adjusted to represent 0, 25, 50, 100, or 200% of the recommended level (1,180 IU/kg of body weight) for guinea pigs. In some experiments, half of the animals in each diet group were vaccinated with Mycobacterium bovis BCG vaccine at the time the diets were introduced. Six weeks later, all guinea pigs were infected by the respiratory route with a low dose of virulent M. tuberculosis H37Rv. Vitamin D-deficient animals exhibited marked reductions in levels of the major vitamin D metabolite, 25-hydroxyvitamin D3, in plasma. Altered vitamin D intake was accompanied by changes in antigen (purified protein derivative)-induced, cell-mediated immune responses both in vivo (tuberculin hypersensitivity) and in vitro (lymphoproliferation). Dermal tuberculin reactivity developed more slowly in vitamin D-deficient guinea pigs but eventually achieved normal levels. The proliferation of splenocytes cultured with purified protein derivative was suppressed by both deficiency and excess of dietary vitamin D. Vitamin D status did not affect the abilities of naive guinea pigs to control primary, pulmonary tuberculosis, nor did it influence the protective efficacy of BCG vaccination. We conclude that changes in dietary vitamin D are associated with alterations in some cellular immune functions but may not be an important determinant of disease outcome in pulmonary tuberculosis, as has been suggested previously.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Modlin R. L., Diz M. M., Barnes P. F. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab. 1989 Aug;69(2):457–460. doi: 10.1210/jcem-69-2-457. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Noff D., Edelstein S., Meyer M., Shibolet S., Goldman R. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33(6):673–676. doi: 10.1007/BF02409507. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Modlin R. L., Bikle D. D., Adams J. S. Transpleural gradient of 1,25-dihydroxyvitamin D in tuberculous pleuritis. J Clin Invest. 1989 May;83(5):1527–1532. doi: 10.1172/JCI114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N. H., Shary J., Shaw S., Turner R. T. Hypercalcemia associated with increased circulating 1,25 dihydroxyvitamin D in a patient with pulmonary tuberculosis. Calcif Tissue Int. 1985 Dec;37(6):588–591. doi: 10.1007/BF02554911. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Gupta S. 1,25 Dihydroxyvitamin D3-dependent inhibition of growth or killing of Mycobacterium avium complex in human macrophages is mediated by TNF and GM-CSF. Cell Immunol. 1990 May;127(2):432–441. doi: 10.1016/0008-8749(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Cadranel J., Hance A. J., Milleron B., Paillard F., Akoun G. M., Garabedian M. Vitamin D metabolism in tuberculosis. Production of 1,25(OH)2D3 by cells recovered by bronchoalveolar lavage and the role of this metabolite in calcium homeostasis. Am Rev Respir Dis. 1988 Oct;138(4):984–989. doi: 10.1164/ajrccm/138.4.984. [DOI] [PubMed] [Google Scholar]
- Chaisson R. E., Slutkin G. Tuberculosis and human immunodeficiency virus infection. J Infect Dis. 1989 Jan;159(1):96–100. doi: 10.1093/infdis/159.1.96. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Ross E. J., May M. H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987 Dec;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. D. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985 Dec;66(4):301–306. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Denis M. Killing of Mycobacterium tuberculosis within human monocytes: activation by cytokines and calcitriol. Clin Exp Immunol. 1991 May;84(2):200–206. doi: 10.1111/j.1365-2249.1991.tb08149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld A. J., Drezner M. K., Llach F. Hypercalcemia and elevated calcitriol in a maintenance dialysis patient with tuberculosis. Arch Intern Med. 1986 Oct;146(10):1941–1945. [PubMed] [Google Scholar]
- Girasole G., Wang J. M., Pedrazzoni M., Pioli G., Balotta C., Passeri M., Lazzarin A., Ridolfo A., Mantovani A. Augmentation of monocyte chemotaxis by 1 alpha,25-dihydroxyvitamin D3. Stimulation of defective migration of AIDS patients. J Immunol. 1990 Oct 15;145(8):2459–2464. [PubMed] [Google Scholar]
