Abstract
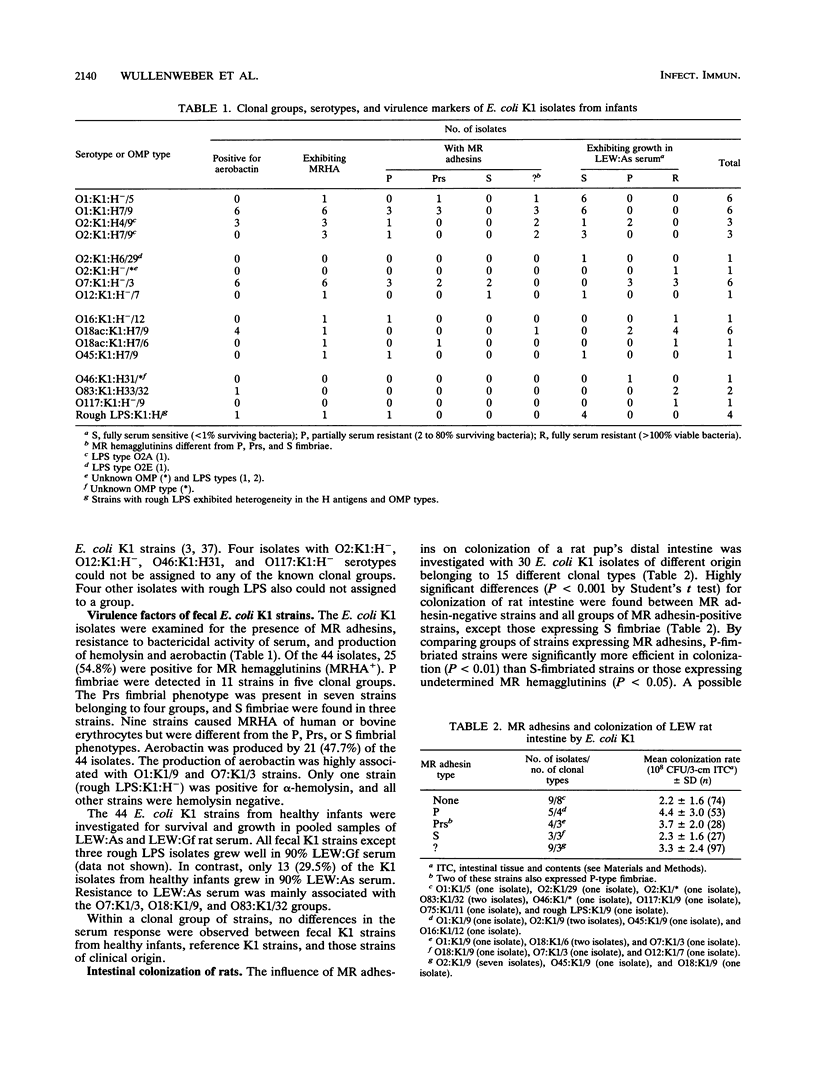
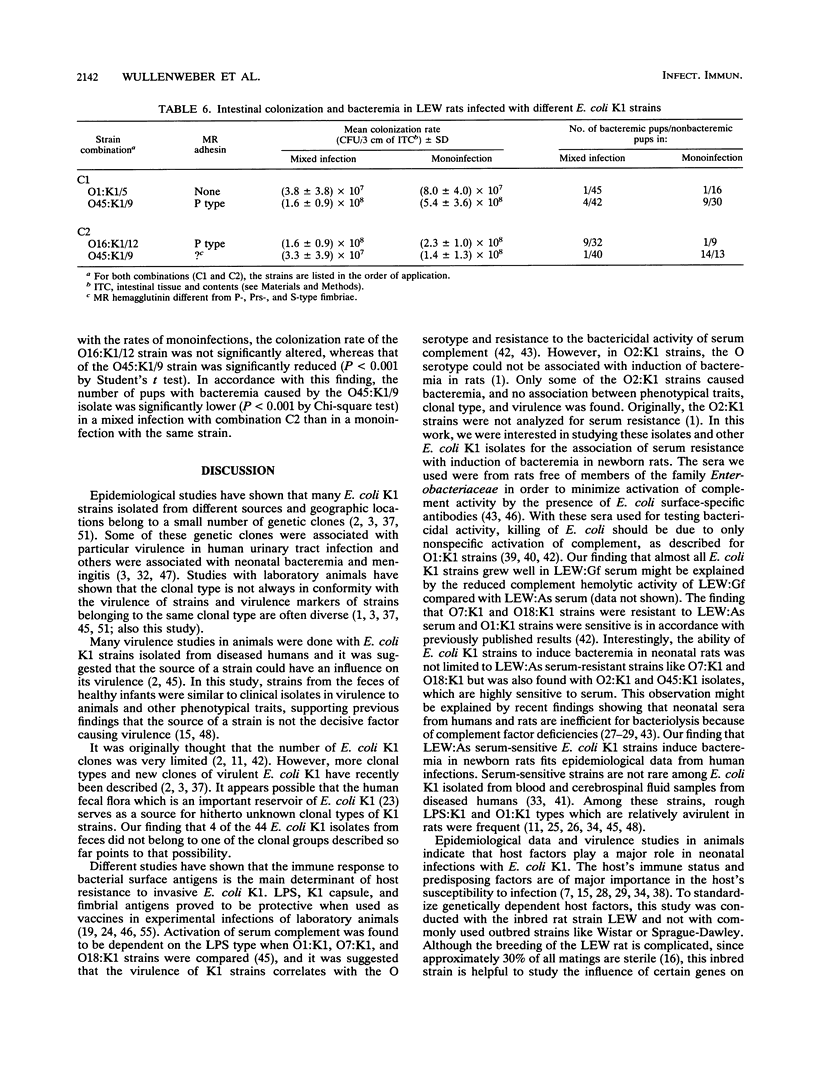
Of 209 healthy infants examined, 44 (21.1%) carried Escherichia coli K1 in their feces. Of these 44 isolates, 36 (81.8%) were attributed to 10 different known clonal groups of E. coli K1 and 4 isolates represented unknown types. The influence of mannose-resistant (MR) adhesins, aerobactin production, and resistance to serum on colonization and invasiveness of E. coli K1 in orally infected inbred LEW baby rats was investigated. Strains expressing MR adhesins had significantly higher colonization and invasion rates than non-MR strains did. Mixed-infection experiments of LEW rats revealed interactions between different types of E. coli K1 strains affecting colonization and invasion rats. P-fimbriated strains appeared to have a selective advantage for colonization. The bacteremic potentials of different E. coli K1 strains could not be associated with their resistance to sera from LEW rats free of members of the family Enterobacteriaceae. No differences in virulence between fecal E. coli K1 isolates and clinical isolates from diseased humans were found. An influence of the major histocompatibility complex on host susceptibility to invasive E. coli K1 was indicated by comparing the parental LEW rat strain with different congenic LEW strains (RT1).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Heuzenroeder M., Kusecek B., Ochman H., Caugant D., Selander R. K., Väisanen-Rhen V., Korhonen T. K., Stuart S., Orskov F. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect Immun. 1986 Jan;51(1):268–276. doi: 10.1128/iai.51.1.268-276.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Berg R. D. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980 Sep;29(3):1073–1081. doi: 10.1128/iai.29.3.1073-1081.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Prada J., Zimmermann S., Stephan R., Orskov I., Orskov F. Enterohemolysin, a new type of hemolysin produced by some strains of enteropathogenic E. coli (EPEC). Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Mar;267(4):576–588. doi: 10.1016/s0176-6724(88)80042-7. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Orndorff P. E. Impaired colonization by and full invasiveness of Escherichia coli K1 bearing a site-directed mutation in the type 1 pilin gene. Infect Immun. 1990 Jan;58(1):275–278. doi: 10.1128/iai.58.1.275-278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolussi R. Escherichia coli infection in neonates: humoral defense mechanisms. Semin Perinatol. 1990 Aug;14(4 Suppl 1):40–43. [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox F., Taylor L. Prevention of Escherichia coli K1 bacteremia in newborn mice by using topical vaginal carbohydrates. J Infect Dis. 1990 Oct;162(4):978–981. doi: 10.1093/infdis/162.4.978. