Abstract
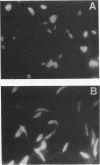
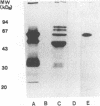
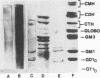
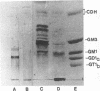
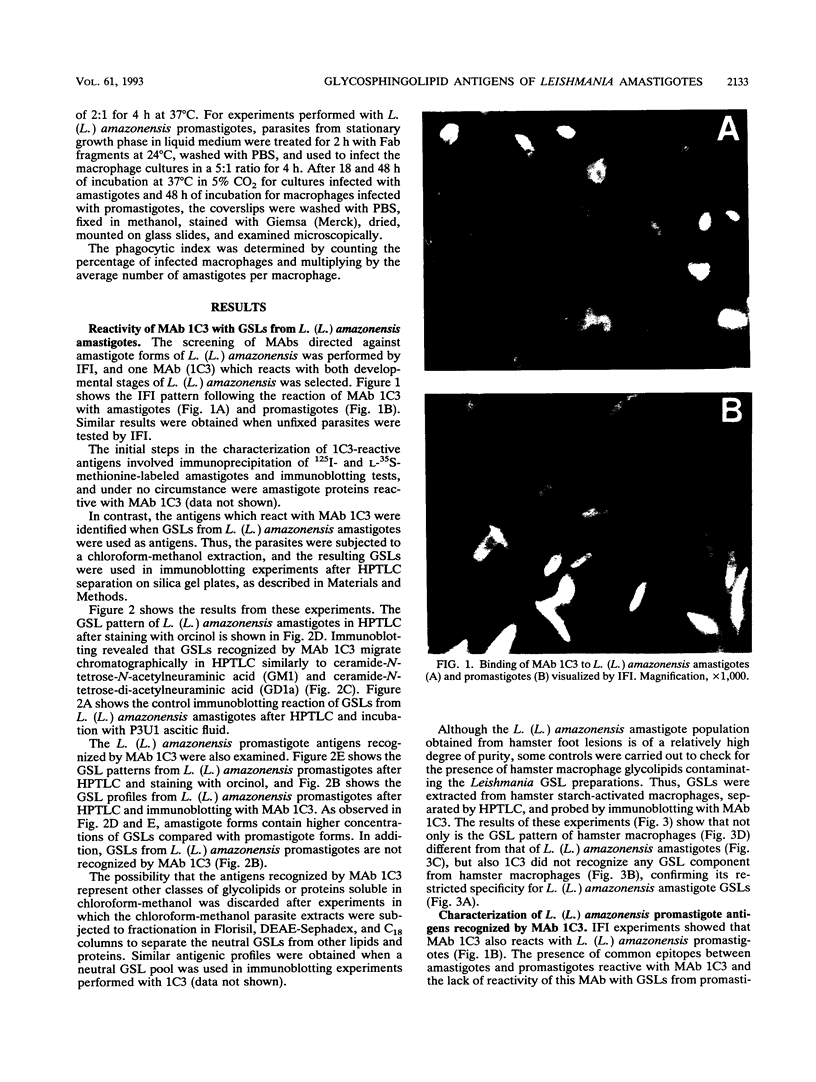
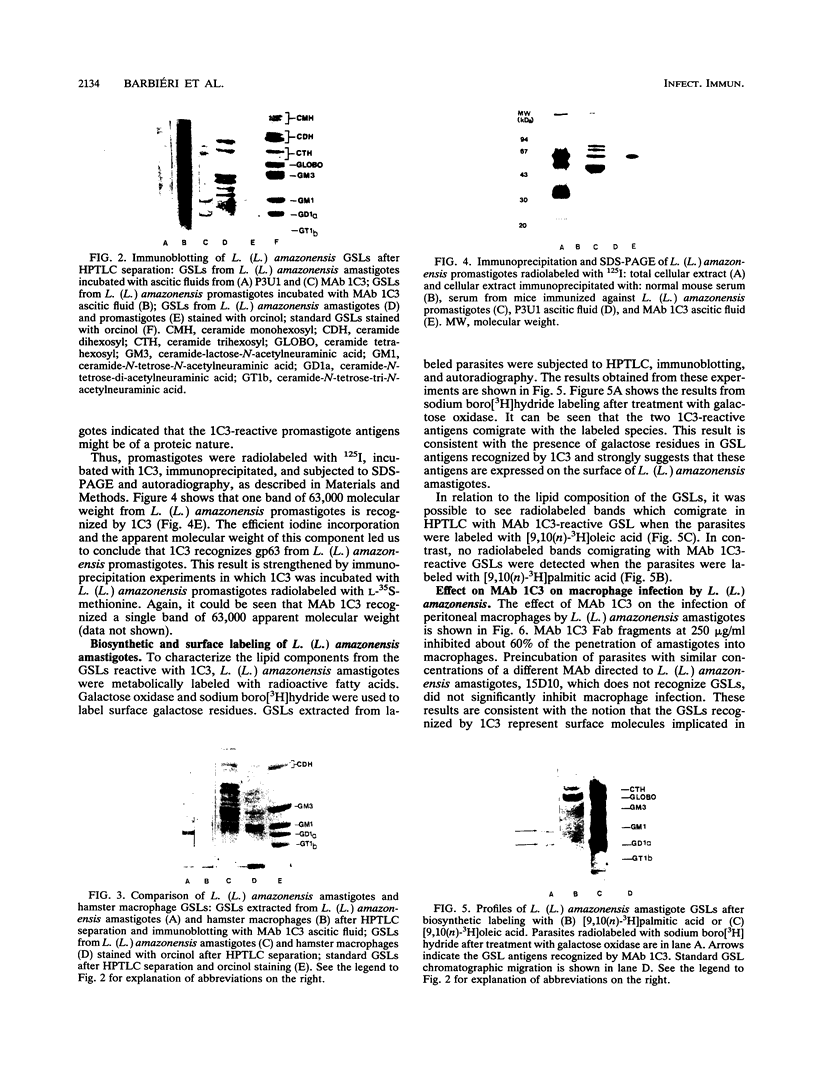
Monoclonal antibodies directed against Leishmania (Leishmania) amazonensis amastigotes were produced. One monoclonal antibody (1C3) selected by indirect immunofluorescence reacted with both amastigotes and promastigotes of L. (L.) amazonensis. Glycolipid extraction from L. (L.) amazonensis amastigotes and separation by high-performance thin-layer chromatography followed by immunoblotting demonstrated that 1C3 reacts with two glycosphingolipids which migrate chromatographically similarly to ceramide-N-acetylneuraminic acid (GM1) and ceramide-N-tetrose-di-acetylneuraminic acid (GD1a). The antibody did not react with glycosphingolipids from L. (L.) amazonensis promastigotes. Immunoprecipitation of 125I- and 35S-methionine-labeled promastigotes demonstrated that 1C3 recognizes gp63 from L. (L.) amazonensis promastigotes. Biosynthetic incorporation of labeled lipids by L. (L.) amazonensis amastigotes indicated that the glycosphingolipids reactive with 1C3 contain oleic acid in their structures. Surface labeling with galactose oxidase and sodium boro[3H]hydride indicated that galactose is present in 1C3-reactive antigens, strongly suggesting that these glycosphingolipids are localized on the surface of L. (L.) amazonensis amastigotes. Inhibition experiments of macrophage infection implicated the 1C3-reactive glycosphingolipids from L. (L.) amazonensis amastigotes in Leishmania invasion. The role of gp63 in promastigote-macrophage attachment was also demonstrated by inhibition experiments performed with 1C3, consistent with data from the literature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony R. L., Williams K. M., Sacci J. B., Rubin D. C. Subcellular and taxonomic specificity of monoclonal antibodies to New World Leishmania. Am J Trop Med Hyg. 1985 Nov;34(6):1085–1094. doi: 10.4269/ajtmh.1985.34.1085. [DOI] [PubMed] [Google Scholar]
- Bouvier J., Etges R., Bordier C. Identification of the promastigote surface protease in seven species of Leishmania. Mol Biochem Parasitol. 1987 May;24(1):73–79. doi: 10.1016/0166-6851(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Button L. L., Reiner N. E., McMaster W. R. Modification of GP63 genes from diverse species of Leishmania for expression of recombinant protein at high levels in Escherichia coli. Mol Biochem Parasitol. 1991 Feb;44(2):213–224. doi: 10.1016/0166-6851(91)90007-s. [DOI] [PubMed] [Google Scholar]
- CAMARGO E. P. GROWTH AND DIFFERENTIATION IN TRYPANOSOMA CRUZI. I. ORIGIN OF METACYCLIC TRYPANOSOMES IN LIQUID MEDIA. Rev Inst Med Trop Sao Paulo. 1964 May-Jun;6:93–100. [PubMed] [Google Scholar]
