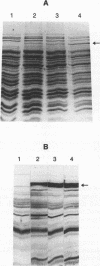
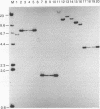
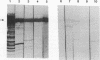
Abstract
Sera from lepromatous leprosy patients were used to screen a Mycobacterium leprae lambda gt11 library. Three positive plaques were picked, and lysogens were constructed. Immunoblot analysis showed that all of the lysogens expressed an apparently identical beta-galactosidase fusion protein which reacted strongly with the sera. The 1.7-kbp insert from one clone was subcloned into the lacZ gene in pUR290; sequence analysis of the end fused to lacZ revealed an open reading frame with no significant homology to previously published sequences. The insert was used to screen an M. leprae cosmid library, and five clones were isolated. The insert was also found to hybridize to clones expressing the M. leprae antigen which had previously been designated class III and 25L. A 1.8-kbp HindIII fragment was subcloned from one of the cosmids and sequenced. The sequence revealed a 1,227-bp open reading frame, encoding a 408-amino-acid protein with a predicted molecular mass of 42,466 Da. The protein contains amino- and carboxy-terminal hydrophobic domains and a hydrophilic central domain; the amino-terminal domain shows some homology to a 51-kDa hypothetical antigen of Mycobacterium tuberculosis, while the hydrophilic region contains a high proportion of serine residues, and we have therefore designated the protein serine-rich antigen (Sra). Some repeated motifs are present in the protein, but their significance is unknown. Seventy-eight percent of serum samples from multibacillary leprosy patients and 68% of serum samples from paucibacillary leprosy patients recognized the fusion protein, showing that this is a major M. leprae antigen. In contrast, all serum samples from endemic controls were negative, while 26% of serum samples from tuberculosis patients were weakly positive.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton W. J., Hellqvist L., Basten A., Raison R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J Immunol. 1985 Dec;135(6):4171–4177. [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Garsia R. J., Basten A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin Exp Immunol. 1987 Jan;67(1):31–42. [PMC free article] [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherayil B. J., Young R. A. A 28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J Immunol. 1988 Dec 15;141(12):4370–4375. [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifis T., Costopoulos C., Radford A. J., Bacic A., Wood P. R. Purification and characterization of major antigens from a Mycobacterium bovis culture filtrate. Infect Immun. 1991 Mar;59(3):800–807. doi: 10.1128/iai.59.3.800-807.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsia R. J., Hellqvist L., Booth R. J., Radford A. J., Britton W. J., Astbury L., Trent R. J., Basten A. Homology of the 70-kilodalton antigens from Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect Immun. 1989 Jan;57(1):204–212. doi: 10.1128/iai.57.1.204-212.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hilkens J., Ligtenberg M. J., Vos H. L., Litvinov S. V. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992 Sep;17(9):359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- Hinshelwood S., Stoker N. G. An Escherichia coli-Mycobacterium shuttle cosmid vector, pMSC1. Gene. 1992 Jan 2;110(1):115–118. doi: 10.1016/0378-1119(92)90453-v. [DOI] [PubMed] [Google Scholar]
- Ivanyi J., Sinha S., Aston R., Cussell D., Keen M., Sengupta U. Definition of species specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin Exp Immunol. 1983 Jun;52(3):528–536. [PMC free article] [PubMed] [Google Scholar]
- Jones D. S., Schofield J. P., Vaudin M. Fluorescent and radioactive solid phase dideoxy sequencing of pcr products in microtitre plates. DNA Seq. 1991;1(4):279–283. doi: 10.3109/10425179109020783. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Klatser P. R., De Wit M. Y., Kolk A. H. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin Exp Immunol. 1985 Dec;62(3):468–473. [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Laal S., Sharma Y. D., Prasad H. K., Murtaza A., Singh S., Tangri S., Misra R. S., Nath I. Recombinant fusion protein identified by lepromatous sera mimics native Mycobacterium leprae in T-cell responses across the leprosy spectrum. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1054–1058. doi: 10.1073/pnas.88.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Oftung F., Deggerdal A., Gill H. K., Young R. A., Godal T. Gene isolation with human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T cells specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J Immunol. 1988 Oct 15;141(8):2729–2733. [PubMed] [Google Scholar]
- Roy A., Lu C. F., Marykwas D. L., Lipke P. N., Kurjan J. The AGA1 product is involved in cell surface attachment of the Saccharomyces cerevisiae cell adhesion glycoprotein a-agglutinin. Mol Cell Biol. 1991 Aug;11(8):4196–4206. doi: 10.1128/mcb.11.8.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Esser R. E., Thole J. E., Clark-Curtiss J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect Immun. 1990 May;58(5):1327–1336. doi: 10.1128/iai.58.5.1327-1336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela S., Thole J. E., Ottenhoff T. H., Clark-Curtiss J. E. Identification of Mycobacterium leprae antigens from a cosmid library: characterization of a 15-kilodalton antigen that is recognized by both the humoral and cellular immune systems in leprosy patients. Infect Immun. 1991 Nov;59(11):4117–4124. doi: 10.1128/iai.59.11.4117-4124.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. G. The serodiagnosis of leprosy. Lepr Rev. 1992 Jun;63(2):97–100. doi: 10.5935/0305-7518.19920012. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Grant K. A., Dockrell H. M., Howard C. R., Jouy N. F., McAdam K. P. High level expression of genes cloned in phage lambda gt11. Gene. 1989 May 15;78(1):93–99. doi: 10.1016/0378-1119(89)90317-x. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Lopez F., Stoker N. G., Locniskar M. F., Dockrell H. M., Grant K. A., McAdam K. P. Recognition of mycobacterial antigens by sera from patients with leprosy. J Clin Microbiol. 1988 Dec;26(12):2474–2479. doi: 10.1128/jcm.26.12.2474-2479.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young D. B., Fohn M. J., Khanolkar S. R., Buchanan T. M. Monoclonal antibodies to a 28,000 mol. wt protein antigen of Mycobacterium leprae. Clin Exp Immunol. 1985 Jun;60(3):546–552. [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]