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Gemski P., Sadoff J. C., Orskov F., Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis. 1984 Feb;149(2):184–193. doi: 10.1093/infdis/149.2.184. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Moxon E. R., Robbins J. B. Pathogenesis of neonatal Escherichia coli meningitis: induction of bacteremia and meningitis in infant rats fed E. coli K1. Infect Immun. 1977 Apr;16(1):75–80. doi: 10.1128/iai.16.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode M. P., Sutton A., Robbins J. B., McCracken G. H., Gotschlich E. C., Kaijser B., Hanson L. A. Neonatal meningitis due of Escherichia coli K1. J Infect Dis. 1977 Aug;136 (Suppl):S93–S97. doi: 10.1093/infdis/136.supplement.s93. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Woodson K., Hirshfeld D., Goldmann D. A. Heterologous protection against invasive Escherichia coli K1 disease in newborn rats by maternal immunization with purified mannose-sensitive pili. Infect Immun. 1989 May;57(5):1568–1572. doi: 10.1128/iai.57.5.1568-1572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Smith S. D., Wadowsky R. M., DePudyt L., Rowe M. I. The effect of E coli virulence on bacterial translocation and systemic sepsis in the neonatal rabbit model. J Pediatr Surg. 1991 Apr;26(4):483–486. doi: 10.1016/0022-3468(91)91000-o. [DOI] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B. M., Cross A. S., Futrovsky S. L., Sidberry H. F., Sadoff J. C. Monoclonal antibodies reactive with K1-encapsulated Escherichia coli lipopolysaccharide are opsonic and protect mice against lethal challenge. Infect Immun. 1986 May;52(2):617–619. doi: 10.1128/iai.52.2.617-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusecek B., Wloch H., Mercer A., Vaisänen V., Pluschke G., Korhonen T., Achtman M. Lipopolysaccharide, capsule, and fimbriae as virulence factors among O1, O7, O16, O18, or O75 and K1, K5, or K100 Escherichia coli. Infect Immun. 1984 Jan;43(1):368–379. doi: 10.1128/iai.43.1.368-379.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter H. A., Christensen R. D., Parker C., Rothstein G. Neutrophil-mediated killing, opsonization, and serum-mediated killing of Escherichia coli K1 by neonatal rats. Biol Neonate. 1988;53(3):156–162. doi: 10.1159/000242777. [DOI] [PubMed] [Google Scholar]
- Lassiter H. A., Tanner J. E., Miller R. D. Inefficient bacteriolysis of Escherichia coli by serum from human neonates. J Infect Dis. 1992 Feb;165(2):290–298. doi: 10.1093/infdis/165.2.290. [DOI] [PubMed] [Google Scholar]