- Chang C. S., Chang K. P. Monoclonal antibody affinity purification of a Leishmania membrane glycoprotein and its inhibition of leishmania-macrophage binding. Proc Natl Acad Sci U S A. 1986 Jan;83(1):100–104. doi: 10.1073/pnas.83.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Pennington J., Uemura K., Scudder P., Goodfellow P. N., Evans M. J., Feizi T. High-molecular-weight glycoproteins are the major carriers of the carbohydrate differentiation antigens I, i and SSEA-1 of mouse teratocarcinoma cells. Biochem J. 1983 Dec 1;215(3):491–503. doi: 10.1042/bj2150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eperon S., McMahon-Pratt D. Extracellular amastigote-like forms of Leishmania panamensis and L. braziliensis. II. Stage- and species-specific monoclonal antibodies. J Protozool. 1989 Sep-Oct;36(5):510–518. doi: 10.1111/j.1550-7408.1989.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Giorgio S., Jasiulionis M. G., Straus A. H., Takahashi H. K., Barbiéri C. L. Inhibition of mouse lymphocyte proliferative response by glycosphingolipids from Leishmania (L.) amazonensis. Exp Parasitol. 1992 Aug;75(1):119–125. doi: 10.1016/0014-4894(92)90127-v. [DOI] [PubMed] [Google Scholar]
- Glaser T. A., Moody S. F., Handman E., Bacic A., Spithill T. W. An antigenically distinct lipophosphoglycan on amastigotes of Leishmania major. Mol Biochem Parasitol. 1991 Apr;45(2):337–344. doi: 10.1016/0166-6851(91)90102-c. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Jr, David J. R., McMahon-Pratt D. Identification and distribution of New World Leishmania species characterized by serodeme analysis using monoclonal antibodies. Am J Trop Med Hyg. 1987 Mar;36(2):270–287. doi: 10.4269/ajtmh.1987.36.270. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Tumor-associated carbohydrate antigens. Annu Rev Immunol. 1984;2:103–126. doi: 10.1146/annurev.iy.02.040184.000535. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe C. L., Rachamim N. Amastigote stage-specific monoclonal antibodies against Leishmania major. Infect Immun. 1989 Dec;57(12):3770–3777. doi: 10.1128/iai.57.12.3770-3777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M., Theander T. G., Handman E., Hey A. S., Kurtzhals J. A., Hviid L., Sørensen A. L., Were J. O., Koech D. K., Kharazmi A. Activation of human T lymphocytes by Leishmania lipophosphoglycan. Scand J Immunol. 1991 Feb;33(2):219–224. doi: 10.1111/j.1365-3083.1991.tb03752.x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981 Apr 3;212(4490):55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Steplewski Z., Koprowski H., Ginsburg V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983 Nov;43(11):5489–5492. [PubMed] [Google Scholar]