- Grange J. M., Davies P. D., Brown R. C., Woodhead J. S., Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985 Sep;66(3):187–191. doi: 10.1016/0041-3879(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Grover A. A., Kim H. K., Wiegeshaus E. H., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at -70 C. J Bacteriol. 1967 Oct;94(4):832–835. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killing intracellular mycobacteria: dogmas and realities. 5th Forum in Microbiology. Proceedings. Res Microbiol. 1990 Feb;141(2):191–270. doi: 10.1016/0923-2508(90)90029-p. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Carlomagno M. A., Mintzer C. L., Tetzlaff C. L. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect Immun. 1985 Nov;50(2):555–559. doi: 10.1128/iai.50.2.555-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray D. N., Mintzer C. L., Tetzlaff C. L., Carlomagno M. A. The influence of dietary protein on the protective effect of BCG in guinea pigs. Tubercle. 1986 Mar;67(1):31–39. doi: 10.1016/0041-3879(86)90029-2. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Yetley E. A. Response to Mycobacterium bovis BCG vaccination in protein- and zinc-deficient guinea pigs. Infect Immun. 1983 Feb;39(2):755–761. doi: 10.1128/iai.39.2.755-761.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K., Svenson M., Bendtzen K. 1 alpha,25-Dihydroxyvitamin D3 and a novel vitamin D analogue MC 903 are potent inhibitors of human interleukin 1 in vitro. Immunol Lett. 1988 Apr;17(4):361–365. doi: 10.1016/0165-2478(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Müller K., Diamant M., Bendtzen K. Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunol Lett. 1991 May;28(2):115–120. doi: 10.1016/0165-2478(91)90108-m. [DOI] [PubMed] [Google Scholar]
- Phalen S. W., McMurray D. N. T-lymphocyte response in a guinea pig model of tuberculous pleuritis. Infect Immun. 1993 Jan;61(1):142–145. doi: 10.1128/iai.61.1.142-145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Norman A. W. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989 Apr 13;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- Rigby W. F., Hamilton B. J., Waugh M. G. 1,25-Dihydroxyvitamin D3 modulates the effects of interleukin 2 independent of IL-2 receptor binding. Cell Immunol. 1990 Feb;125(2):396–414. doi: 10.1016/0008-8749(90)90094-8. [DOI] [PubMed] [Google Scholar]
- Rigby W. F., Stacy T., Fanger M. W. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol). J Clin Invest. 1984 Oct;74(4):1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- Schieffelbein C. W., Jr, Snider D. E., Jr Tuberculosis control among homeless populations. Arch Intern Med. 1988 Aug;148(8):1843–1846. [PubMed] [Google Scholar]
- Sergeev I. N., Arkhapchev Y. P., Spirichev V. B. Ascorbic acid effects on vitamin D hormone metabolism and binding in guinea pigs. J Nutr. 1990 Oct;120(10):1185–1190. doi: 10.1093/jn/120.10.1185. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Wiegeshaus E. H. What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S385–S393. doi: 10.1093/clinids/11.supplement_2.s385. [DOI] [PubMed] [Google Scholar]
- Ströder J., Kasal P. Evaluation of phagocytosis in rickets. Acta Paediatr Scand. 1970 May;59(3):288–292. doi: 10.1111/j.1651-2227.1970.tb09005.x. [DOI] [PubMed] [Google Scholar]
- Styblo K. Overview and epidemiologic assessment of the current global tuberculosis situation with an emphasis on control in developing countries. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S339–S346. doi: 10.1093/clinids/11.supplement_2.s339. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Provvedini D. M., Manolagas S. C. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984 Jun 29;224(4656):1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- Wiegeshaus E. H., McMurray D. N., Grover A. A., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970 Sep;102(3):422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]