- Lassiter H. A., Watson S. W., Seifring M. L., Tanner J. E. Complement factor 9 deficiency in serum of human neonates. J Infect Dis. 1992 Jul;166(1):53–57. doi: 10.1093/infdis/166.1.53. [DOI] [PubMed] [Google Scholar]
- Leying H., Suerbaum S., Kroll H. P., Stahl D., Opferkuch W. The capsular polysaccharide is a major determinant of serum resistance in K-1-positive blood culture isolates of Escherichia coli. Infect Immun. 1990 Jan;58(1):222–227. doi: 10.1128/iai.58.1.222-227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Mulder C. J., van Alphen L., Zanen H. C. Neonatal meningitis caused by Escherichia coli in The Netherlands. J Infect Dis. 1984 Dec;150(6):935–940. doi: 10.1093/infdis/150.6.935. [DOI] [PubMed] [Google Scholar]
- Mårild S., Jodal U., Orskov I., Orskov F., Svanborg Edén C. Special virulence of the Escherichia coli O1:K1:H7 clone in acute pyelonephritis. J Pediatr. 1989 Jul;115(1):40–45. doi: 10.1016/s0022-3476(89)80326-9. [DOI] [PubMed] [Google Scholar]
- Orskov F., Sorenson K. B. Escherichia coli serogroups in breast-fed and bottle-fed infants. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):25–30. [PubMed] [Google Scholar]
- Ott M., Bender L., Blum G., Schmittroth M., Achtman M., Tschäpe H., Hacker J. Virulence patterns and long-range genetic mapping of extraintestinal Escherichia coli K1, K5, and K100 isolates: use of pulsed-field gel electrophoresis. Infect Immun. 1991 Aug;59(8):2664–2672. doi: 10.1128/iai.59.8.2664-2672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J. C., Jr Neonatal bacterial meningitis. Analysis of predisposing factors and outcome compared with matched control subjects. J Pediatr. 1970 Apr;76(4):499–511. doi: 10.1016/s0022-3476(70)80399-7. [DOI] [PubMed] [Google Scholar]
- Pelkonen S., Pluschke G. Recombinant interleukin-1 stimulates clearance of Escherichia coli K1 bacteraemia. Microb Pathog. 1989 Jun;6(6):415–424. doi: 10.1016/0882-4010(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Pelkonen S., Pluschke G. Roles of spleen and liver in the clearance of Escherichia coli K1 bacteraemia in infant rats. Microb Pathog. 1989 Feb;6(2):93–102. doi: 10.1016/0882-4010(89)90012-0. [DOI] [PubMed] [Google Scholar]
- Pitt J. K-1 antigen of Escherichia coli: epidemiology and serum sensitivity of pathogenic strains. Infect Immun. 1978 Oct;22(1):219–224. doi: 10.1128/iai.22.1.219-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Antibodies to O-antigen of lipopolysaccharide are protective against neonatal infection with Escherichia coli K1. Infect Immun. 1985 Aug;49(2):365–370. doi: 10.1128/iai.49.2.365-370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infect Immun. 