- Marcus D. M. A review of the immunogenic and immuno-modulatory properties of glycosphingolipids. Mol Immunol. 1984 Nov;21(11):1083–1091. doi: 10.1016/0161-5890(84)90118-4. [DOI] [PubMed] [Google Scholar]
- McConville M. J., Bacic A. A family of glycoinositol phospholipids from Leishmania major. Isolation, characterization, and antigenicity. J Biol Chem. 1989 Jan 15;264(2):757–766. [PubMed] [Google Scholar]
- McConville M. J., Bacic A., Mitchell G. F., Handman E. Lipophosphoglycan of Leishmania major that vaccinates against cutaneous leishmaniasis contains an alkylglycerophosphoinositol lipid anchor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8941–8945. doi: 10.1073/pnas.84.24.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M. J., Blackwell J. M. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991 Aug 15;266(23):15170–15179. [PubMed] [Google Scholar]
- Medina-Acosta E., Karess R. E., Schwartz H., Russell D. G. The promastigote surface protease (gp63) of Leishmania is expressed but differentially processed and localized in the amastigote stage. Mol Biochem Parasitol. 1989 Dec;37(2):263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- Mendonça S. C., Russell D. G., Coutinho S. G. Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin Exp Immunol. 1991 Mar;83(3):472–478. doi: 10.1111/j.1365-2249.1991.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A. ENZYMATIC DIGESTION OF RABBIT GAMMA GLOBULIN AND ANTIBODY AND CHROMATOGRAPHY OF DIGESTION PRODUCTS. Methods Med Res. 1964;10:134–141. [PubMed] [Google Scholar]
- Pratt D. M., David J. R. Monoclonal antibodies that distinguish between New World species of Leishmania. Nature. 1981 Jun 18;291(5816):581–583. doi: 10.1038/291581a0. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Russo D. M., Burns J. M., Jr, Carvalho E. M., Armitage R. J., Grabstein K. H., Button L. L., McMaster W. R., Reed S. G. Human T cell responses to gp63, a surface antigen of Leishmania. J Immunol. 1991 Nov 15;147(10):3575–3580. [PubMed] [Google Scholar]
- Russo D. M., Turco S. J., Burns J. M., Jr, Reed S. G. Stimulation of human T lymphocytes by Leishmania lipophosphoglycan-associated proteins. J Immunol. 1992 Jan 1;148(1):202–207. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Schoenenberger C., Ball R., Braun D. G., Rosenfelder G. Glycosphingolipid-blotting: an immunological detection procedure after separation by thin layer chromatography. J Immunol Methods. 1984 Sep 4;72(2):471–479. doi: 10.1016/0022-1759(84)90015-2. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Sacks D. L. Expression of a stage-specific lipophosphoglycan in Leishmania major amastigotes. Mol Biochem Parasitol. 1991 Mar;45(1):91–99. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Wilkerson M. A., Clawson D. R. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984 Mar 25;259(6):3883–3889. [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]