1984 Feb;43(2):684–692. doi: 10.1128/iai.43.2.684-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mercer A., Kusećek B., Pohl A., Achtman M. Induction of bacteremia in newborn rats by Escherichia coli K1 is correlated with only certain O (lipopolysaccharide) antigen types. Infect Immun. 1983 Feb;39(2):599–608. doi: 10.1128/iai.39.2.599-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles M. F., Mandine E., Zalisz R., Guenounou M., Smets P. Protective effects of murine monoclonal antibodies in experimental septicemia: E. coli antibodies protect against different serotypes of E. coli. J Infect Dis. 1989 Apr;159(4):641–647. doi: 10.1093/infdis/159.4.641. [DOI] [PubMed] [Google Scholar]
- Sandberg T., Kaijser B., Lidin-Janson G., Lincoln K., Orskov F., Orskov I., Stokland E., Svanborg-Edén C. Virulence of Escherichia coli in relation to host factors in women with symptomatic urinary tract infection. J Clin Microbiol. 1988 Aug;26(8):1471–1476. doi: 10.1128/jcm.26.8.1471-1476.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Saukkonen K. M., Nowicki B., Leinonen M. Role of type 1 and S fimbriae in the pathogenesis of Escherichia coli O18:K1 bacteremia and meningitis in the infant rat. Infect Immun. 1988 Apr;56(4):892–897. doi: 10.1128/iai.56.4.892-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco F. A., Guerina N. G., Goldmann D. A. Comparison of virulence and colonizing capacity of Escherichia coli K1 and non-K1 strains in neonatal rats. Infect Immun. 1982 Aug;37(2):830–832. doi: 10.1128/iai.37.2.830-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Korhonen T. K., Väisänen-Rhen V., Williams P. H., Pattison P. E., Caugant D. A. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986 Apr;52(1):213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen E. K., Berg R. D., Deitch E. A. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis. 1988 May;157(5):1032–1038. doi: 10.1093/infdis/157.5.1032. [DOI] [PubMed] [Google Scholar]
- Vermeulen C., Cross A., Byrne W. R., Zollinger W. Quantitative relationship between capsular content and killing of K1-encapsulated Escherichia coli. Infect Immun. 1988 Oct;56(10):2723–2730. doi: 10.1128/iai.56.10.2723-2730.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuopio-Varkila J., Mäkelä P. H. Killing of Escherichia coli in the peritoneal cavity of convalescent mice; role of specific and non-specific immune mechanisms. J Med Microbiol. 1988 Mar;25(3):205–211. doi: 10.1099/00222615-25-3-205. [DOI] [PubMed] [Google Scholar]
- Vuopio-Varkila J., Nurminen M., Pyhälä L., Mäkelä P. H. Lipopolysaccharide-induced non-specific resistance to systemic Escherichia coli infection in mice. J Med Microbiol. 1988 Mar;25(3):197–203. doi: 10.1099/00222615-25-3-197. [DOI] [PubMed] [Google Scholar]


